Effects of Dietary Alfalfa Meal Supplementation on the Growth Performance, Nutrient Apparent Digestibility, Serum Parameters, and Intestinal Microbiota of Raccoon Dogs (Nyctereutes procyonoides)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Diets and Experimental Design
2.2. Sample Collection and Processing
2.3. Chemical Analysis and Calculation
2.4. Determination of Serum Parameters
2.5. Bacterial DNA Extraction, PCR Amplification, and Illumina MiSeq Sequencing
2.6. Bio-Informational Analysis
2.7. Determination of Short-Chain Fatty Acids (SCFAs)
2.8. Data Statistics and Analysis
3. Results
3.1. Effects of Alfalfa Meal Supplementation on Growth Performance of Raccoon Dogs
3.2. Effects of Alfalfa Meal Supplementation on Nutrient Apparent Digestibility of Raccoon Dogs
3.3. Effects of Alfalfa Meal Supplementation on Serum Parameters of Raccoon Dogs
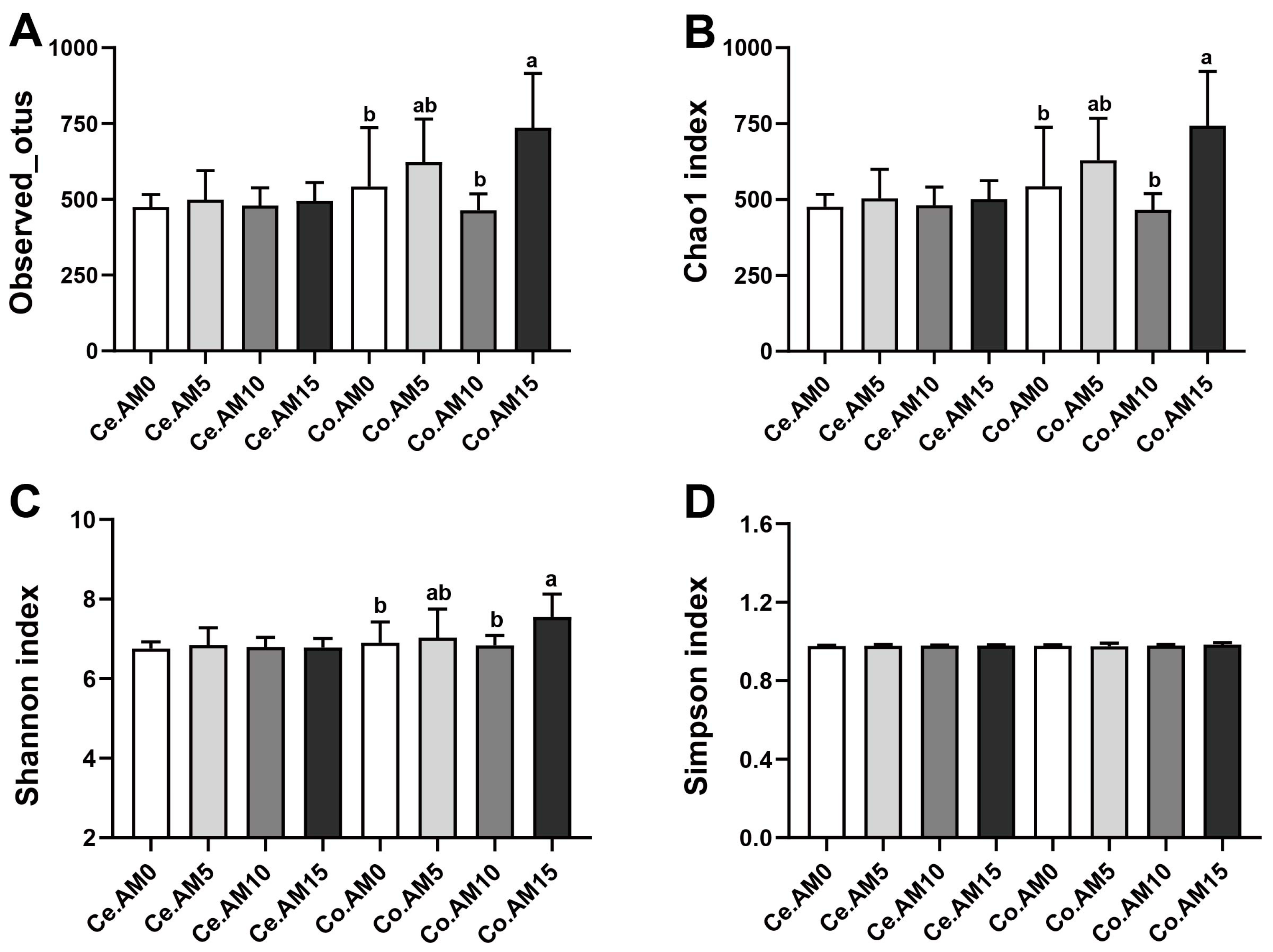
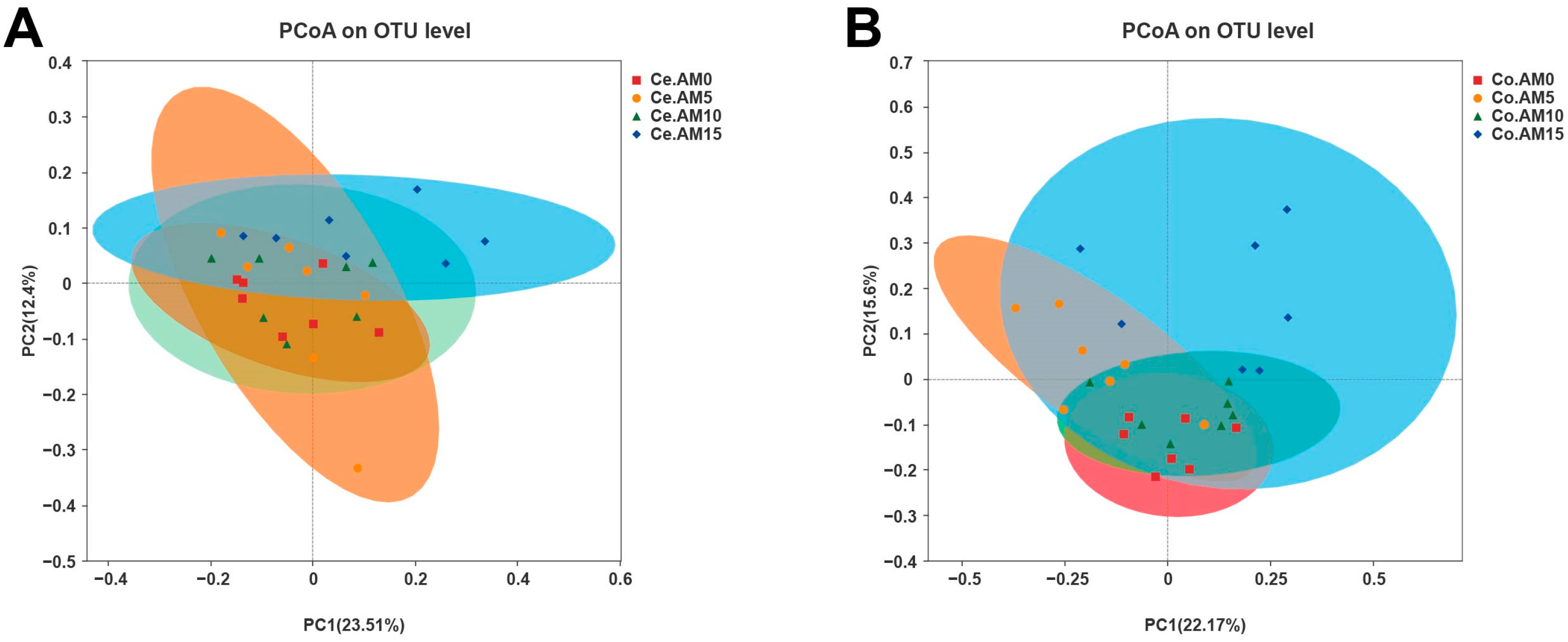
3.4. Effects of Alfalfa Meal Supplementation on Microbial Diversity of Raccoon Dogs
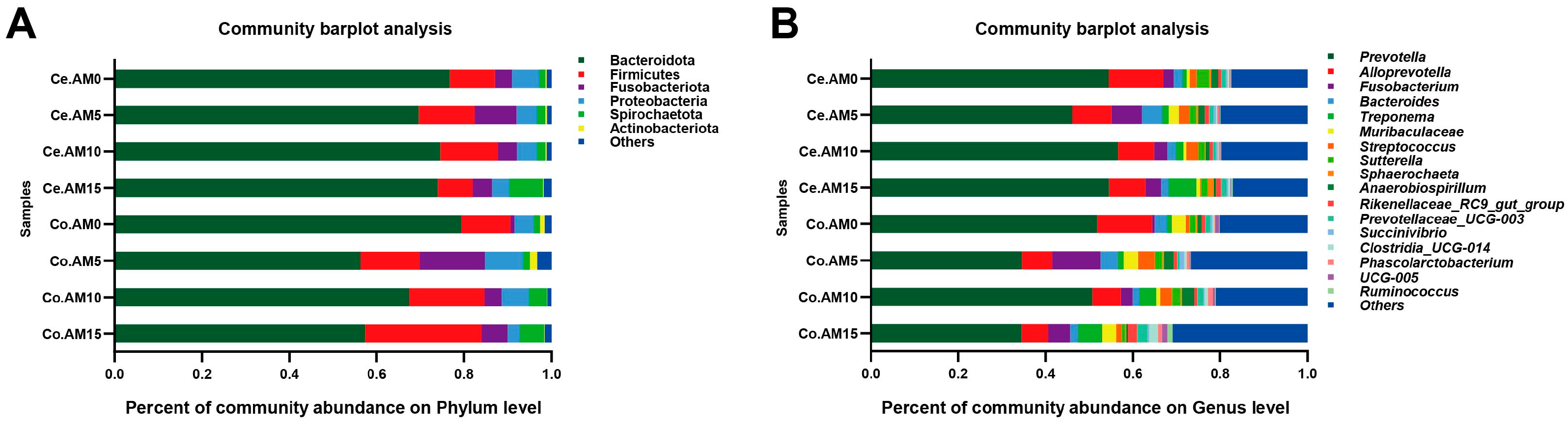
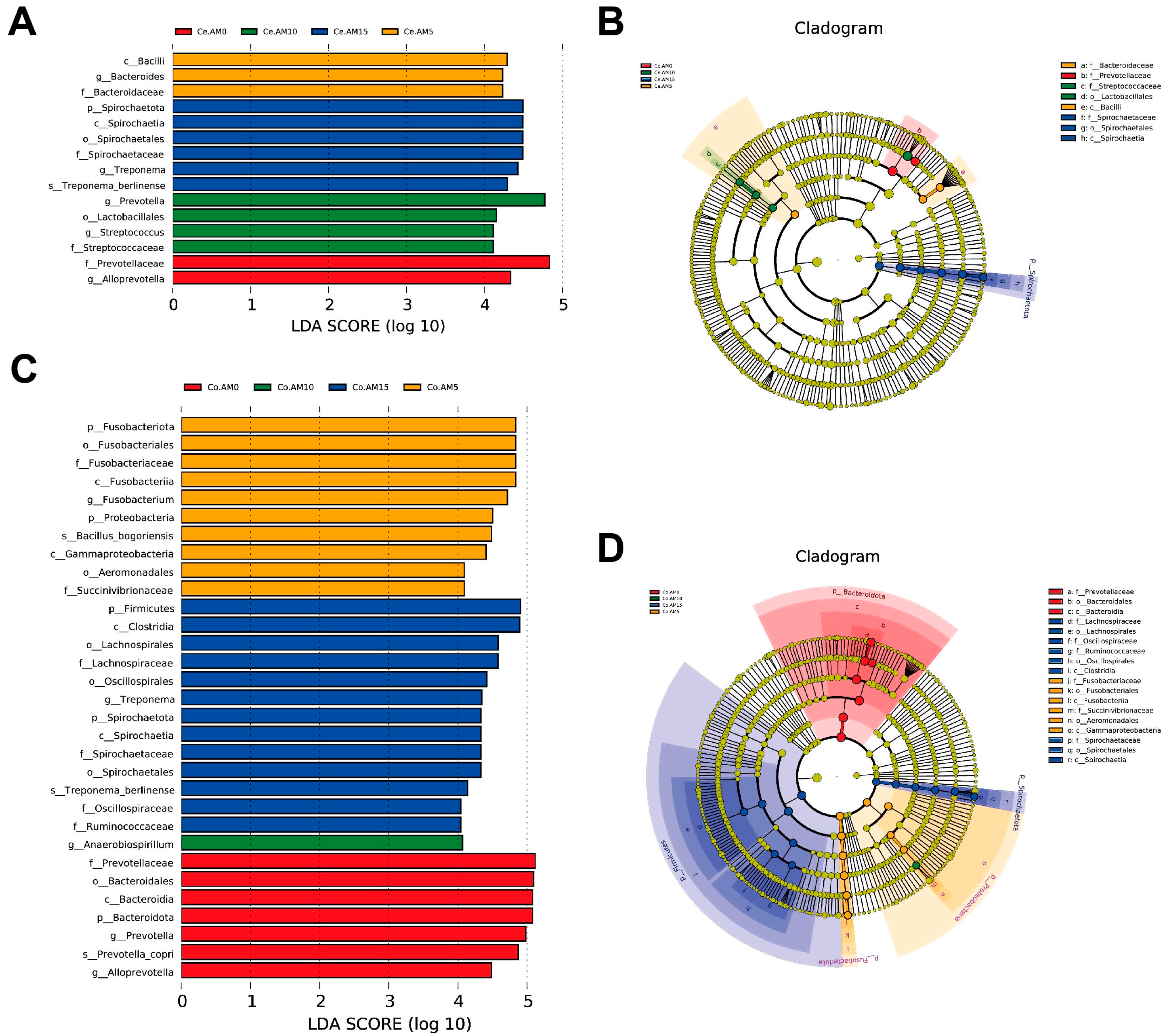
3.5. Composition and Differences of Intestinal Microbiota in Raccoon Dogs
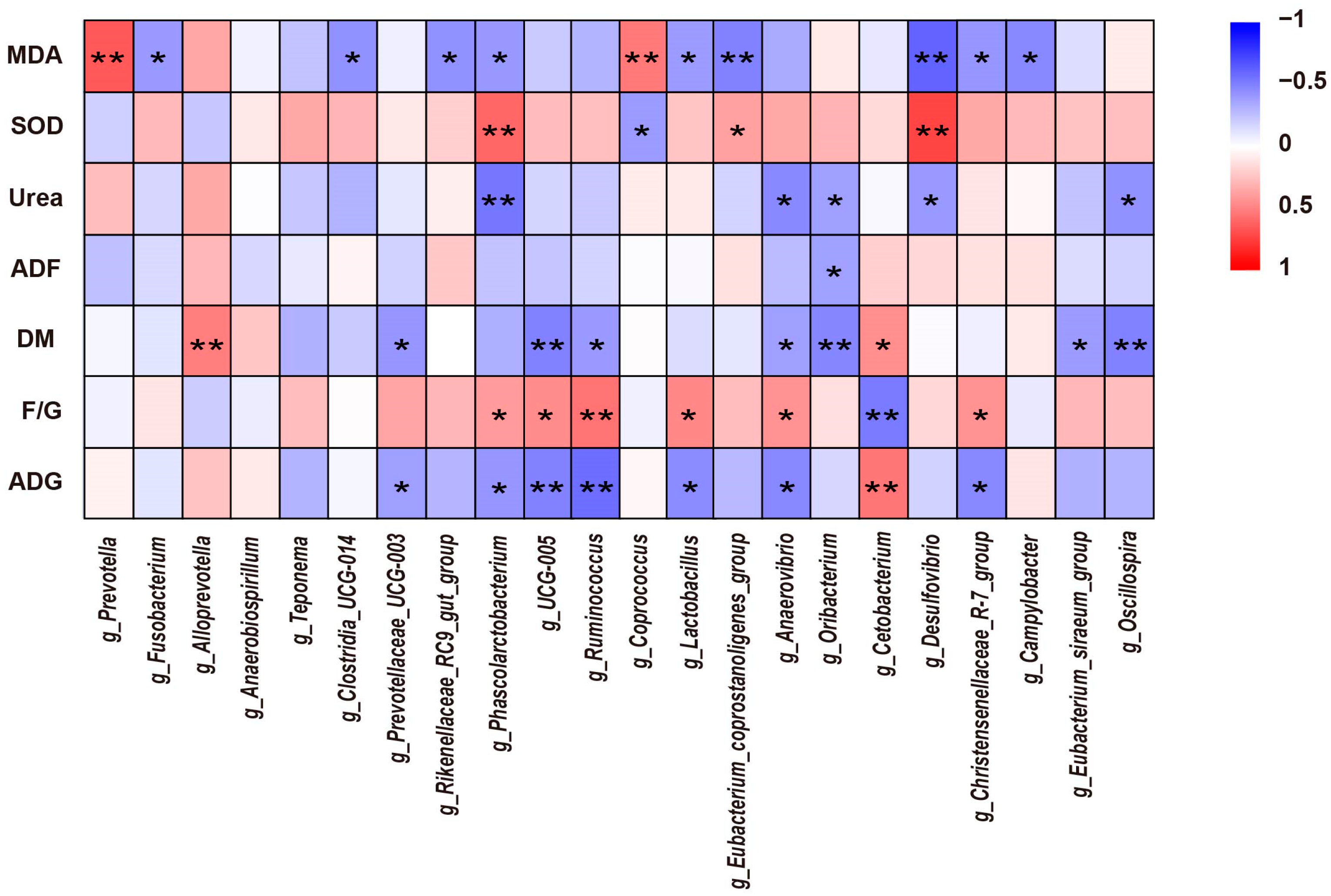
3.6. Correlation Analysis between Significantly Different Colon Microbiota and Growth Performance, Nutrient Apparent Digestibility, and Serum Parameters
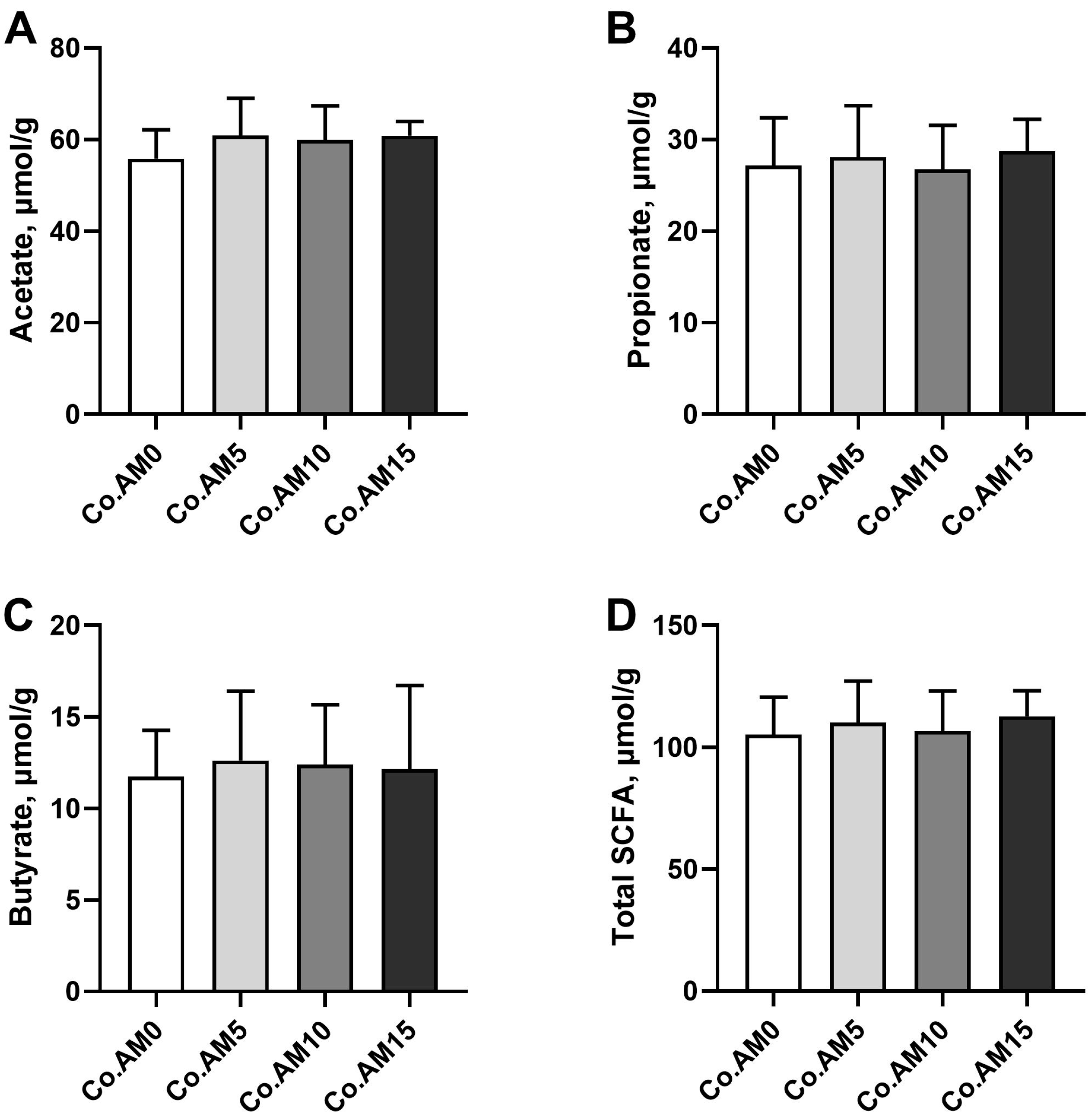
3.7. Concentrations of SCFA in Colonic Contents
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.; Chen, X.L.; Yuan, W.T.; Zhang, X.L.; Mao, A.P.; Zhao, W.G.; Yao, N.Q.; Deng, X.M.; Xu, C. Effects of Platycladus orientalis leaf extract on the growth performance, fur-production, serum parameters, and intestinal microbiota of raccoon dogs. Animals 2023, 13, 3151. [Google Scholar] [CrossRef]
- Drygala, F.; Werner, U.; Zoller, H. Diet composition of the invasive raccoon dog (Nyctereutes procyonoides) and the native red fox (Vulpes vulpes) in north-east Germany. Hystrix 2013, 24, 190–194. [Google Scholar]
- Gao, W.Y. Biological characteristics and production application of raccoon dogs. Anim. Husb. Vet. Med. 2005, 37, 30–31. [Google Scholar]
- Jha, R.; Berrocoso, J.D. Review: Dietary fiber utilization and its effects on physiological functions and gut health of swine. Animal 2015, 9, 1441–1452. [Google Scholar] [CrossRef]
- Cheng, P.H.; Wang, Y.; Liang, J.B.; Wu, Y.B.; Wright, A.; Liao, X.D. Exploratory analysis of the microbiological potential for efficient utilization of fiber between Lantang and Duroc pigs. Front. Microbiol. 2018, 9, 1342. [Google Scholar] [CrossRef]
- Venter, C.; Meyer, R.W.; Greenhawt, M.; Pali-Schöll, I.; Nwaru, B.; Roduit, C.; Untersmayr, E.; Adel-Patient, K.; Agache, I.; Agostoni, C.; et al. Role of dietary fiber in promoting immune health-An EAACI position paper. Allergy 2022, 77, 3185–3198. [Google Scholar] [CrossRef]
- Haetinger, V.S.; Park, C.S.; Adeola, O. Energy values of copra meal and cornstarch for broiler chickens. Poult. Sci. 2021, 100, 858–864. [Google Scholar] [CrossRef]
- Rocha, H.R.; Coelho, M.C.; Gomes, A.M.; Pintado, M.E. Carotenoids diet: Digestion, gut microbiota modulation, and inflammatory diseases. Nutrients 2023, 15, 2265. [Google Scholar] [CrossRef]
- Gao, Q.T.; Liu, Z.Q.; Li, K.; Bai, G.S.; Liu, L.; Zhong, R.Q.; Chen, L.; Zhang, H.F. Time-course effects of different fiber-rich ingredients on energy values, microbiota composition and SCFA profile in growing pigs. Anim. Nutr. 2023, 12, 263–275. [Google Scholar] [CrossRef]
- Knudsen, K.E.B. Carbohydrate and lignin contents of plant materials used in animal feeding. Anim. Feed Sci. Technol. 1997, 67, 319–338. [Google Scholar] [CrossRef]
- Tucak, M.; Ravlić, M.; Horvat, D.; Čupić, T. Improvement of forage nutritive quality of alfalfa and red clover through plant breeding. Agronomy 2021, 11, 2176. [Google Scholar] [CrossRef]
- Wang, J.; Singh, A.K.; Kong, F.; Kim, W.K. Effect of almond hulls as an alternative ingredient on broiler performance, nutrient digestibility, and cecal microbiota diversity. Poult. Sci. 2021, 100, 100853. [Google Scholar] [CrossRef]
- Horvat, D.; Vuletić, M.V.; Andrić, L.; Baličević, R.; Babić, M.K.; Tucak, M. Characterization of forage quality, phenolic profiles, and antioxidant activity in alfalfa (Medicago sativa L.). Plants 2022, 11, 2735. [Google Scholar] [CrossRef]
- Gao, Q.T.; Sun, G.M.; Duan, J.J.; Luo, C.Z.; Yangji, C.D.; Zhong, R.Q.; Chen, L.; Zhu, Y.B.; Wangdui, B.S.; Zhang, H.F. Alterations in gut microbiota improve SCFA production and fiber utilization in Tibetan pigs fed alfalfa diet. Front. Microbiol. 2022, 13, 969524. [Google Scholar] [CrossRef]
- Cui, Y.; Diao, Z.P.; Fan, W.T.; Wei, J.L.; Zhou, J.S.; Zhu, H.Y.; Li, D.S.; Guo, L.W.; Tian, Y.M.; Song, H.; et al. Effects of dietary inclusion of alfalfa meal on laying performance, egg quality, intestinal morphology, caecal microbiota and metabolites in Zhuanghe Dagu chickens. Ital. J. Anim. Sci. 2022, 21, 831–846. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Mink and Foxes, 2nd ed.; National Academy Press: Washington, DC, USA, 1982. [Google Scholar]
- AOAC. Official Methods of Analysis, 19th ed.; Associatin of Official Agricultural Chemists: Arlington, VA, USA, 2012. [Google Scholar]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Haas, B.J.; Gevers, D.; Earl, A.M.; Feldgarden, M.; Ward, D.V.; Giannoukos, G.; Ciulla, D.; Tabbaa, D.; Highlander, S.K.; Sodergren, E.; et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 2011, 21, 494–504. [Google Scholar] [CrossRef]
- Li, M.J.; Shao, D.T.; Zhou, J.C.; Gu, J.H.; Qin, J.J.; Chen, W.; Wei, W.Q. Signatures within esophageal microbiota with progression of esophageal squamous cell carcinoma. Chin. J. Cancer Res. 2020, 32, 755–767. [Google Scholar] [CrossRef]
- Tang, S.L.; Zhang, S.F.; Zhong, R.Q.; Su, D.; Xia, B.; Liu, L.; Chen, L.; Zhang, H.F. Time-course alterations of gut microbiota and short-chain fatty acids after short-term lincomycin exposure in young swine. Appl. Microbiol. Biotechnol. 2021, 105, 8441–8456. [Google Scholar] [CrossRef]
- Nadeau, E.; de Sousa, D.O.; Magnusson, A.; Hedlund, S.; Richardt, W.; Nørgaard, P. Digestibility and protein utilization in wethers fed whole-crop barley or grass silages harvested at different maturity stages, with or without protein supplementation. J. Anim. Sci. 2019, 97, 2188–2201. [Google Scholar] [CrossRef]
- Lan, T.M.; Li, H.M.; Yang, S.C.; Shi, M.H.; Han, L.; Sahu, S.K.; Lu, Y.X.; Wang, J.G.; Zhou, M.C.; Liu, H.; et al. The chromosome-scale genome of the raccoon dog: Insights into its evolutionary characteristics. iScience 2022, 25, 25. [Google Scholar] [CrossRef]
- Johnson, E.J.; Marlett, J.A. A simple method to estimate neutral detergent fiber content of typical daily menus. Am. J. Clin. Nutr 1986, 44, 127–134. [Google Scholar] [CrossRef]
- Zhong, R.Q.; Adeola, O. Energy values of solvent-extracted canola meal and expeller-derived canola meal for broiler chickens and growing pigs determined using the regression method. J. Anim. Sci. 2019, 97, 3415–3425. [Google Scholar] [CrossRef]
- Brandl, B.; Rennekamp, R.; Reitmeier, S.; Pietrynik, K.; Dirndorfer, S.; Haller, D.; Hofmann, T.; Skurk, T.; Hauner, H. Offering fiber-enriched foods increases fiber intake in adults with or without cardiometabolic risk: A randomized controlled trial. Front. Nutr. 2022, 9, 816299. [Google Scholar] [CrossRef]
- Men, Z.Y.; Cao, M.; Gong, Y.C.; Hua, L.; Zhang, R.H.; Zhu, X.; Tang, L.C.; Jiang, X.M.; Xu, S.Y.; Li, J.; et al. Microbial and metabolomic mechanisms mediating the effects of dietary inulin and cellulose supplementation on porcine oocyte and uterine development. J. Anim. Sci. Biotechnol. 2022, 13, 16. [Google Scholar] [CrossRef]
- Flores, K.R.; Fahrenholz, A.C.; Grimes, J.L. Effect of feed form, soybean meal protein content, and Rovabio Advance on poult live performance to 3 wk of age. Poult. Sci. 2020, 99, 6705–6714. [Google Scholar] [CrossRef]
- Batonon-Alavo, D.I.; Faruk, M.U.; Lescoat, P.; Weber, G.M.; Bastianelli, D. Inclusion of sorghum, millet and cottonseed meal in broiler diets: A meta-analysis of effects on performance. Animal 2015, 9, 1120–1130. [Google Scholar] [CrossRef]
- Ogbuewu, I.P.; Emenalom, O.O.; Okoli, I.C. Alternative feedstuffs and their effects on blood chemistry and haematology of rabbits and chickens: A review. Comp. Clin. Pathol. 2017, 26, 277–286. [Google Scholar] [CrossRef]
- Guo, S.C.; Lei, J.X.; Liu, L.L.; Qu, X.Y.; Li, P.; Liu, X.; Guo, Y.; Gao, Q.Q.; Lan, F.L.; Xiao, B.; et al. Effects of Macleaya cordata extract on laying performance, egg quality, and serum indices in Xuefeng black-bone chicken. Poult. Sci. 2021, 100, 101031. [Google Scholar] [CrossRef]
- Cheng, C.; Tang, R.Q.; Xiong, L.; Hector, R.E.; Bai, F.W.; Zhao, X.Q. Association of improved oxidative stress tolerance and alleviation of glucose repression with superior xylose-utilization capability by a natural isolate of Saccharomyces cerevisiae. Biotechnol. Biofuels 2018, 11, 28. [Google Scholar] [CrossRef]
- Momo, C.M.; Ferdinand, N.; Bebe, N.O.; Marquise, M.A.; Augustave, K.; Narcisse, V.B.; Herve, T.; Joseph, T. Oxidative effects of potassium dichromate on biochemical, hematological characteristics, and hormonal levels in rabbit doe (Oryctolagus cuniculus). Vet. Sci. 2019, 6, 30. [Google Scholar] [CrossRef]
- Sun, Y.K.; Hou, T.Y.; Yu, Q.Y.; Zhang, C.R.; Zhang, Y.G.; Xu, L.J. Mixed oats and alfalfa improved the antioxidant activity of mutton and the performance of goats by affecting intestinal microbiota. Front. Microbiol. 2023, 13, 1056315. [Google Scholar] [CrossRef]
- Chen, S.; Li, X.; Liu, X.; Wang, N.; An, Q.; Ye, X.M.; Zhao, Z.T.; Zhao, M.; Han, Y.; Ouyang, K.H.; et al. Investigation of chemical composition, antioxidant activity, and the effects of alfalfa flavonoids on growth performance. Oxidative Med. Cell. Longev. 2020, 2020, 8569237. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, S.; Li, L.; Huang, J.H. Synergistic fermentation of Lactobacillus plantarum and Saccharomyces cerevisiae to improve the quality of wheat bran dietary fiber-steamed bread. Food Chem. X 2022, 16, 100528. [Google Scholar] [CrossRef]
- Koh, A.; Bäckhed, F. From association to causality: The role of the gut microbiota and its functional products on host metabolism. Mol. Cell 2020, 78, 584–596. [Google Scholar] [CrossRef]
- Liu, H.L.; Li, Z.P.; Si, H.Z.; Zhong, W.; Fan, Z.Y.; Li, G.Y. Comparative analysis of the gut microbiota of the blue fox (Alopex lagopus) and raccoon dog (Nyctereutes procyonoides). Arch. Microbiol. 2020, 202, 135–142. [Google Scholar] [CrossRef]
- Mao, K.; Lu, G.W.; Li, Y.J.; Zang, Y.T.; Zhao, X.H.; Qiu, Q.H.; Qu, M.R.; Ouyang, K.H. Effects of rumen-protected creatine pyruvate on blood biochemical parameters and rumen fluid characteristics in transported beef cattle. BMC Vet. Res. 2022, 18, 35. [Google Scholar] [CrossRef]
- Nathani, N.M.; Patel, A.K.; Mootapally, C.S.; Bhaskar, R.; Shah, S.V.; Lunagaria, P.M.; Kothari, R.K.; Joshi, C.G. Effect of roughage on rumen microbiota composition in the efficient feed converter and sturdy Indian Jaffrabadi buffalo (Bubalus bubalis). BMC Genom. 2015, 16, 1116. [Google Scholar] [CrossRef]
- Chen, S.Y.; Yan, C.; Liu, W.; Chen, K.C.; Xing, L.M.; Li, H.; Zhao, X.B. Research Note: Integrated gut microbiome and short-chain fatty acids responds to dominance hierarchy in roosters. Poult. Sci. 2022, 101, 101670. [Google Scholar] [CrossRef]
- Li, H.; Ma, L.T.; Li, Z.Q.; Yin, J.; Tan, B.; Chen, J.S.; Jiang, Q.; Ma, X.K. Evolution of the gut microbiota and its fermentation characteristics of Ningxiang pigs at the young stage. Animals 2021, 11, 638. [Google Scholar] [CrossRef]
- Zhang, H.L.; Lang, X.; Zhang, Y.S.; Wang, C.L. Distribution of bacteria in different regions of the small intestine with Zanthoxylum bungeanum essential oil supplement in small-tailed Han sheep. Front. Microbiol. 2022, 13, 1062077. [Google Scholar] [CrossRef]
- Mao, X.B.; Ding, X.M.; Zhang, Q.F.; Bai, S.P.; Zhang, K.Y.; Chen, D.W.; Yu, B.; He, J.; Yu, J.; Yan, H.; et al. The effect of dietary pectic oligosaccharide supplementation on intestinal health of broiler breeders with different egg-laying rates. Poult. Sci. 2021, 100, 100938. [Google Scholar] [CrossRef]
- Hong, J.S.; Ndou, S.P.; Adams, S.; Scaria, J.; Woyengo, T.A. Canola meal in nursery pig diets: Growth performance and gut health. J. Anim. Sci. 2020, 98, skaa338. [Google Scholar] [CrossRef]
- Sanchez, H.N.; Moroney, J.B.; Gan, H.Q.; Shen, T.; Im, J.L.; Li, T.B.; Taylor, J.R.; Zan, H.; Casali, P. B cell-intrinsic epigenetic modulation of antibody responses by dietary fiber-derived short-chain fatty acids. Nat. Commun. 2020, 11, 60. [Google Scholar] [CrossRef]
- Witten, G.Q.; Richardson, F.D. Competition of three aggregated microbial species for four substrates in the rumen. Ecol. Model. 2003, 164, 121–135. [Google Scholar] [CrossRef]
- Zhao, J.D.; Liu, L.L.; Xin, L.; Lu, Y.X.; Yang, X.J.; Hou, Y.; Shi, M.; Han, S.; Zhou, H.; Liu, Y.H.; et al. The protective effects of a modified Xiaohua Funing decoction against acute liver failure in mice induced by D-Gal and LPS. Evid.-Based Complement Altern. Med. 2022, 2022, 6611563. [Google Scholar] [CrossRef]
- Nogueira, J.P.D.; He, F.; Mangian, H.F.; Oba, P.M.; De Godoy, M.R.C. Dietary supplementation of a fiber-prebiotic and saccharin-eugenol blend in extruded diets fed to dogs. J. Anim. Sci. 2019, 97, 4519–4531. [Google Scholar] [CrossRef]
- Cheng, C.S.; Wei, H.K.; Yu, H.C.; Xu, C.H.; Jiang, S.W.; Peng, J. Metabolic syndrome during perinatal period in sows and the link with gut microbiota and metabolites. Front. Microbiol. 2018, 9, 1989. [Google Scholar] [CrossRef]
- Pilla, R.; Gaschen, F.P.; Barr, J.W.; Olson, E.; Honneffer, J.; Guard, B.C.; Blake, A.B.; Villanueva, D.; Khattab, M.R.; AlShawaqfeh, M.K.; et al. Effects of metronidazole on the fecal microbiome and metabolome in healthy dogs. J. Vet. Intern. Med. 2020, 34, 1853–1866. [Google Scholar] [CrossRef]
- Liu, Y.H.; Ajami, N.J.; El-Serag, H.B.; Hair, C.; Graham, D.Y.; White, D.L.; Chen, L.; Wang, Z.S.; Plew, S.; Kramer, J.; et al. Dietary quality and the colonic mucosa-associated gut microbiome in humans. Am. J. Clin. Nutr. 2019, 110, 701–712. [Google Scholar] [CrossRef]
- Sugden, S.; Sanderson, D.; Ford, K.; Stein, L.Y.; St Clair, C.C. An altered microbiome in urban coyotes mediates relationships between anthropogenic diet and poor health. Sci. Rep. 2020, 10, 22207. [Google Scholar] [CrossRef]
- Ribeiro, G.O.; Gruninger, R.J.; Jones, D.R.; Beauchemin, K.A.; Yang, W.; Wang, Y.; Abbott, D.W.; Tsang, A.; McAllister, T.A. Effect of ammonia fiber expansion-treated wheat straw and a recombinant fibrolytic enzyme on rumen microbiota and fermentation parameters, total tract digestibility, and performance of lambs. J. Anim. Sci. 2020, 98, skaa116. [Google Scholar] [CrossRef]
- Wang, K.; Nan, X.M.; Tong, J.J.; Zhao, G.Y.; Jiang, L.S.; Xiong, B.H. Steam explosion pretreatment changes ruminal fermentationin in vitro of corn stover by shifting archaeal and bacterial community structure. Front. Microbiol. 2020, 11, 2027. [Google Scholar]
- Zhang, L.; Chung, J.S.; Jiang, Q.Q.; Sun, R.; Zhang, J.; Zhong, Y.J.; Ren, N.Q. Characteristics of rumen microorganisms involved in anaerobic degradation of cellulose at various pH values. RSC Adv. 2017, 7, 40303–40310. [Google Scholar] [CrossRef]
- Lv, X.K.; Chai, J.M.; Diao, Q.Y.; Huang, W.Q.; Zhuang, Y.M.; Zhang, N.F. The signature microbiota drive rumen function shifts in goat kids introduced to solid diet regimes. Microorganisms 2019, 7, 516. [Google Scholar] [CrossRef]
- Kilburn, L.R.; Allenspach, K.; Jergens, A.E.; Bourgois-Mochel, A.; Mochel, J.P.; Serao, M.C.R. Apparent total tract digestibility, fecal characteristics, and blood parameters of healthy adult dogs fed high-fat diets. J. Anim. Sci. 2020, 98, skaa043. [Google Scholar] [CrossRef]
- Cui, J.; Yang, X.Y.; Wang, F.X.; Liu, S.D.; Han, S.J.; Chen, B.J. Effects of ammonia on growth performance, lipid metabolism and cecal microbial community of rabbits. PLoS ONE 2021, 16, e0252065. [Google Scholar] [CrossRef]
- Wang, J.W.; Qin, C.F.; He, T.; Qiu, K.; Sun, W.J.; Zhang, X.; Jiao, N.; Zhu, W.Y.; Yin, J.D. Alfalfa-containing diets alter luminal microbiota structure and short chain fatty acid sensing in the caecal mucosa of pigs. J. Anim. Sci. Biotechnol. 2018, 9, 11. [Google Scholar] [CrossRef]
- Ellner, C.; Wessels, A.G.; Zentek, J. Effects of dietary cereal and protein source on fiber digestibility, composition, and metabolic activity of the intestinal microbiota in weaner piglets. Animals 2022, 12, 109. [Google Scholar] [CrossRef]
| Item | AM0 | AM5 | AM10 | AM15 |
|---|---|---|---|---|
| Ingredients | ||||
| Extruded corn | 46.50 | 46.50 | 45.50 | 43.50 |
| Soybean meal | 15.00 | 14.00 | 14.00 | 13.00 |
| Alfalfa meal 1 | 0.00 | 5.00 | 10.00 | 15.00 |
| Wheat bran | 4.00 | 2.00 | 0.00 | 0.00 |
| Corn germ meal | 6.00 | 6.00 | 6.00 | 6.00 |
| Corn skin | 6.00 | 4.00 | 2.00 | 0.00 |
| Meat and bone meal | 5.00 | 5.00 | 5.00 | 5.00 |
| Fish meal | 2.50 | 2.50 | 2.50 | 2.50 |
| Chicken meal | 2.00 | 2.00 | 2.00 | 2.00 |
| Plasma protein powder | 1.00 | 1.00 | 1.00 | 1.00 |
| Chicken oil | 4.00 | 4.00 | 4.00 | 4.00 |
| Glucose | 1.50 | 1.50 | 1.50 | 1.50 |
| Milk powder | 2.50 | 2.50 | 2.50 | 2.50 |
| Limestone | 0.30 | 0.30 | 0.30 | 0.30 |
| Dicalcium phosphate | 0.60 | 0.60 | 0.60 | 0.60 |
| L-Lysine | 0.40 | 0.40 | 0.40 | 0.40 |
| DL-Methionine | 0.30 | 0.30 | 0.30 | 0.30 |
| Sodium chloride | 0.40 | 0.40 | 0.40 | 0.40 |
| Vitamin–mineral premix 2 | 2.00 | 2.00 | 2.00 | 2.00 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 |
| Nutrient and energy composition 3 | ||||
| Dry matter | 92.88 | 92.96 | 93.19 | 92.61 |
| Crude protein | 19.90 | 20.22 | 20.07 | 20.68 |
| Ether extract | 7.62 | 7.31 | 7.52 | 7.36 |
| Crude fiber | 7.76 | 9.21 | 11.04 | 12.21 |
| Neutral detergent fiber | 14.84 | 15.72 | 19.05 | 23.17 |
| Acid detergent fiber | 5.28 | 7.25 | 9.01 | 10.65 |
| Crude ash | 6.17 | 6.60 | 6.80 | 7.20 |
| Calcium | 1.13 | 1.24 | 1.28 | 1.38 |
| Total phosphorus | 1.38 | 1.37 | 1.31 | 1.24 |
| Gross energy, MJ/kg | 18.99 | 18.90 | 18.95 | 18.44 |
| Item | AM0 | AM5 | AM10 | AM15 | SEM | p-Value |
|---|---|---|---|---|---|---|
| IBW, kg | 4.60 | 4.60 | 4.51 | 4.57 | 0.05 | 0.879 |
| FBW, kg | 7.49 a | 7.44 a | 7.20 ab | 6.96 b | 0.06 | 0.004 |
| ADG, g/d | 48.11 a | 47.33 a | 44.83 a | 39.83 b | 0.71 | <0.001 |
| ADFI, g/d | 308.01 a | 307.08 b | 306.10 c | 305.49 d | 0.13 | <0.001 |
| F/G | 6.47 b | 6.53 b | 6.87 b | 7.80 a | 0.11 | <0.001 |
| Item | AM0 | AM5 | AM10 | AM15 | SEM | p-Value |
|---|---|---|---|---|---|---|
| DM intake, g | 882.75 a | 791.83 b | 792.96 b | 788.74 c | 7.65 | <0.001 |
| DM output, g | 249.34 ab | 230.05 b | 259.01 a | 255.20 a | 4.23 | 0.064 |
| DM digestibility, % | 71.76 a | 70.95 a | 67.34 b | 67.64 b | 0.58 | 0.004 |
| CP digestibility, % | 68.93 | 69.41 | 67.04 | 68.85 | 0.51 | 0.391 |
| EE digestibility, % | 87.73 | 86.50 | 83.06 | 82.80 | 0.83 | 0.078 |
| NDF digestibility, % | 64.44 | 62.23 | 62.78 | 66.00 | 0.70 | 0.219 |
| ADF digestibility, % | 42.05 a | 40.32 ab | 31.41 b | 38.35 ab | 1.47 | 0.045 |
| Item | AM0 | AM5 | AM10 | AM15 | SEM | p-Value |
|---|---|---|---|---|---|---|
| TP, g/L | 58.53 | 57.39 | 56.78 | 57.08 | 0.90 | 0.920 |
| ALB, g/L | 29.31 | 27.99 | 28.06 | 27.59 | 0.56 | 0.742 |
| GLB g/L | 29.22 | 29.39 | 28.72 | 29.50 | 1.13 | 0.996 |
| LDH, U/L | 295.25 | 302.83 | 293.58 | 293.48 | 5.10 | 0.914 |
| AST, U/L | 56.58 | 55.28 | 51.30 | 51.48 | 1.20 | 0.303 |
| ALT, U/L | 18.82 | 17.01 | 16.91 | 16.26 | 0.74 | 0.669 |
| ALP, U/L | 23.44 | 22.66 | 21.23 | 21.47 | 0.63 | 0.591 |
| Urea, mmol/L | 9.99 a | 8.26 ab | 6.70 b | 7.65 b | 0.31 | <0.001 |
| T-CHO, mmol/L | 2.92 | 2.54 | 2.68 | 2.57 | 0.10 | 0.526 |
| TG, mmol/L | 0.38 | 0.36 | 0.36 | 0.35 | 0.01 | 0.869 |
| HDL-C, mmol/L | 2.20 | 1.94 | 1.90 | 1.98 | 0.07 | 0.535 |
| LDL-C, mmol/L | 0.10 | 0.09 | 0.10 | 0.09 | 0.01 | 0.745 |
| GLU, mmol/L | 2.68 | 2.48 | 2.67 | 2.65 | 0.07 | 0.763 |
| Item | AM0 | AM5 | AM10 | AM15 | SEM | p-Value |
|---|---|---|---|---|---|---|
| T-AOC, mM | 0.34 | 0.36 | 0.37 | 0.37 | 0.01 | 0.543 |
| SOD activity, U/mL | 10.17 b | 12.32 a | 12.31 a | 12.71 a | 0.32 | 0.012 |
| MDA content, nmol/mL | 5.63 a | 4.59 b | 4.27 b | 3.91 b | 0.17 | 0.001 |
| Item | Cecum | Colon | ||
|---|---|---|---|---|
| R2 | p-Value | R2 | p-Value | |
| AM0 vs. AM5 | 0.0971 | 0.135 | 0.1776 | 0.005 |
| AM0 vs. AM10 | 0.0979 | 0.181 | 0.1257 | 0.031 |
| AM0 vs. AM15 | 0.1796 | 0.020 | 0.2306 | 0.001 |
| AM5 vs. AM10 | 0.0970 | 0.119 | 0.1813 | 0.005 |
| AM5 vs. AM15 | 0.1571 | 0.020 | 0.2070 | 0.004 |
| AM10 vs. AM15 | 0.1560 | 0.028 | 0.1753 | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Li, X.; Chen, D.; Zhao, W.; Zhang, X.; Yuan, W.; Si, H.; Deng, X.; Du, R.; Xu, C. Effects of Dietary Alfalfa Meal Supplementation on the Growth Performance, Nutrient Apparent Digestibility, Serum Parameters, and Intestinal Microbiota of Raccoon Dogs (Nyctereutes procyonoides). Animals 2024, 14, 623. https://doi.org/10.3390/ani14040623
Chen X, Li X, Chen D, Zhao W, Zhang X, Yuan W, Si H, Deng X, Du R, Xu C. Effects of Dietary Alfalfa Meal Supplementation on the Growth Performance, Nutrient Apparent Digestibility, Serum Parameters, and Intestinal Microbiota of Raccoon Dogs (Nyctereutes procyonoides). Animals. 2024; 14(4):623. https://doi.org/10.3390/ani14040623
Chicago/Turabian StyleChen, Xiaoli, Xiao Li, Danyang Chen, Weigang Zhao, Xiuli Zhang, Weitao Yuan, Huazhe Si, Xuming Deng, Rui Du, and Chao Xu. 2024. "Effects of Dietary Alfalfa Meal Supplementation on the Growth Performance, Nutrient Apparent Digestibility, Serum Parameters, and Intestinal Microbiota of Raccoon Dogs (Nyctereutes procyonoides)" Animals 14, no. 4: 623. https://doi.org/10.3390/ani14040623
APA StyleChen, X., Li, X., Chen, D., Zhao, W., Zhang, X., Yuan, W., Si, H., Deng, X., Du, R., & Xu, C. (2024). Effects of Dietary Alfalfa Meal Supplementation on the Growth Performance, Nutrient Apparent Digestibility, Serum Parameters, and Intestinal Microbiota of Raccoon Dogs (Nyctereutes procyonoides). Animals, 14(4), 623. https://doi.org/10.3390/ani14040623






