Species Richness and Composition of Forest Birds in Urban Parks and Reserves of Buenos Aires City, Argentina
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
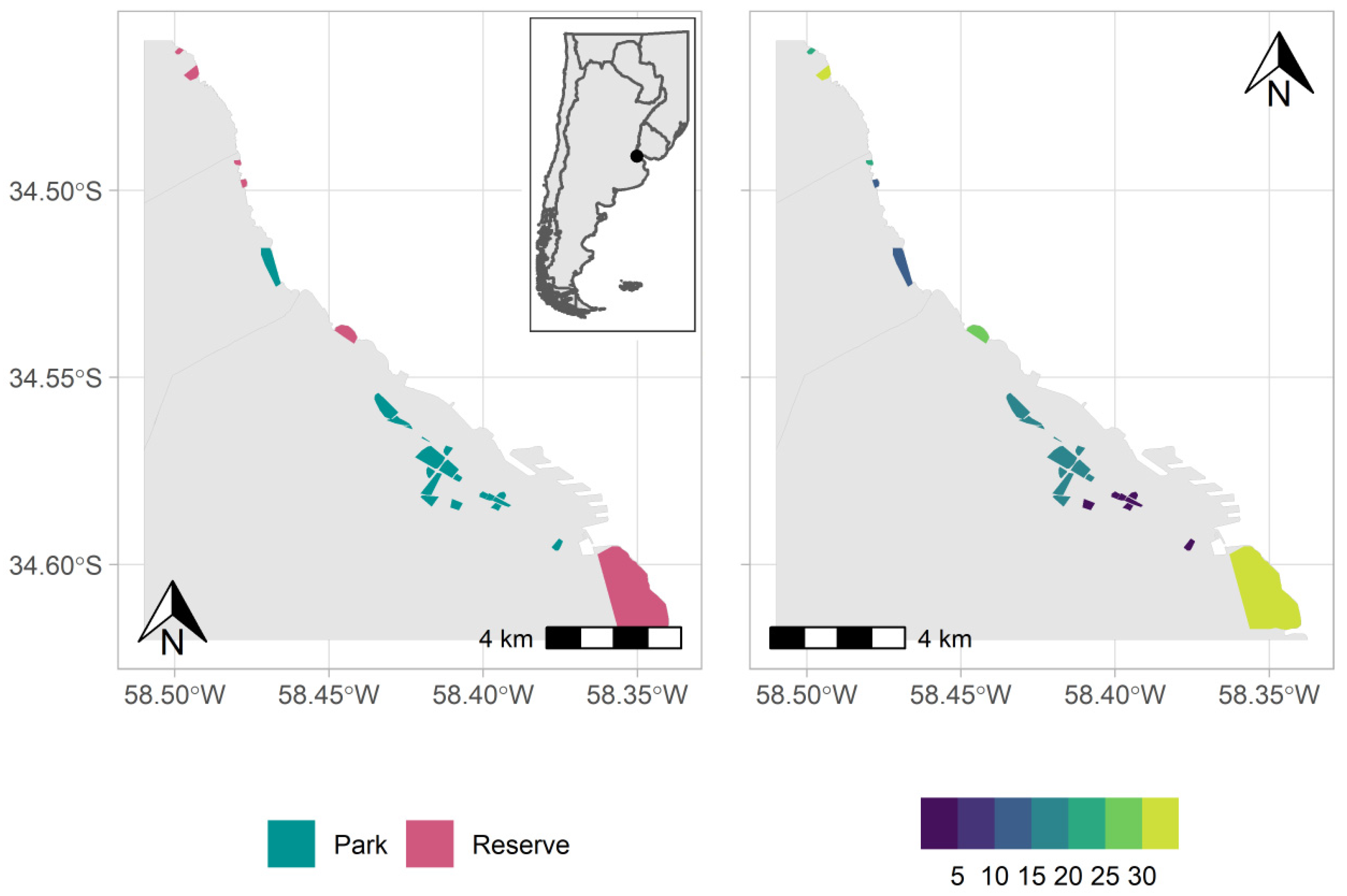
2.1. Study Area
2.2. Forest Birds
2.3. eBird Data
2.4. Green Space Types
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dallimer, M.; Tang, Z.; Bibby, P.R.; Brindley, P.; Gaston, K.J.; Davies, Z.G. Temporal changes in greenspace in a highly urbanized region. Biol. Lett. 2011, 7, 763–766. [Google Scholar] [CrossRef] [PubMed]
- Morello, J.; Buzai, G.D.; Baxendale, C.A.; Rodríguez, A.F.; Matteucci, S.D.; Godagnone, R.E.; Casas, R.R. Urbanization and the consumption of fertile land and other ecological changes: The case of Buenos Aires. Environ. Urban. 2000, 12, 119–131. [Google Scholar] [CrossRef]
- Vergnes, A.; Pellissier, V.; Lemperiere, G.; Rollard, C.; Clergeau, P. Urban densification causes the decline of ground-dwelling arthropods. Biodivers. Conserv. 2014, 23, 1859–1877. [Google Scholar] [CrossRef]
- Aronson, M.; Lepczyk, C.; Evans, K.; Goddard, M.; Lerman, S.; MacIvor, J.S.; Nilon, C.; Vargo, T. Biodiversity in the city: Key challenges for urban green space management. Front. Ecol. Environ. 2017, 15, 189–196. [Google Scholar] [CrossRef]
- Savard, J.-P.L.; Clergeau, P.; Mennechez, G. Biodiversity concepts and urban ecosystems. Landsc. Urban Plan. 2000, 48, 131–142. [Google Scholar] [CrossRef]
- Canedoli, C.; Manenti, R.; Padoa-Schioppa, E. Birds biodiversity in urban and periurban forests: Environmental determinants at local and landscape scales. Urban Ecosyst. 2018, 21, 779–793. [Google Scholar] [CrossRef]
- Marzluff, J.M.; Ewing, K. Restoration of Fragmented Landscapes for the Conservation of Birds: A General Framework Specific Recommendations for Urbanizing Landscapes. In Urban Ecology: An International Perspective on the Interaction Between Humans and Nature; Marzluff, J.M., Shulenberger, E., Endlicher, W., Alberti, M., Bradley, G., Ryan, C., Simon, U., ZumBrunnen, C., Eds.; Springer: Boston, MA, USA, 2008; pp. 739–755. [Google Scholar] [CrossRef]
- Villaseñor, N.R.; Chiang, L.A.; Hernández, H.J.; Escobar MA, H. Vacant lands as refuges for native birds: An opportunity for biodiversity conservation in cities. Urban For. Urban Green. 2020, 49, 126632. [Google Scholar] [CrossRef]
- Aerts, R.; Honnay, O.; Van Nieuwenhuyse, A. Biodiversity and human health: Mechanisms and evidence of the positive health effects of diversity in nature and green spaces. Br. Med. Bull. 2018, 127, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Bertram, C.; Rehdanz, K. The role of urban green space for human well-being. Ecol. Econ. 2015, 120, 139–152. [Google Scholar] [CrossRef]
- Fernández-Juricic, E. Spatial and temporal analysis of the distribution of forest specialists in an urban-fragmented landscape (Madrid, Spain): Implications for local and regional bird conservation. Landsc. Urban Plan. 2004, 69, 17–32. [Google Scholar] [CrossRef]
- Gunnarsson, B.; Heyman, E.; Vowles, T. Bird predation effects on bush canopy arthropods in suburban forests. For. Ecol. Manag. 2009, 257, 619–627. [Google Scholar] [CrossRef]
- Heyman, E. Clearance of understory in urban woodlands: Assessing impact on bird abundance and diversity. For. Ecol. Manag. 2010, 260, 125–131. [Google Scholar] [CrossRef]
- Fenoglio, M.S.; Rossetti, M.R.; Videla, M. Negative effects of urbanization on terrestrial arthropod communities: A meta-analysis. Glob. Ecol. Biogeogr. 2020, 29, 1412–1429. [Google Scholar] [CrossRef]
- Threlfall, C.G.; Mata, L.; Mackie, J.A.; Hahs, A.K.; Stork, N.E.; Williams, N.S.; Livesley, S.J. Increasing biodiversity in urban green spaces through simple vegetation interventions. J. Appl. Ecol. 2017, 54, 1874–1883. [Google Scholar] [CrossRef]
- Jokimäki, J. Occurrence of breeding bird species in urban parks: Effects of park structure and broad-scale variables. Urban Ecosyst. 1999, 3, 21–34. [Google Scholar] [CrossRef]
- Nielsen, A.B.; van den Bosch, M.; Maruthaveeran, S.; van den Bosch, C.K. Species richness in urban parks and its drivers: A review of empirical evidence. Urban Ecosyst. 2014, 17, 305–327. [Google Scholar] [CrossRef]
- Minor, E.; Urban, D. Forest bird communities across a gradient of urban development. Urban Ecosyst. 2010, 13, 51–71. [Google Scholar] [CrossRef]
- White, J.G.; Antos, M.J.; Fitzsimons, J.A.; Palmer, G.C. Non-uniform bird assemblages in urban environments: The influence of streetscape vegetation. Landsc. Urban Plan. 2005, 71, 123–135. [Google Scholar] [CrossRef]
- Trollope, S.T.; White, J.G.; Cooke, R. The response of ground and bark foraging insectivorous birds across an urban–forest gradient. Landsc. Urban Plan. 2009, 93, 142–150. [Google Scholar] [CrossRef]
- Major, R.E.; Christie, F.J.; Gowing, G. Influence of remnant and landscape attributes on Australian woodland bird communities. Biol. Conserv. 2001, 102, 47–66. [Google Scholar] [CrossRef]
- Kaushik, M.; Tiwari, S.; Manisha, K. Habitat patch size and tree species richness shape the bird community in urban green spaces of rapidly urbanizing Himalayan foothill region of India. Urban Ecosyst. 2022, 25, 423–436. [Google Scholar] [CrossRef]
- Patterson, B.D.; Atmar, W. Nested subsets and the structure of insular mammalian faunas and archipelagos. Biol. J. Linn. Soc. 1986, 28, 65–82. [Google Scholar] [CrossRef]
- Callaghan, C.T.; Bino, G.; Major, R.E.; Martin, J.M.; Lyons, M.B.; Kingsford, R.T. Heterogeneous urban green areas are bird diversity hotspots: Insights using continental-scale citizen science data. Landsc. Ecol. 2019, 34, 1231–1246. [Google Scholar] [CrossRef]
- La Sorte, F.A.; Aronson MF, J.; Lepczyk, C.A.; Horton, K.G. Area is the primary correlate of annual and seasonal patterns of avian species richness in urban green spaces. Landsc. Urban Plan. 2020, 203, 103892. [Google Scholar] [CrossRef]
- Leveau, L.M.; Gorleri, F.C.; Roesler, I.; González-Táboas, F. What Makes an Urban Raptor? Ibis 2022, 164, 1213–1226. [Google Scholar] [CrossRef]
- Ruggera, R.A.; Yapura, A.; Chocobar, N.; Baffa-Trasci, N.G.; Caldano, S.A.; Schaaf, A.A. Ecología urbana del Tucán Grande (Ramphastos toco) en Jujuy: Resultados preliminares de un proyecto de ciencia ciudadana. El Hornero 2022, 37, 14. [Google Scholar] [CrossRef]
- Cueto, V.R.; de Casenave, J.L. Determinants of bird species richness: Role of climate and vegetation structure at a regional scale. J. Biogeogr. 1999, 26, 487–492. [Google Scholar] [CrossRef]
- Haene, E. Biocorredores de la Ciudad Autónoma de Buenos Aires, un Modelo Demostrativo Para la Argentina; Universidad de Belgrano: Buenos Aires, Argentina, 2020. [Google Scholar]
- INDEC. Datos definitivos. Censo Nacional de Población, Hogares y Viviendas. 2022. Available online: https://censo.gob.ar/index.php/datos_definitivos/ (accessed on 11 December 2023).
- Oyarzabal, M.; Clavijo, J.; Oakley, L.; Biganzoli, F.; Tognetti, P.; Barberis, I.; Maturo, H.M.; Aragón, R.; Campanello, P.I.; Prado, D.; et al. Vegetation units of Argentina. Ecol. Austral 2018, 28, 40–63. [Google Scholar] [CrossRef]
- Wilman, H.; Belmaker, J.; Simpson, J.; de la Rosa, C.; Rivadeneira, M.M.; Jetz, W. EltonTraits 1.0: Species-level foraging attributes of the world’s birds and mammals. Ecology 2014, 95, 2027. [Google Scholar] [CrossRef]
- Kirwan, G.M.; Shah, S.S.; Barbosa, K. Benteveo Rayado (Myiodynastes Maculatus), Version 2.0. Birds of the World. 2022. Available online: https://doi.org/10.2173/bow.strfly1.02species_shared.bow.project_name (accessed on 5 July 2022).
- Vitório, J.G.; de Frenedozo, R.C.; de Barbosa, K.V.C. Habitat use and home range of a migratory bird, Myiodynastes maculatus solitarius, in an urban park in the Atlantic Forest, Brazil. Rev. Bras. Ornitol. 2019, 27, 115–121. [Google Scholar] [CrossRef]
- Sullivan, B.L.; Aycrigg, J.L.; Barry, J.H.; Bonney, R.E.; Bruns, N.; Cooper, C.B.; Damoulas, T.; Dhondt, A.A.; Dietterich, T.; Farnsworth, A.; et al. The eBird enterprise: An integrated approach to development and application of citizen science. Biol. Conserv. 2014, 169, 31–40. [Google Scholar] [CrossRef]
- Johnston, A.; Hochachka, W.M.; Strimas-Mackey, M.E.; Ruiz Gutierrez, V.; Robinson, O.J.; Miller, E.T.; Auer, T.; Kelling, S.T.; Fink, D. Analytical guidelines to increase the value of community science data: An example using eBird data to estimate species distributions. Divers. Distrib. 2021, 27, 1265–1277. [Google Scholar] [CrossRef]
- Chain, D.G. Manual de Diseño Urbano, 1st ed.; Ministerio de Desarrollo Urbano del Gobierno de la Ciudad Autónoma de Buenos Aires: Buenos Aires, Argentina, 2015. [Google Scholar]
- Faggi, A.; Ignatieva, M. Urban green spaces in Buenos Aires and Christchurch. Proc. Inst. Civ. Eng. Munic. Eng. 2009, 162, 241–250. [Google Scholar] [CrossRef]
- Kalesnik, F.; Cagnoni, M.; Bertolini, P.; Quintana, R.; Madanes, N.; Malvárez, A. La vegetación del refugio educativo de la Ribera Norte, Provincia de Buenos Aires, Argentina. Invasión de especies exóticas. Miscelánea 2005, 14, 140. [Google Scholar]
- Melzi Fiorenza, R.; Sirolli, H.; Picca, P.I. Vegetación De Un Área Polderizada Del Río De La Plata En La Ciudad De Buenos Aires: La Reserva Ecológica Ciudad Universitaria—Costanera Norte. Darwiniana 2020, 8, 460–478. [Google Scholar] [CrossRef]
- Carpintero, D.L.; de Biase, S.; Damer, L.; Konopko, S.A. Chinches (Hemiptera: Heteroptera) de la Reserva Ecológica Vicente López. Hist. Nat. 2016, 6, 61–74. [Google Scholar]
- Rodríguez Tourón, G.; Gasparri, B. Actualización de la flora del Parque Natural Municipal Ribera Norte, San Isidro, provincia de Buenos Aires, Argentina. Hist. Nat. 2017, 7, 129–144. [Google Scholar]
- San Isidro Municipio. Áreas Protegidas|San Isidro. 2018. Available online: https://www.sanisidro.gob.ar/areas-protegidas (accessed on 10 June 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 20 June 2023).
- Vanegas, L.; Rondón, L.; Paula, G. glmtoolbox: Set of Tools to Data Analysis Using Generalized Linear Models. R Package Version 0.1.3. 2022. Available online: https://CRAN.R-project.org/package=glmtoolbox (accessed on 20 June 2023).
- Palacio, F.X.; Apodaca, M.J.; Crisci, J.V. Análisis Multivariado para Datos Biológicos: Teoría y su Aplicación Utilizando el Lenguaje R, 1st ed.; Fundación de Historia Natural Félix de Azara: Ciudad Autónoma de Buenos Aires, Argentina, 2020. [Google Scholar]
- Jaccard, P. Nouvelles recherches sur la distribution floral. Bull. Société Vaudoise Des Sci. Nat. 1908, 44, 223–270. [Google Scholar]
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. vegan: Community Ecology Package. R Package Version 2.6-2. 2022. Available online: https://CRAN.R-project.org/package=vegan (accessed on 20 June 2023).
- Almeida-Neto, M.; Guimarães, P.; Guimarães, P.R., Jr.; Loyola, R.D.; Ulrich, W. A consistent metric for nestedness analysis in ecological systems: Reconciling concept and measurement. Oikos 2008, 117, 1227–1239. [Google Scholar] [CrossRef]
- Strona, G.; Galli, P.; Seveso, D.; Montano, S.; Fattorini, S. Nestedness for Dummies (NeD): A User-Friendly Web Interface for Exploratory Nestedness Analysis. J. Stat. Softw. 2014, 59, 1–9. [Google Scholar] [CrossRef]
- Gavareski, C.A. Relation of Park Size and Vegetation to Urban Bird Populations in Seattle, Washington. Condor 1976, 78, 375–382. [Google Scholar] [CrossRef]
- Beninde, J.; Veith, M.; Hochkirch, A. Biodiversity in cities needs space: A meta-analysis of factors determining intra-urban biodiversity variation. Ecol. Lett. 2015, 18, 581–592. [Google Scholar] [CrossRef]
- Evans, K.L.; Newson, S.E.; Gaston, K.J. Habitat influences on urban avian assemblages. Ibis 2009, 151, 19–39. [Google Scholar] [CrossRef]
- Kang, W.; Minor, E.S.; Park, C.-R.; Lee, D. Effects of habitat structure, human disturbance, and habitat connectivity on urban forest bird communities. Urban Ecosyst. 2015, 18, 857–870. [Google Scholar] [CrossRef]
- Lepczyk, C.A.; Aronson MF, J.; Evans, K.L.; Goddard, M.A.; Lerman, S.B.; MacIvor, J.S. Biodiversity in the City: Fundamental Questions for Understanding the Ecology of Urban Green Spaces for Biodiversity Conservation. BioScience 2017, 67, 799–807. [Google Scholar] [CrossRef]
- Leveau, L.M. Relaciones Aves–Habitat en el Sector Suburbano de Mar del Plata, Argentina. 2013. Available online: https://ri.conicet.gov.ar/handle/11336/25879 (accessed on 23 October 2023).
- Threlfall, C.G.; Williams NS, G.; Hahs, A.K.; Livesley, S.J. Approaches to urban vegetation management and the impacts on urban bird and bat assemblages. Landsc. Urban Plan. 2016, 153, 28–39. [Google Scholar] [CrossRef]
- Tilghman, N.G. Characteristics of urban woodlands affecting breeding bird diversity and abundance. Landsc. Urban Plan. 1987, 14, 481–495. [Google Scholar] [CrossRef]
- Ferger, S.W.; Schleuning, M.; Hemp, A.; Howell, K.M.; Böhning-Gaese, K. Food resources and vegetation structure mediate climatic effects on species richness of birds. Glob. Ecol. Biogeogr. 2014, 23, 541–549. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, Y.; Li, S.; von Gadow, K. The Effects of habitat area, vegetation structure and insect richness on breeding bird populations in Beijing urban parks. Urban For. Urban Green. 2015, 14, 1027–1039. [Google Scholar] [CrossRef]
- Archer, J.-M.J.; Hostetler, M.E.; Acomb, G.; Blair, R. A systematic review of forest bird occurrence in North American forest fragments and the built environment. Landsc. Urban Plan. 2019, 185, 1–23. [Google Scholar] [CrossRef]
- Chace, J.F.; Walsh, J.J. Urban effects on native avifauna: A review. Landsc. Urban Plan. 2006, 74, 46–69. [Google Scholar] [CrossRef]
- Dale, S. Urban bird community composition influenced by size of urban green spaces, presence of native forest, and urbanization. Urban Ecosyst. 2018, 21, 1–14. [Google Scholar] [CrossRef]
- Rottenborn, S.C. Predicting the impacts of urbanization on riparian bird communities. Biol. Conserv. 1999, 88, 289–299. [Google Scholar] [CrossRef]
- Burghardt, K.T.; Tallamy, D.W.; Gregory Shriver, W. Impact of Native Plants on Bird and Butterfly Biodiversity in Suburban Landscapes. Conserv. Biol. 2009, 23, 219–224. [Google Scholar] [CrossRef]
- Narango, D.L.; Tallamy, D.W.; Marra, P.P. Native plants improve breeding and foraging habitat for an insectivorous bird. Biol. Conserv. 2017, 213, 42–50. [Google Scholar] [CrossRef]
- Kane, B.; Warren, P.S.; Lerman, S.B. A broad scale analysis of tree risk, mitigation and potential habitat for cavity-nesting birds. Urban For. Urban Green. 2015, 14, 1137–1146. [Google Scholar] [CrossRef]
- Stagoll, K.; Manning, A.D.; Knight, E.; Fischer, J.; Lindenmayer, D.B. Using bird–habitat relationships to inform urban planning. Landsc. Urban Plan. 2010, 98, 13–25. [Google Scholar] [CrossRef]
- Callaghan, C.; Major, R.; Lyons, M.; Martin, J.; Kingsford, R. The effects of local and landscape habitat attributes on bird diversity in urban greenspaces. Ecosphere 2018, 9, e02347. [Google Scholar] [CrossRef]
- Donnelly, R.; Marzluff, J.M. Importance of Reserve Size and Landscape Context to Urban Bird Conservation. Conserv. Biol. 2004, 18, 733–745. [Google Scholar] [CrossRef]
- Matthies, S.A.; Rüter, S.; Schaarschmidt, F.; Prasse, R. Determinants of species richness within and across taxonomic groups in urban green spaces. Urban Ecosyst. 2017, 20, 897–909. [Google Scholar] [CrossRef]
- Fernández-Juricic, E.; Jokimäki, J. A habitat island approach to conserving birds in urban landscapes: Case studies from southern and northern Europe. Biodivers. Conserv. 2001, 10, 2023–2043. [Google Scholar] [CrossRef]
- Whitcomb, R.F.; Lynch, J.F.; Opler, P.A.; Robbins, C.S. Island Biogeography and Conservation: Strategy and Limitations. Science 1976, 193, 1030–1102. [Google Scholar] [CrossRef]
- Dri, G.F.; Fontana, C.S.; de Sales Dambros, C. Estimating the impacts of habitat loss induced by urbanization on bird local extinctions. Biol. Conserv. 2021, 256, 109064. [Google Scholar] [CrossRef]
- Ridgely, R.S.; Tudor, G. The Birds of South America: Volume 1: The Oscine Passerines; University of Texas Press: Austin, TX, USA, 1989. [Google Scholar]
- Ridgely, R.S.; Tudor, G. The Birds of South America: Volume 2: The Suboscine Passerines; University of Texas Press: Austin, TX, USA, 1994. [Google Scholar]
- De la Peña, M.R. Nidos y reproducción de las aves argentinas. Ediciones Biológica. Ser. Nat. Conserv. Soc. 2013, 8, 590. [Google Scholar]
- Leveau, L.M.; Ruggiero, A.; Matthews, T.J.; Isabel Bellocq, M. A global consistent positive effect of urban green area size on bird richness. Avian Res. 2019, 10, 30. [Google Scholar] [CrossRef]
- Gorleri, F.C.; Areta, J.I. Misidentifications in citizen science bias the phenological estimates of two hard-to-identify Elaenia flycatchers. Ibis 2022, 164, 13–26. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, P.; Chen, S.; Zheng, G. Nestedness of bird assemblages on urban woodlots: Implications for conservation. Landsc. Urban Plan. 2013, 111, 59–67. [Google Scholar] [CrossRef]
- Fernández-Juricic, E. Can human disturbance promote nestedness? A case study with breeding birds in urban habitat fragments. Oecologia 2002, 131, 269–278. [Google Scholar] [CrossRef]



| English Name | Scientific Name | Code | Reserves | Parks |
|---|---|---|---|---|
| Ash-colored Cuckoo | Coccycua cinerea | Coc.cin | 3 | 1 |
| Dark-billed Cuckoo | Coccyzus melacoryphus | Coc.mel | 4 | 2 |
| Yellow-billed Cuckoo | Coccyzus americanus | Coc.ame | 1 | 0 |
| White-barred Piculet | Picumnus cirratus | Pic.cir | 4 | 1 |
| White-fronted Woodpecker | Melanerpes cactorum | Mel.cac | 1 | 0 |
| Checkered Woodpecker | Dryobates mixtus | Dry.mix | 6 | 4 |
| Green-barred Woodpecker | Colaptes melanochloros | Col.mel | 6 | 6 |
| Rufous-capped Antshrike | Thamnophilus ruficapillus | Tha.ruf | 3 | 0 |
| Variable Antshrike | Thamnophilus caerulescens | Tha.cae | 4 | 0 |
| Narrow-billed Woodcreeper | Lepidocolaptes angustirostris | Lep.ang | 6 | 5 |
| Tufted Tit-Spinetail | Leptasthenura platensis | Lep.pla | 3 | 0 |
| Freckle-breasted Thornbird | Phacellodomus sibilatrix | Pha.sib | 1 | 0 |
| Stripe-crowned Spinetail | Cranioleuca pyrrhophia | Cra.pyr | 3 | 0 |
| Spix’s Spinetail | Synallaxis spixi | Syn.spi | 5 | 0 |
| Sooty-fronted Spinetail | Synallaxis frontalis | Syn.fro | 6 | 1 |
| Green-backed Becard | Pachyramphus viridis | Pac.vir | 1 | 0 |
| White-winged Becard | Pachyramphus polychopterus | Pac.pol | 5 | 1 |
| Mottle-cheeked Tyrannulet | Phylloscartes ventralis | Phy.ven | 5 | 2 |
| Large Elaenia | Elaenia spectabilis | Ela.spe | 2 | 0 |
| Small-billed Elaenia | Elaenia parvirostris | Ela.par | 4 | 1 |
| Suiriri Flycatcher | Suiriri suiriri | Sui.sui | 2 | 1 |
| Southern Beardless-Tyrannulet | Camptostoma obsoletum | Cam.obs | 4 | 0 |
| White-crested Tyrannulet | Serpophaga subcristata | Ser.sub | 6 | 5 |
| Straneck’s Tyrannulet | Serpophaga griseicapilla | Ser.gri | 6 | 2 |
| Streaked Flycatcher | Myiodynastes maculatus | Myi.mac | 5 | 3 |
| Bran-colored Flycatcher | Myiophobus fasciatus | Myi.fas | 6 | 1 |
| Euler’s Flycatcher | Lathrotriccus euleri | Lat.eul | 3 | 0 |
| Rufous-browed Peppershrike | Cyclarhis gujanensis | Cyc.guj | 5 | 2 |
| Chivi Vireo | Vireo chivi | Vir.chi | 6 | 3 |
| Plush-crested Jay | Cyanocorax chrysops | Cya.chr | 2 | 1 |
| Masked Gnatcatcher | Polioptila dumicola | Pol.dum | 6 | 3 |
| Solitary Black Cacique | Cacicus solitarius | Cac.sol | 6 | 2 |
| Golden-winged Cacique | Cacicus chrysopterus | Cac.chr | 2 | 0 |
| Variable Oriole | Icterus pyrrhopterus | Ict.pyr | 5 | 3 |
| Tropical Parula | Setophaga pitiayumi | Set.pit | 5 | 4 |
| Golden-crowned Warbler | Basileuterus culicivorus | Bas.cul | 5 | 3 |
| Hepatic Tanager | Piranga flava | Pir.fla | 6 | 4 |
| Variable | Estimate | Std. Error | z | p |
|---|---|---|---|---|
| Intercept | 1.555 | 0.221 | 7.050 | <0.001 |
| Reserve | 0.720 | 0.160 | 4.497 | <0.001 |
| Number of Checklists (log) | 0.383 | 0.087 | 4.396 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godoy, I.N.; Gorleri, F.C.; Cristaldi, M.A.; Leveau, L.M. Species Richness and Composition of Forest Birds in Urban Parks and Reserves of Buenos Aires City, Argentina. Animals 2024, 14, 602. https://doi.org/10.3390/ani14040602
Godoy IN, Gorleri FC, Cristaldi MA, Leveau LM. Species Richness and Composition of Forest Birds in Urban Parks and Reserves of Buenos Aires City, Argentina. Animals. 2024; 14(4):602. https://doi.org/10.3390/ani14040602
Chicago/Turabian StyleGodoy, Ianina N., Fabricio C. Gorleri, Maximiliano A. Cristaldi, and Lucas M. Leveau. 2024. "Species Richness and Composition of Forest Birds in Urban Parks and Reserves of Buenos Aires City, Argentina" Animals 14, no. 4: 602. https://doi.org/10.3390/ani14040602





