Patterns of Equine Small Strongyle Species Infection after Ivermectin Intervention in Thailand: Egg Reappearance Period and Nemabiome Metabarcoding Approach
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval and Informed Consent
2.2. Study Design and Fecal Sample Collection
2.3. Fecal Egg Count, Larval Culture, and Harvesting
2.4. DNA Preparation, ITS-2 Barcodes Amplification, and Sequencing
2.5. Bioinformatic Analysis
2.6. Statistical Analysis
2.6.1. Assessing IVM Effectiveness and Determination of ERP
2.6.2. Evaluation of Animal Weights
2.6.3. Diversity Indices
3. Results
3.1. Fecal Egg Counts
3.2. Animal Body Weights
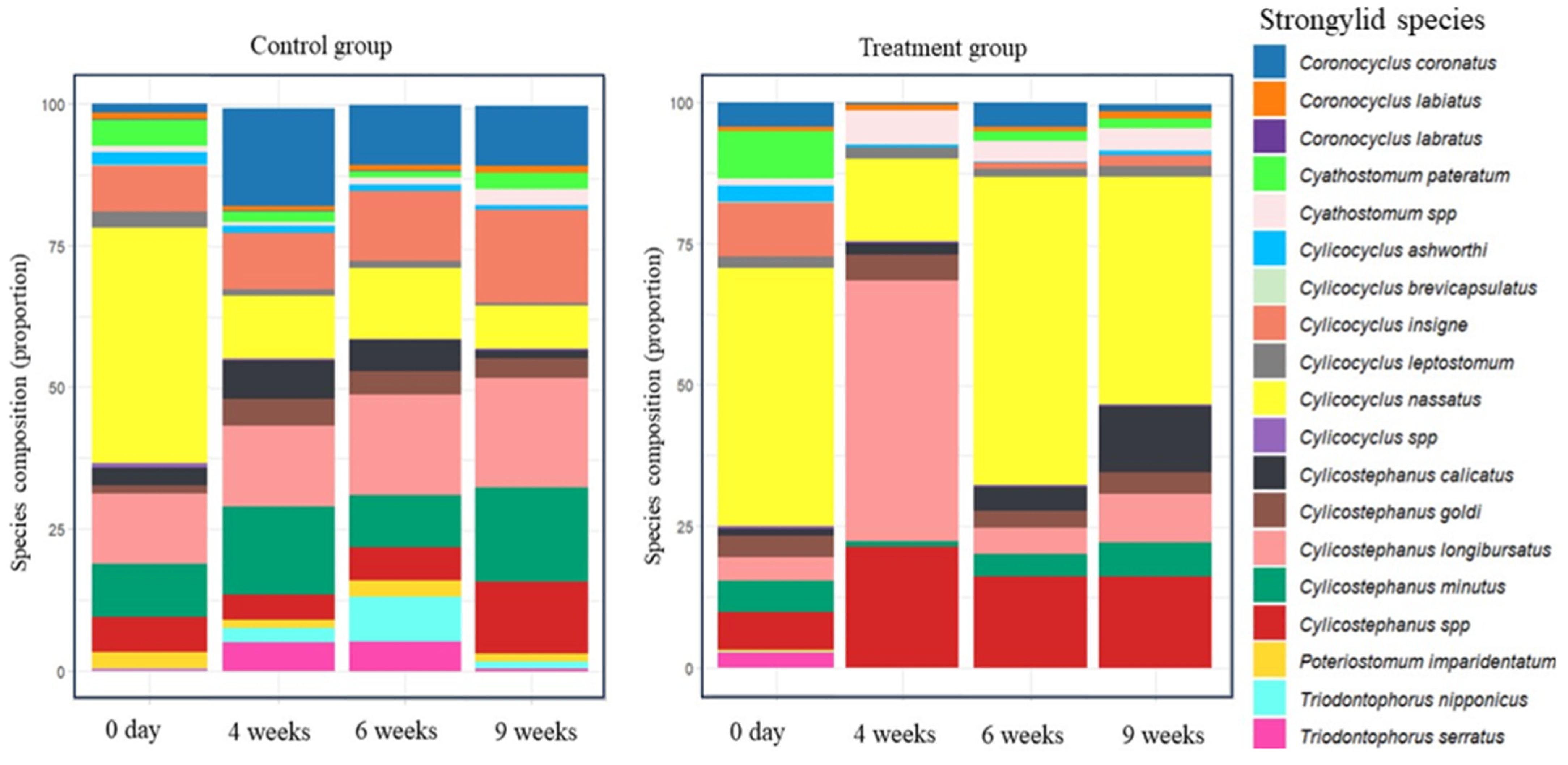
3.3. ITS-2 Nemabiome Metabarcoding and Diversity Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lichtenfels, J.R.; Kharchenko, V.A.; Dvojnos, G.M. Illustrated identification keys to strongylid parasites (Strongylidae: Nematoda) of horses, zebras and asses (Equidae). Vet. Parasitol. 2008, 156, 4–161. [Google Scholar] [CrossRef]
- Kuzmina, T.; Kornas, S.; Basiaga, M.; Kharchenko, V.; Vyniarska, A. Biodiversity of strongylids (Nematoda: Strongylidae) communities in domestic horses from Poland and Ukraine. Helminthologia 2011, 48, 77–84. [Google Scholar] [CrossRef]
- Matthews, J. An update on cyathostomins: Anthelmintic resistance and worm control. Equine Vet. Educ. 2008, 20, 552–560. [Google Scholar] [CrossRef]
- Kuzmina, T.A.; Dzeverin, I.; Kharchenko, V.A. Strongylids in domestic horses: Influence of horse age, breed and deworming programs on the strongyle parasite community. Vet. Parasitol. 2016, 227, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Lyons, E.T.; Tolliver, S.C.; Drudge, J.H. Historical perspective of cyathostomes: Prevalence, treatment and control programs. Vet. Parasitol. 1999, 85, 97–111; discussion 111–112, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Bredtmann, C.M.; Krucken, J.; Murugaiyan, J.; Kuzmina, T.; von Samson-Himmelstjerna, G. Nematode species identification-current status, challenges and future perspectives for cyathostomins. Front. Cell. Infect. Microbiol. 2017, 7, 283. [Google Scholar] [CrossRef] [PubMed]
- Bredtmann, C.M.; Krucken, J.; Murugaiyan, J.; Balard, A.; Hofer, H.; Kuzmina, T.A.; von Samson-Himmelstjerna, G. Concurrent proteomic fingerprinting and molecular analysis of cyathostomins. Proteomics 2019, 19, e1800290. [Google Scholar] [CrossRef] [PubMed]
- Bellaw, J.L.; Nielsen, M.K. Meta-analysis of cyathostomin species-specific prevalence and relative abundance in domestic horses from 1975–2020: Emphasis on geographical region and specimen collection method. Parasit. Vectors 2020, 13, 509. [Google Scholar] [CrossRef] [PubMed]
- Love, S.; Murphy, D.; Mellor, D. Pathogenicity of cyathostome infection. Vet. Parasitol. 1999, 85, 113–121; discussion 121–2, 215–25. [Google Scholar] [CrossRef]
- Salem, S.E.; Abd El-Ghany, A.M.; Hamad, M.H.; Abdelaal, A.M.; Elsheikh, H.A.; Hamid, A.A.; Saud, M.A.; Daniels, S.P.; Ras, R. Prevalence of gastrointestinal nematodes, parasite control practices and anthelmintic resistance patterns in a working horse population in Egypt. Equine Vet. J. 2021, 53, 339–348. [Google Scholar] [CrossRef]
- Duncan, J.; Bairden, K.; Abbott, E. Elimination of mucosal cyathostome larvae by five daily treatments with fenbendazole. Vet. Rec. 1998, 142, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Malan, F.S.; Reinecke, R.K.; Scialdo, R.C. Anthelmintic efficacy fenbendazole paste in equines. J. S. Afr. Vet. Assoc. 1981, 52, 127–130. [Google Scholar] [PubMed]
- Lyons, E.; Drudge, J.; Tolliver, S. Critical tests of three salts of pyrantel against internal parasites of the horse. Am. J. Vet. Res. 1975, 35, 1515–1522. [Google Scholar]
- Slocombe, J.O.; Smart, J. Evaluation of pyrantel pamoate against strongyles in horses. Can. Vet. J. 1975, 16, 310–312. [Google Scholar] [PubMed]
- Traversa, D. The little-known scenario of anthelmintic resistance in equine cyathostomes in Italy. Ann. N. Y. Acad. Sci. 2008, 1149, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Brady, H.A.; Nichols, W.T. Drug resistance in equine parasites: An emerging global problem. J. Equine Vet. Sci. 2009, 29, 285–295. [Google Scholar] [CrossRef]
- Peregrine, A.S.; Molento, M.B.; Kaplan, R.M.; Nielsen, M.K. Anthelmintic resistance in important parasites of horses: Does it really matter? Vet. Parasitol. 2014, 201, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.K.; Steuer, A.E.; Anderson, H.P.; Gavriliuc, S.; Carpenter, A.B.; Redman, E.M.; Gilleard, J.S.; Reinemeyer, C.R.; Poissant, J. Shortened egg reappearance periods of equine cyathostomins following ivermectin or moxidectin treatment: Morphological and molecular investigation of efficacy and species composition. Int. J. Parasitol. 2022, 52, 787–798. [Google Scholar] [CrossRef]
- Kuzmina, T.A.; Zvegintsova, N.S.; Yasyntelska, N.I.; Kharchenko, V.A. Anthelmintic resistance in strongylids (Nematoda: Strongylidae) parasitizing wild and domestic equids in the Askania Nova Biosphere Reserve, Ukraine. Ann. Parasitol. 2020, 66, 49–60. [Google Scholar] [CrossRef]
- Relf, V.E.; Morgan, E.R.; Hodgkinson, J.E.; Matthews, J.B. A questionnaire study on parasite control practices on UK breeding Thoroughbred studs. Equine Vet. J. 2012, 44, 466–471. [Google Scholar] [CrossRef]
- Nielsen, M.K.; Monrad, J.; Olsen, S.N. Prescription-only anthelmintics--a questionnaire survey of strategies for surveillance and control of equine strongyles in Denmark. Vet. Parasitol. 2006, 135, 47–55. [Google Scholar] [CrossRef]
- Bellaw, J.L.; Krebs, K.; Reinemeyer, C.R.; Norris, J.K.; Scare, J.A.; Pagano, S.; Nielsen, M.K. Anthelmintic therapy of equine cyathostomin nematodes–larvicidal efficacy, egg reappearance period, and drug resistance. Int. J. Parasitol. 2018, 48, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Elghryani, N.; McOwan, T.; Mincher, C.; Duggan, V.; de Waal, T. Estimating the prevalence and factors affecting the shedding of helminth eggs in Irish equine populations. Animals 2023, 13, 581. [Google Scholar] [CrossRef]
- Alm, Y.H.; Osterman-Lind, E.; Martin, F.; Lindfors, R.; Roepstorff, N.; Hedenstrom, U.; Fredriksson, I.; Halvarsson, P.; Tyden, E. Retained efficacy of ivermectin against cyathostomins in Swedish horse establishments practicing selective anthelmintic treatment. Vet. Parasitol. 2023, 322, 110007. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Nisar, A.; Yuan, J.; Luo, X.; Dou, X.; Liu, F.; Zhao, X.; Li, J.; Ahmad, H.; Mehmood, S.A.; et al. A whole genome re-sequencing based GWA analysis reveals candidate genes associated with ivermectin resistance in Haemonchus contortus. Genes 2020, 11, 367. [Google Scholar] [CrossRef] [PubMed]
- Robert, M.; Hu, W.; Nielsen, M.; Stowe, C. Attitudes towards implementation of surveillance-based parasite control on Kentucky Thoroughbred farms–current strategies, awareness and willingness-to-pay. Equine Vet. J. 2015, 47, 694–700. [Google Scholar] [CrossRef]
- Raza, A.; Qamar, A.G.; Hayat, K.; Ashraf, S.; Williams, A.R. Anthelmintic resistance and novel control options in equine gastrointestinal nematodes. Parasitology 2019, 146, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Lyons, E.T.; Tolliver, S.C.; Ionita, M.; Lewellen, A.; Collins, S.S. Field studies indicating reduced activity of ivermectin on small strongyles in horses on a farm in Central Kentucky. Parasitol. Res. 2008, 103, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Lyons, E.; Tolliver, S. Further indication of lowered activity of ivermectin on immature small strongyles in the intestinal lumen of horses on a farm in Central Kentucky. Parasitol. Res. 2013, 112, 889–891. [Google Scholar] [CrossRef]
- Coles, G.; Bauer, C.; Borgsteede, F.; Geerts, S.; Klei, T.; Taylor, M.; Waller, P. World Association for the Advancement of Veterinary Parasitology (WAAVP) methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 1992, 44, 35–44. [Google Scholar] [CrossRef]
- Kaplan, R.M.; Denwood, M.J.; Nielsen, M.K.; Thamsborg, S.M.; Torgerson, P.R.; Gilleard, J.S.; Dobson, R.J.; Vercruysse, J.; Levecke, B. World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) guideline for diagnosing anthelmintic resistance using the faecal egg count reduction test in ruminants, horses and swine. Vet. Parasitol. 2023, 318, 109936. [Google Scholar] [CrossRef] [PubMed]
- Sangster, N.C. Pharmacology of anthelmintic resistance in cyathostomes: Will it occur with the avermectin/milbemycins? Vet. Parasitol. 1999, 85, 189–201; discussion 201–4, 215–25. [Google Scholar] [CrossRef]
- Nielsen, M.K. Anthelmintic resistance in equine nematodes: Current status and emerging trends. Int. J. Parasitol. Drugs Drug Resist. 2022, 20, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.K.; von Samson-Himmelstjerna, G.; Kuzmina, T.A.; van Doorn, D.C.K.; Meana, A.; Rehbein, S.; Elliott, T.; Reinemeyer, C.R. World association for the advancement of veterinary parasitology (WAAVP): Third edition of guideline for evaluating the efficacy of equine anthelmintics. Vet. Parasitol. 2022, 303, 109676. [Google Scholar] [CrossRef] [PubMed]
- Buono, F.; Roncoroni, C.; Pacifico, L.; Piantedosi, D.; Neola, B.; Barile, V.L.; Fagiolo, A.; Várady, M.; Veneziano, V. Cyathostominae egg reappearance period after treatment with major horse anthelmintics in donkeys. J. Equine Vet. Sci. 2018, 65, 6–11. [Google Scholar] [CrossRef]
- Demeulenaere, D.; Vercruysse, J.; Dorny, P.; Claerebout, E. Comparative studies of ivermectin and moxidectin in the control of naturally acquired cyathostome infections in horses. Vet. Rec. 1997, 141, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Piche, C.A.; Kennedy, M.J.; Herbers, H.A.; Newcomb, K.M. Comparison of ivermectin, oxibendazole, and pyrantel pamoate in suppressing fecal egg output in horses. Can. Vet. J. 1991, 32, 104–107. [Google Scholar]
- Porr, C.A.S.; Hedinger, V.F.; Hamm, L.R.; Ernst, M.M.; Papajeski, B.M.; Santiago, M.L.; Davis, A.J. Effects of ivermectin and moxidectin on fecal egg count and egg reappearance rate in horses. J. Equine Vet. Sci. 2017, 57, 51–55. [Google Scholar] [CrossRef]
- Baranova, M.V.; Panova, O.A.; Polukhina, D.N.; Panova, D.S. Reduction of the nematode egg reappearance period in horses after anthelmintic therapy. Vet. World 2022, 15, 1530–1534. [Google Scholar] [CrossRef]
- Corning, S. Equine cyathostomins: A review of biology, clinical significance and therapy. Parasit. Vectors 2009, 2 (Suppl. S2), S1. [Google Scholar] [CrossRef]
- Johnson, A.C.B.; Biddle, A.S. The use of molecular profiling to track equine reinfection rates of cyathostomin species following anthelmintic administration. Animals 2021, 11, 1345. [Google Scholar] [CrossRef]
- Molena, R.A.; Peachey, L.E.; Di Cesare, A.; Traversa, D.; Cantacessi, C. Cyathostomine egg reappearance period following ivermectin treatment in a cohort of UK Thoroughbreds. Parasit. Vectors 2018, 11, 61. [Google Scholar] [CrossRef]
- Sargison, N.; Chambers, A.; Chaudhry, U.; Júnior, L.C.; Doyle, S.R.; Ehimiyein, A.; Evans, M.; Jennings, A.; Kelly, R.; Sargison, F. Faecal egg counts and nemabiome metabarcoding highlight the genomic complexity of equine cyathostomin communities and provide insight into their dynamics in a Scottish native pony herd. Int. J. Parasitol. 2022, 52, 763–774. [Google Scholar] [CrossRef]
- Poissant, J.; Gavriliuc, S.; Bellaw, J.; Redman, E.M.; Avramenko, R.W.; Robinson, D.; Workentine, M.L.; Shury, T.K.; Jenkins, E.J.; McLoughlin, P.D.; et al. A repeatable and quantitative DNA metabarcoding assay to characterize mixed strongyle infections in horses. Int. J. Parasitol. 2021, 51, 183–192. [Google Scholar] [CrossRef]
- Avramenko, R.W.; Bras, A.; Redman, E.M.; Woodbury, M.R.; Wagner, B.; Shury, T.; Liccioli, S.; Windeyer, M.C.; Gilleard, J.S. High species diversity of trichostrongyle parasite communities within and between Western Canadian commercial and conservation bison herds revealed by nemabiome metabarcoding. Parasit. Vectors 2018, 11, 299. [Google Scholar] [CrossRef] [PubMed]
- Pafco, B.; Cizkova, D.; Kreisinger, J.; Hasegawa, H.; Vallo, P.; Shutt, K.; Todd, A.; Petrzelkova, K.J.; Modry, D. Metabarcoding analysis of strongylid nematode diversity in two sympatric primate species. Sci. Rep. 2018, 8, 5933. [Google Scholar] [CrossRef] [PubMed]
- van Wyk, J.A. Refugia-overlooked as perhaps the most potent factor concerning the development of anthelmintic resistance. Onderstepoort J. Vet. 2001, 68, 55–67. [Google Scholar]
- Lester, H.E.; Morgan, E.R.; Hodgkinson, J.E.; Matthews, J.B. Analysis of strongyle egg shedding consistency in horses and factors that affect it. J. Equine Vet. Sci. 2018, 60, 113–119.e1. [Google Scholar] [CrossRef]
- Nielsen, M.K.; Mittel, L.; Grice, A.; Erskine, M.; Graves, E.; Vaala, W.; Tully, R.C.; French, D.D.; Bowman, R.; Kaplan, R.M. AAEP Parasite Control Guidelines. Online at American Association of Equine Practitioners. 2019. Available online: www.aaep.org (accessed on 1 December 2020).
- Dias de Castro, L.L.; Abrahão, C.L.H.; Buzatti, A.; Molento, M.B.; Bastianetto, E.; Rodrigues, D.S.; Lopes, L.B.; Silva, M.X.; de Freitas, M.G.; Conde, M.H.; et al. Comparison of McMaster and Mini-FLOTAC fecal egg counting techniques in cattle and horses. Vet. Parasitol. Reg. Stud. Rep. 2017, 10, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Manual of Veterinary Parasitological Laboratory Techniques, 3rd ed.; Ref. Book 418; Ministry of Agriculture Fisheries and Food: London, UK, 1986; p. 160.
- Gasser, R.B.; Chilton, N.B.; Hoste, H.; Beveridge, I. Rapid sequencing of rDNA from single worms and eggs of parasitic helminths. Nucleic Acids Res. 1993, 21, 2525–2526. [Google Scholar] [CrossRef] [PubMed]
- Boisseau, M.; Dhorne-Pollet, S.; Bars-Cortina, D.; Courtot, É.; Serreau, D.; Annonay, G.; Lluch, J.; Gesbert, A.; Reigner, F.; Sallé, G. Species interactions, stability, and resilience of the gut microbiota-helminth assemblage in horses. iScience 2023, 26, 106044. [Google Scholar] [CrossRef]
- Jalili, V.; Afgan, E.; Gu, Q.; Clements, D.; Blankenberg, D.; Goecks, J.; Taylor, J.; Nekrutenko, A. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2020 update. Nucleic Acids Res. 2020, 48, W395–W402. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson-Palme, J.; Ryberg, M.; Hartmann, M.; Branco, S.; Wang, Z.; Godhe, A.; De Wit, P.; Sánchez-García, M.; Ebersberger, I.; de Sousa, F. Improved software detection and extraction of ITS1 and ITS2 from ribosomal ITS sequences of fungi and other eukaryotes for analysis of environmental sequencing data. Methods Ecol. Evol. 2013, 4, 914–919. [Google Scholar] [CrossRef]
- Wright, E.S.; Vetsigian, K.H. Quality filtering of Illumina index reads mitigates sample cross-talk. BMC Genom. 2016, 17, 876. [Google Scholar] [CrossRef] [PubMed]
- Avramenko, R.W.; Redman, E.M.; Lewis, R.; Yazwinski, T.A.; Wasmuth, J.D.; Gilleard, J.S. Exploring the gastrointestinal “Nemabiome”: Deep amplicon sequencing to quantify the species composition of parasitic nematode communities. PLoS ONE 2015, 10, e0143559. [Google Scholar] [CrossRef] [PubMed]
- Denwood, M.J.; Kaplan, R.M.; McKendrick, I.J.; Thamsborg, S.M.; Nielsen, M.K.; Levecke, B. A statistical framework for calculating prospective sample sizes and classifying efficacy results for faecal egg count reduction tests in ruminants, horses and swine. Vet. Parasitol. 2023, 314, 109867. [Google Scholar] [CrossRef]
- Torgerson, P.R.; Paul, M.; Furrer, R. Evaluating faecal egg count reduction using a specifically designed package “eggCounts” in R and a user friendly web interface. Int. J. Parasitol. 2014, 44, 299–303. [Google Scholar] [CrossRef]
- Wang, C.; Torgerson, P.R.; Kaplan, R.M.; George, M.M.; Furrer, R. Modelling anthelmintic resistance by extending eggCounts package to allow individual efficacy. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 386–393. [Google Scholar] [CrossRef]
- Wickham, H. Getting Started with ggplot2. In ggplot2: Elegant Graphics for Data Analysis; Wickham, H., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 11–31. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P. Community Ecology Package. Available online: https://cran.r-project.org/package=vegan (accessed on 15 October 2023).
- Hill, M.O. Diversity and evenness: A unifying notation and its consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Fissiha, W.; Kinde, M.Z. Anthelmintic resistance and its mechanism: A Review. Infect. Drug Resist. 2021, 14, 5403–5410. [Google Scholar] [CrossRef] [PubMed]
- Dauparaite, E.; Kupcinskas, T.; von Samson-Himmelstjerna, G.; Petkevicius, S. Anthelmintic resistance of horse strongyle nematodes to ivermectin and pyrantel in Lithuania. Acta Vet. Scand. 2021, 63, 5. [Google Scholar] [CrossRef] [PubMed]
- Denwood, M.; Innocent, G.; Prentice, J.; Matthews, L.; Reid, S.; Pipper, C.; Levecke, B.; Kaplan, R.; Kotze, A.; Keiser, J. A hypothesis testing framework for the ratio of means of two negative binomial distributions: Classifying the efficacy of anthelmintic treatment against intestinal parasites. arXiv 2019, arXiv:1910.06667. [Google Scholar]
- Traversa, D.; von Samson-Himmelstjerna, G.; Demeler, J.; Milillo, P.; Schürmann, S.; Barnes, H.; Otranto, D.; Perrucci, S.; di Regalbono, A.F.; Beraldo, P. Anthelmintic resistance in cyathostomin populations from horse yards in Italy, United Kingdom and Germany. Parasit. Vectors 2009, 2 (Suppl. 2), S2. [Google Scholar] [CrossRef] [PubMed]
- Daniels, S.P.; Proudman, C.J. Shortened egg reappearance after ivermectin or moxidectin use in horses in the UK. Vet. J. 2016, 218, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Hinney, B.; Wirtherle, N.C.; Kyule, M.; Miethe, N.; Zessin, K.H.; Clausen, P.H. A questionnaire survey on helminth control on horse farms in Brandenburg, Germany and the assessment of risks caused by different kinds of management. Parasitol. Res. 2011, 109, 1625–1635. [Google Scholar] [CrossRef]
- Nielsen, M.K.; Branan, M.A.; Wiedenheft, A.M.; Digianantonio, R.; Garber, L.P.; Kopral, C.A.; Phillippi-Taylor, A.M.; Traub-Dargatz, J.L. Parasite control strategies used by equine owners in the United States: A national survey. Vet. Parasitol. 2018, 250, 45–51. [Google Scholar] [CrossRef]
- Saeed, M.A.; Beveridge, I.; Abbas, G.; Beasley, A.; Bauquier, J.; Wilkes, E.; Jacobson, C.; Hughes, K.J.; El-Hage, C.; O’Handley, R.; et al. Systematic review of gastrointestinal nematodes of horses from Australia. Parasit. Vectors 2019, 12, 188. [Google Scholar] [CrossRef]
- Larsen, M.L.; Ritz, C.; Petersen, S.L.; Nielsen, M.K. Determination of ivermectin efficacy against cyathostomins and Parascaris equorum on horse farms using selective therapy. Vet. J. 2011, 188, 44–47. [Google Scholar] [CrossRef]
- van Doorn, D.C.; Ploeger, H.W.; Eysker, M.; Geurden, T.; Wagenaar, J.A.; Kooyman, F.N. Cylicocyclus species predominate during shortened egg reappearance period in horses after treatment with ivermectin and moxidectin. Vet. Parasitol. 2014, 206, 246–252. [Google Scholar] [CrossRef]
- von Samson-Himmelstjerna, G.; Fritzen, B.; Demeler, J.; Schürmann, S.; Rohn, K.; Schnieder, T.; Epe, C. Cases of reduced cyathostomin egg-reappearance period and failure of Parascaris equorum egg count reduction following ivermectin treatment as well as survey on pyrantel efficacy on German horse farms. Vet. Parasitol. 2007, 144, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.K. Sustainable equine parasite control: Perspectives and research needs. Vet. Parasitol. 2012, 185, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, T.; Bauer, C.; Sasse, H.; Baumgartner, W.; Rey-Moreno, C.; Hermosilla, C.; Damriyasa, I.M.; Zahner, H. Small strongyle infection: Consequences of larvicidal treatment of horses with fenbendazole and moxidectin. Vet. Parasitol. 2006, 139, 115–131. [Google Scholar] [CrossRef] [PubMed]
- Seyoum, Z.; Tesfaye, M.; Derso, S. Prevalence, intensity and risk factors of infestation with major gastrointestinal nematodes in equines in and around Shashemane, Southern Ethiopia. Trop. Anim. Health Prod. 2015, 47, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, J.P.; Drolet, R.; Parsons, D.; Leguillette, R.; Sauvageau, R.; Shapiro, J.; Houle, L.; Halle, G.; Gebhart, C.J. Equine proliferative enteropathy: A cause of weight loss, colic, diarrhoea and hypoproteinaemia in foals on three breeding farms in Canada. Equine Vet. J. 2000, 32, 418–425. [Google Scholar] [CrossRef]
- Ionita, M.; Howe, D.K.; Lyons, E.T.; Tolliver, S.C.; Kaplan, R.M.; Mitrea, I.L.; Yeargan, M. Use of a reverse line blot assay to survey small strongyle (Strongylida: Cyathostominae) populations in horses before and after treatment with ivermectin. Vet. Parasitol. 2010, 168, 332–337. [Google Scholar] [CrossRef]
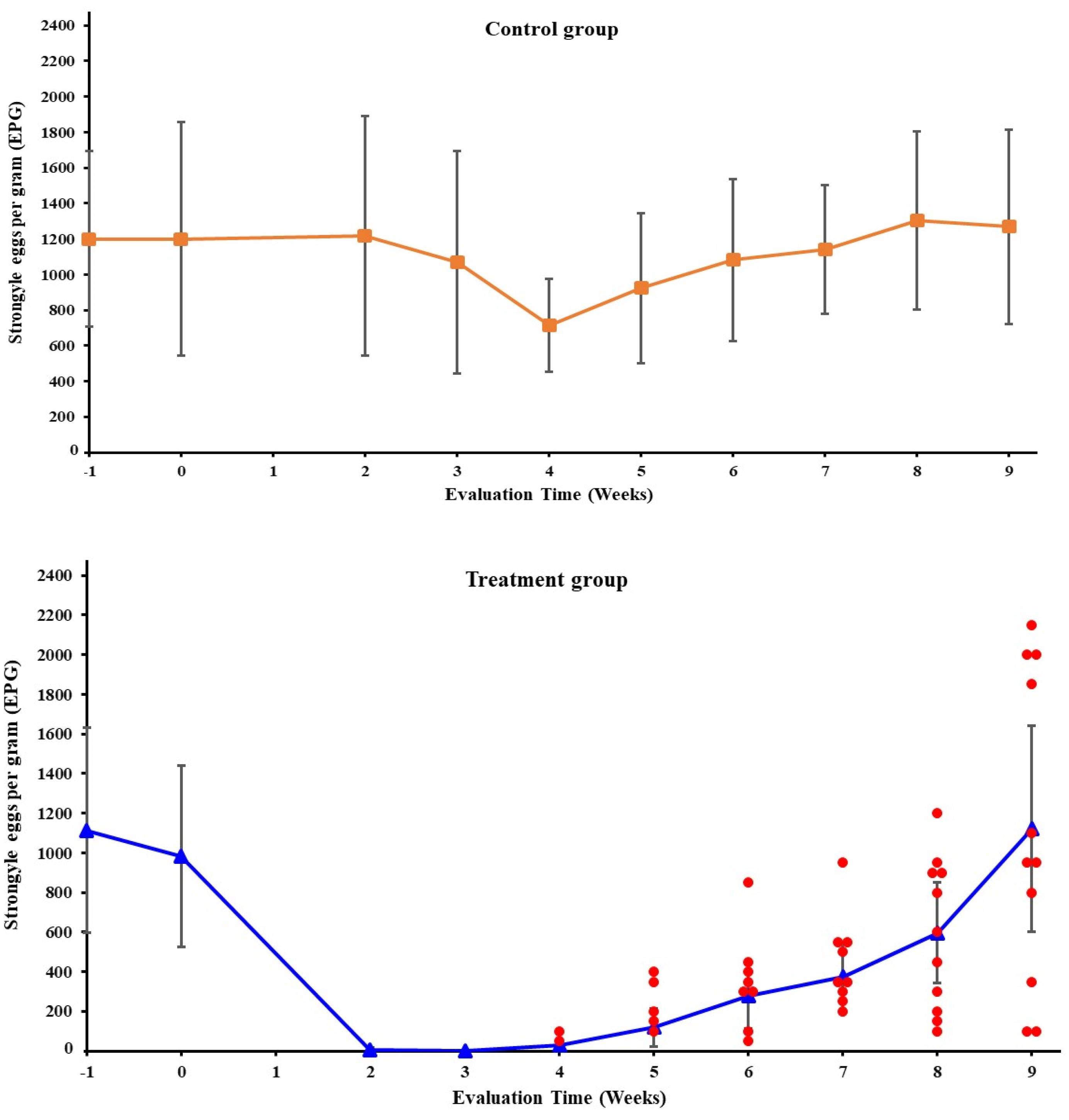

| Weeks Post-IVM-Treatment | ||||||||
|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| FECR 1 | 99.4 | 99.8 | 97.1 | 87.7 | 70.4 | 57.1 | 37.9 | 26.2 |
| CI 2 | 97.2–100 | 98.4–100 | 92.9–99.5 | 70.5–96.6 | 46.1–84.0 | 29.8–76.9 | 16.7–61.9 | 12.9–51.1 |
| Group | Control | Treatment | ||||||
|---|---|---|---|---|---|---|---|---|
| Diversity Indices | 0 Day | 4 Weeks | 6 Weeks | 9 Weeks | 0 Day | 4 Weeks | 6 Weeks | 9 Weeks |
| Species richness | 16 | 15 | 15 | 15 | 15 | 9 | 11 | 12 |
| Species evenness | 0.654 | 0.815 | 0.826 | 0.698 | 0.646 | 0.486 | 0.505 | 0.604 |
| Shannon | 1.813 | 2.207 | 2.239 | 1.891 | 1.750 | 1.068 | 1.211 | 1.501 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamad, M.H.; Islam, S.I.; Jitsamai, W.; Chinkangsadarn, T.; Naraporn, D.; Ouisuwan, S.; Taweethavonsawat, P. Patterns of Equine Small Strongyle Species Infection after Ivermectin Intervention in Thailand: Egg Reappearance Period and Nemabiome Metabarcoding Approach. Animals 2024, 14, 574. https://doi.org/10.3390/ani14040574
Hamad MH, Islam SI, Jitsamai W, Chinkangsadarn T, Naraporn D, Ouisuwan S, Taweethavonsawat P. Patterns of Equine Small Strongyle Species Infection after Ivermectin Intervention in Thailand: Egg Reappearance Period and Nemabiome Metabarcoding Approach. Animals. 2024; 14(4):574. https://doi.org/10.3390/ani14040574
Chicago/Turabian StyleHamad, Mohamed H., Sk Injamamul Islam, Wanarit Jitsamai, Teerapol Chinkangsadarn, Darm Naraporn, Suraseha Ouisuwan, and Piyanan Taweethavonsawat. 2024. "Patterns of Equine Small Strongyle Species Infection after Ivermectin Intervention in Thailand: Egg Reappearance Period and Nemabiome Metabarcoding Approach" Animals 14, no. 4: 574. https://doi.org/10.3390/ani14040574






