Effect of Myrtus communis L. Plant Extract as a Milk Supplement on the Performance, Selected Blood Parameters and Immune Response of Holstein Calves
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amaral-Phillips, D.M. Dairy Calf Management Practices Impact Future Production. 2021. University of Kentucky. USA. Available online: https://afs.ca.uky.edu/content/dairy-calf-management-practices-impact-future-production (accessed on 12 December 2023).
- Langford, F.M.; Weary, D.M.; Fisher, L. Antibiotic resistance in gut bacteria from dairy calves: A dose response to the level of antibiotics fed in milk. J. Dairy Sci. 2003, 86, 3963–3966. [Google Scholar] [CrossRef]
- Uyeno, Y.; Shigemori, S.; Shimosato, T. Effect of Probiotics/Prebiotics on Cattle Health and Productivity. Microbes Environ. 2015, 30, 126–132. [Google Scholar] [CrossRef]
- Volpato, A.; Crecencio, R.B.; Tomasi, T.; Galli, G.M.; Griss, L.G.; Da Silva, A.D.; Schetinger, M.R.C.; Schogor, A.L.B.; Baldissera, M.D.; Stefani, L.M.; et al. Phytogenic as feed additive for suckling dairy calves’ has a beneficial effect on animal health and performance. An. Acad. Bras. Cienc. 2019, 91, e20180747. [Google Scholar] [CrossRef]
- Myrtus communis. Wikipedia. 2021. Available online: https://en.wikipedia.org/wiki/Myrtus_communis (accessed on 12 December 2023).
- Raeiszadeh, M.; Pardakhty, A.; Sharififar, F.; Mehrabani, M.; Mehrabani, M. Phytoniosome: A novel drug delivery for myrtle extract. Iran. J. Pharm. Res. 2018, 17, 804–817. [Google Scholar]
- Qader, K.O.; Al-Saadi, S.A.M.; Al-Saadi, T.A. Chemical composition of Myrtus communis L. (Myrtaceae) fruits. J. Appl. Life Sci. Int. 2017, 12, 1–8. [Google Scholar] [CrossRef]
- Alipour, G.; Dashti, S.; Hosseinzadeh, H. Review of pharmacological effects of Myrtus communis L. and its active constituents. Phytother. Res. 2014, 28, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Miraj, S.; Kiani, S. A review study of therapeutic effects of Myrtus communis. Der. Pharm. Lett. 2016, 8, 281–285. [Google Scholar]
- Bardzardi, M.M.; Ghazanfari, S.; Salehi, A.; Sharifi, S.D. Growth performance, carcass characteristics, antibody titer and blood parameters in broiler chickens fed dietary myrtle (Myrtus communis) essential oil as an alternative to antibiotic growth promoter. Poult. Sci. J. 2014, 2, 37–49. [Google Scholar]
- Goudarzi, M.; Samiei, I.; Nanekarani, S.; Nasrolahi, F. The Effect of Myrtus Communis Oil Extract on Growth Performance and Immune Responses in Ross and Cobb Strain Broilers. J. Adv. Agric. Technol. 2016, 3, 10–14. [Google Scholar] [CrossRef]
- Gultepe, E.E.; Iqbal, A.; Cetingul, I.S.; Uyarlar, C.; Ozcinar, U.; Bayram, I. Effect of Myrtus communis L. plant extract as a drinking water supplement on performance, some blood parameters, egg quality and immune response of older laying hens. Kafkas Univ. Vet. Fak. Derg. 2020, 26, 9–16. [Google Scholar]
- Aljebory, A.M.K. Effect of Myrtus communis leaves extract on blood sugar in rabbits. J. Purity Util. React. Environ. 2014, 3, 263–272. [Google Scholar]
- Hashemipour, M.A.; Lotfi, S.; Torabi, M.; Sharifi, F.; Ansari, M.; Ghassemi, A.; Sheikhshoaie, S. Evaluation of the Effects of Three Plant Species (Myrtus communis L., Camellia sinensis L., Zataria multiflora Boiss.) on the Healing Process of Intraoral Ulcers in Rats. J. Dent. 2017, 18, 127–135. [Google Scholar]
- Hsouna, A.B.; Dhibi, S.; Dhifi, W.; Mnif, W.; Nasr, H.B.; Hfaiedn, N. Chemical composition and hepatoprotective effect of essential oil from Mrytus communis L. Flowers agains CCL4 induced acute hepatotoxicity in rats. RSC Adv. 2019, 9, 3777–3787. [Google Scholar] [CrossRef] [PubMed]
- Biricik, H.; Yesilbag, D.; Gezen, S.S.; Bulbul, T. Effects of dietary myrtle oil (Myrtus communis L.) supplementation on growth performance, meat oxidative stability, meat quality and erythrocyte parameters in quails. Revue. Méd. Vét. 2012, 163, 131–138. [Google Scholar]
- Sumbul, S.; Ahmad, M.A.; Asif, M.; Akhtar, M. Myrtus communis Linn.—A review. Indian J. Nat. Prod. Resour. 2011, 2, 395–402. [Google Scholar]
- Romani, A.; Mulinacci, N.; Pinelli, P.; Vincieri, F.F.; Tattini, M. Identification and quantation of polyphenols in leaves Myrtus communis L. Chromatographia 1999, 49, 17–20. [Google Scholar] [CrossRef]
- Ozek, T.; Demirci, B.; Baser, K.H.C. Chemical composition of Turkish myrtle oil. J. Essent. Oil. Res. 2000, 12, 541–544. [Google Scholar] [CrossRef]
- Hayder, N.; Skandrani, I.; Kilani, S.; Bouhlel, I.; Abdelwahed, A.; Ben Amar, R.; Mahmoud, A.; Ghedira, K.; Chekir-Ghedira, L. In vitro antioxidant and antigenotoxic potentials of myricetin-3-o-galactoside and myricetin-3-o-rhamnoside from Myrtus communis: Modulation of expression of genes involved in cell defence system using cDNA microarray. Toxicol. In Vitro. 2008, 22, 567–581. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Dairy Cattle, 6th ed.; National Academy of Sciences: Washington, DC, USA, 2001. [Google Scholar]
- Association of Official Analytical Chemists. Official Method of Analysis of the Association of Official Analytical Chemists; No. 934.06; AOAC: Arlington, VA, USA, 1990. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Heinrichs, A.J.; Jones, C. Feeding the New Born Dairy Calf; The Pennsylvania State University: Pennsylvania, PA, USA, 2003; p. 3434. [Google Scholar]
- Çetin, Ş.; Bek, Y. Covariance Models in Repeated Measured Data. Turk. Klin. J. Biostat. 2019, 11, 239–253. [Google Scholar] [CrossRef]
- Littell, R.C.; Stroup, W.W.; Freund, R.J. SAS for Linear Models, 4th ed.; SAS Institute Inc.: Cary, NC, USA, 2002. [Google Scholar]
- Bülbül, T.; Yesilbag, D.; Ulutas, E.; Biricik, H.; Gezen, S.S.; Bülbül, A. Effect of myrtle (Myrtus communis L.) oil on performance, egg quality, some biochemical values and hatchability in laying quails. Rev. Méd. Vét. 2014, 165, 280–288. [Google Scholar]
- Saei, M.M.; Sadeghi, A.A.; Ahmadvand, H. The effect of Myrtus communis oil extract on growth performance, serum biochemistry and humoral immune responses in broiler chicks fed diet containing aflatoxin B1. Arch. Anim. Breed. 2013, 56, 842–850. [Google Scholar] [CrossRef]
- Taee, H.M.; Hajimoradloo1, A.; Hoseinifar, S.H.; Ahmadvand, H. The effects of dietary Myrtle (Myrtus communis L.) supplementations on growth performance and some innate immune responses in rainbow trout (Oncorhynchus mykiss). Int. J. Aquat. Biol. 2017, 5, 252–259. [Google Scholar]
- Bakova, E.Y.; Plugar, Y.V.; Bakova, N.N.; Konovalov, D.A. Mineral and Amino Acid Composition of Myrtus communis L. Leaves. Chem. Plant Raw Mater. 2019, 3, 217–223. [Google Scholar] [CrossRef]
- Aremu, M.O.; Nweze, C.C.; Alade, P. Evaluation of protein and amino acid composition of selected spices grown in the middle belt region of Nigeria. Pak. J. Nutr. 2011, 10, 991–995. [Google Scholar] [CrossRef]
- Mouritsen, O.G. Umami flavour as a mean of regulating food intake and improving nutrition and health. Nutr. Health 2012, 21, 56–75. [Google Scholar] [CrossRef]
- Camilleri, M. Peripheral mechanisms in appetite regulation. Gastroenterology 2015, 148, 1219–1233. [Google Scholar] [CrossRef]
- Barlas, N.; Özer, S.; Karabulut, G. The estrogenic effects of apigenin, phloretin and myricetin based on uterotrophic assay in immature Wistar albino rats. Toxicol. Lett. 2014, 226, 35–42. [Google Scholar] [CrossRef]
- Abidli, N.; Ghaly, I.S.; Hassanane, M.M.; Ahmed, E.S.; Khalil, W.K.B. Myrtus Species Prevents Reproductive Toxicity Induced by Doxorubicin in Male Mice. Asian J. Pharm. Clin. Res. 2015, 8, 169–175. [Google Scholar]
- Vakili, T.S.T.; Mohaysenzadeh, E.; Mohammadabadi, T.; Zarei, M. Effect of dietary Myrtus communis leaf powder on Quantitative and Qualitative Characteristics of Sperm and Antioxidant Function of Semen and Blood in Arabi Ram. Vet. Res. Biol. Prod. 2020, 33, 102–111. [Google Scholar]
- Overton, T.R.; Waldron, M.R. Nutritional management of transition dairy cows: Strategies to optimize metabolic health. J. Dairy Sci. 2004, 87, 105–119. [Google Scholar] [CrossRef]
- Grummer, R.R. Etiology of lipid-related metabolic disorders in periparturient dairy cows. J. Dairy Sci. 1993, 76, 3882–3896. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, H.; Huhtanen, P. Effects of the ratio of ruminal propionate to butyrate on milk yield and blood metabolites in dairy cows. J. Dairy Sci. 1996, 79, 851–861. [Google Scholar] [CrossRef]
- Coverdale, J.A.; Tyler, H.D.; Quigley, J.D., III; Brumm, J.A. Effect of various levels of forage and form of diet on rumen development and growth in calves. J. Dairy Sci. 2004, 87, 2554–2562. [Google Scholar] [CrossRef]
- Khan, M.A.; Weary, D.M.; von Keyserlingk, M.A.G. Hay intake improves performance and rumen development of calves fed higher quantities of milk. J. Dairy Sci. 2011, 94, 3547–3553. [Google Scholar] [CrossRef] [PubMed]
- Budny-Walczak, A.; Śpitalniak-Bajerska, K.; Szołtysik, M.; Pogoda-Sewerniak, K.; Kupczyński, R. Effects of Iron Supplementation on Metabolism in Calves Receiving Whole Milk. Animals 2023, 13, 477. [Google Scholar] [CrossRef]
- Ong, K.C.; Khoo, H.E. Insulinomimetic effects of myricetin on lipogenesis and glucose transport in rat adipocytes but not glucose transporttranslocation. Biochem. Pharmacol. 1996, 51, 423–429. [Google Scholar] [CrossRef]
- Ong, K.C.; Khoo, H.E. Effects of myricetin on glycemia and glycogen metabolism in diabetic rats. Life Sci. 2000, 67, 1695–1705. [Google Scholar] [CrossRef]
- Jiménez, R.; Andriambeloson, E.; Duarte, J.; Andriantsitohaina, R.; Jiménez, J.; Pérez-Vizcaino, F.; Zarzuelo, A.; Tamargo, J. Involvement of thromboxane A2 in the endothelium-dependent contractions induced by myricetin in rat isolated aorta. Br. J. Pharmacol. 1999, 127, 1539–1544. [Google Scholar] [CrossRef]
- Kang, B.Y.; Kim, S.H.; Cho, D.; Kim, T.S. Inhibition of interleukin-12 production in mouse macrophages via decreased nuclear factor-kappaB DNA binding activity by myricetin, a naturally occurring flavonoid. Arch. Pharm. Res. 2005, 28, 274–279. [Google Scholar] [CrossRef]
- Khamisabadi, H.; Kafilzadeh, F.; Charaien, B. Effect of thyme (Thymus vulgaris) or peppermint (Mentha piperita) on performance, digestibility and blood metabolites of fattening Sanjabi lambs. Biharean Biol. 2016, 10, 118–122. [Google Scholar]
- Ster, C.; Loiselle, M.C.; Lacasse, P. Effect of post-calving serum non-esterified fatty acids concentration on the functionality of bovine immune cells. J. Dairy Sci. 2012, 95, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Salehifar, E.; Abbasi, M.; Bahari-Kashani, R. Effects of Myrtle (Myrtus communis) essential oil on growth performance, carcass characteristics, intestinal morphology, immune response and blood parameters in broiler chickens. J. Livest. Sci. 2017, 8, 63–71. [Google Scholar]
| Ingredient | % |
|---|---|
| Corn grain | 26 |
| D.D.G.S. (Corn) | 15 |
| Soybean meal (CP 48%) | 14.7 |
| Corn Bran (%18P CP) | 12.6 |
| Wheat bran | 12 |
| Wheat grain | 8 |
| Sunflower meal (Dehulled, %36 CP) | 7.9 |
| Limestone | 2.9 |
| Salt | 0.7 |
| Premix * | 0.2 |
| Chemical Composition | |
| Dry Matter, % | 88.19 |
| Crude Protein, % | 20.03 |
| Metabolizable energy (KCal/kg) | 2.622 |
| Crude Fat, % | 3.65 |
| Crude Cellulose, % | 7.39 |
| Crude Ash, % | 7.62 |
| Ca, % | 1.28 |
| P, % | 0.53 |
| NDF, % | 20.21 |
| ADF, % | 8.64 |
| Amino Acid Contents in the Myrtle Extract | |||||||||||||||||||
| Alanine | Arginine | Aspartic Acid | Cystine | Glutamic Acid | Glycine | Histidine | Isoleucine | Leucine | Lysine | Methionine | Proline | Serine | Threonine | Trytophan | Valine | Tyrosine | Phenylalanine | Norvaline | |
| Leaf (mg/L) | 8436.47 | 5484.26 | 10,193.61 | 1591.77 | 10,207.99 | 5759.73 | 3692.57 | 4567.86 | 7517.24 | 5971.68 | 900.21 | 32,886.81 | 11,543.61 | 9808.00 | 1386.40 | 7031.16 | 7265.74 | 4205.79 | 2982.68 |
| Stem (mg/L) | 6733.67 | 4268.64 | 9656.13 | 1366.17 | 9874.28 | 4572.21 | 3190.05 | 4260.04 | 5009.63 | 4329.44 | 769.37 | 4116.44 | 8799.76 | 7481.94 | 648.44 | 5547.04 | 5594.37 | 3607.42 | 252.44 |
| Active Ingredients of Myrtle Extract | |||||||||||||||||||
| Leaf | Phthalic acid mono-2 ethylexyl ester | Gallic acid | Quinic Acid | Hydroxyhydroquinone/phloroglucin/Pyrogallol | Esculetin/Daphnetin | ||||||||||||||
| Stem | Phthalic acid mono-2 ethylexyl ester | Hexadecylamine | Sodium tetradecyl sulphate | Nobuletin | 3,5-di-tert-Butyl-4-hydroxybenzaldehyde | ||||||||||||||
| Leaf plus Stem | Gallic acid | Vanillic acid | Catechin | Quercetin | Protocatechuic acid | Ellagic acid | Rosmarinic acid | Gentisic acid | |||||||||||
| Mineral Contents in Myrtle Extract | |||||||||||||||||||
| Unit (mg/L) | Cadmium (Cd) | Lead (Pb) | Arsenic (As) | Mercury (Hg) | Magnesium (Mg) | Sodium (Na) | Iron (Fe) | Tin (Sn) | Aluminum (Al) | ||||||||||
| Leaf | Not detected | 0.008 | 0.002 | 0.002 | 36,000 | 335,000 | 1.46 | 1 | 1.26 | ||||||||||
| Stem | Not detected | 0.007 | 0.002 | 0.003 | 25,000 | 328,000 | 2.04 | 1 | 1.24 | ||||||||||
| Feedstuffs | 72 Days–6 Months | 6–10 Months | 6 Months—Pregnancy |
|---|---|---|---|
| % DM | % DM | % DM | |
| Corn silage. (Moderate Quality) | 0 | 13.48 | 20.58 |
| Alfalfa hay | 35.6 | 30.82 | 21.23 |
| Wheat straw | 16.82 | 14.56 | 17.16 |
| Corn. grain. fine ground | 34.87 | 30.18 | 27.81 |
| Canola meal (%34 CP) | 4.89 | 4.23 | 6.69 |
| Sunflower meal (Dehulled. %36 CP) | 4.85 | 4.21 | 4.28 |
| DCP | 1.74 | 1.45 | 1.12 |
| Salt | 1.06 | 0.92 | 0.97 |
| Premix * | 0.17 | 0.15 | 0.16 |
| Chemical Composition | |||
| Dry Matter. % | 89.91 | 70.26 | 62.87 |
| Crude Protein. % | 12.66 | 12.02 | 12.04 |
| Metabolizable energy (KCal/kg) | 2.19 | 2.21 | 2.22 |
| Crude Fat. % | 2.68 | 2.73 | 2.71 |
| Crude Cellulose. % | 21.48 | 22.51 | 22.49 |
| Crude Ash. % | 9.27 | 8.58 | 7.83 |
| NDF. % | 39.28 | 40.58 | 41.78 |
| ADF. % | 27.15 | 27.73 | 27.65 |
| Item | Treatment | p-Values | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose Response Leaves (%0–25–50) 6 | Dose Response Root (%0–25–50) 6 | ||||||||||||||
| Control | MPEL 25 | MPEL 50 | MPER 25 | MPER 50 | SEM 5 | Treatment | Time | T × T ≠ | C vs. T | Linear | Quadratic | C vs. T | Linear | Quadratic | |
| BW 2*, kg | 52.68 | 53.21 | 54.55 | 53.87 | 54.84 | 1.08 | 0.68 | <0.0001 | 0.61 | 0.45 | 0.31 | 0.78 | 0.24 | 0.21 | 0.89 |
| Feed Intake, g | 9316.3 b | 9515.7 ab | 9830.7 a | 9611.8 ab | 9765.6 a | 90.0 | 0.002 | <0.0001 | 0.99 | 0.01 | 0.001 | 0.48 | 0.01 | 0.004 | 0.80 |
| FCR 3,g feed/g BW 2* | 0.1647 | 0.1651 | 0.1663 | 0.1650 | 0.1649 | 0.003 | 0.98 | <0.0001 | 0.82 | 0.73 | 0.67 | 0.97 | 0.94 | 0.92 | 0.98 |
| BW 2** on day 300, kg | 257.99 b | 266.71 ab | 268.41 a | 268.82 a | 268.62 a | 2.44 | 0.01 | - | - | 0.005 | 0.01 | 0.27 | 0.001 | 0.005 | 0.08 |
| BW 2*** on day 410, kg | 341.4 | 350.7 | 353.1 | 353.5 | 353.6 | 3.2 | 0.64 | - | - | 0.02 | 0.02 | 0.42 | 0.004 | 0.01 | 0.13 |
| First AI 4, day | 442.1 | 439.8 | 431.9 | 439.1 | 433.1 | 5.9 | 0.67 | - | - | 0.44 | 0.28 | 0.74 | 0.34 | 0.22 | 0.81 |
| No. of AI/conception | 1.6 | 1.5 | 1.2 | 1.3 | 1.2 | 0.2 | 0.39 | - | - | 0.31 | 0.16 | 0.68 | 0.11 | 0.11 | 0.64 |
| Item | Treatment | p-Values | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose Response Leaves (%0–25–50) 6 | Dose Response Root (%0–25–50) 6 | ||||||||||||||
| Control | MPEL 25 | MPEL 50 | MPER 25 | MPER 50 | SEM 5 | Treatment | Time | T × T | C vs. T | Linear | Quadratic | C vs. T | Linear | Quadratic | |
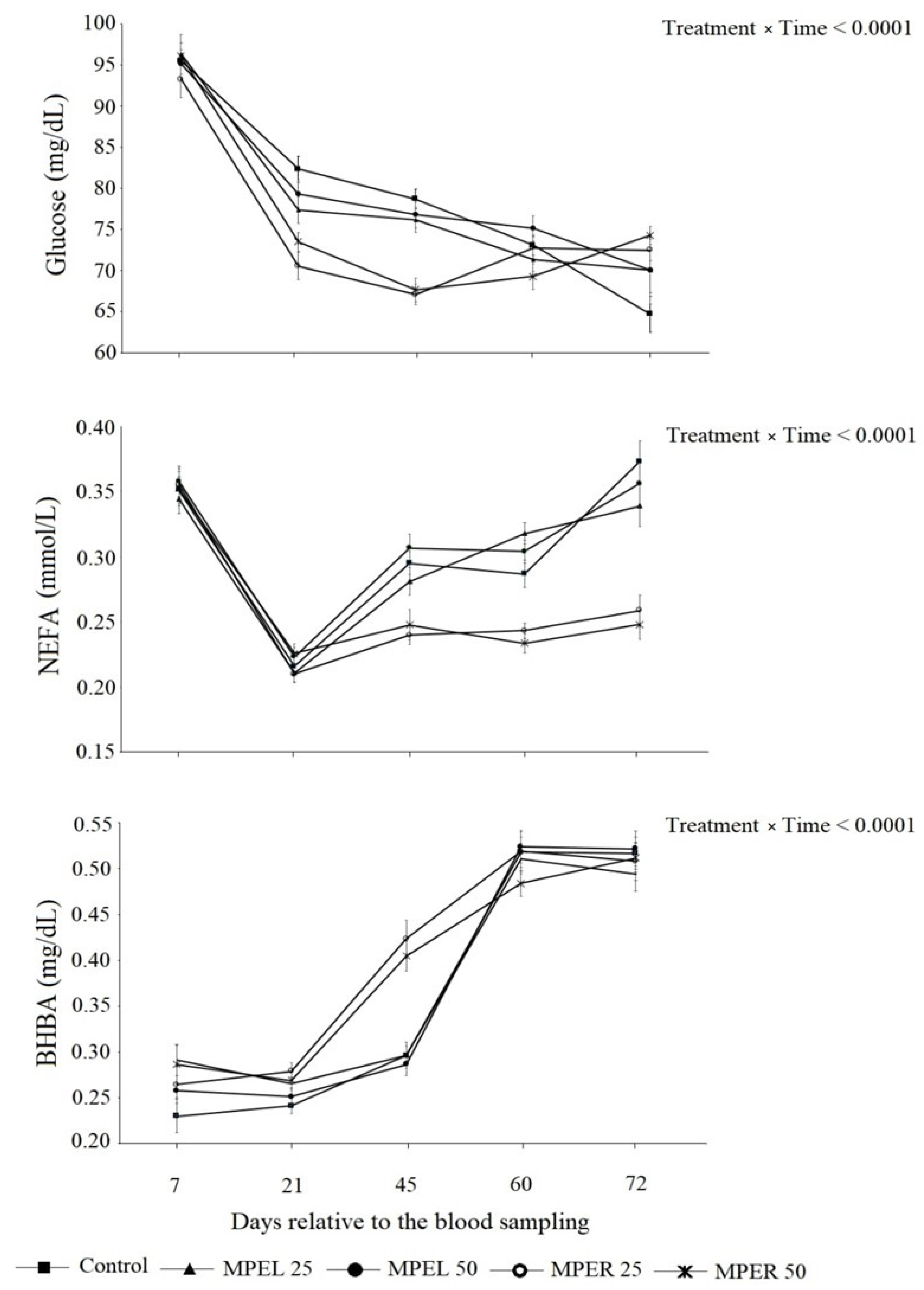
| Glucose, mg/dL | 78.84 a | 78.22 ab | 76.13 ab | 79.25 a | 75.20 b | 0.84 | 0.01 | <0.0001 | <0.0001 | 0.14 | 0.05 | 0.54 | 0.15 | 0.005 | 0.02 |
| NEFA 2, mmol/L | 0.305 a | 0.299 a | 0.261 b | 0.309 a | 0.262 b | 0.005 | <0.0001 | <0.0001 | <0.0001 | 0.002 | <0.0001 | 0.02 | 0.01 | <0.0001 | 0.0004 |
| BHBA 3, mmol/L | 0.360 c | 0.372 abc | 0.391 ab | 0.368 bc | 0.399 a | 0.007 | 0.0004 | <0.0001 | <0.0001 | 0.005 | 0.001 | 0.93 | 0.003 | 0.0001 | 0.23 |
| Total protein, g/dL | 6.52 | 6.62 | 6.73 | 6.74 | 6.69 | 0.07 | 0.15 | 0.01 | 0.93 | 0.08 | 0.04 | 0.89 | 0.02 | 0.09 | 0.11 |
| BUN 4, mg/dL | 15.35 | 15.26 | 16.01 | 15.86 | 16.23 | 0.26 | 0.56 | <0.0001 | 0.42 | 0.36 | 0.07 | 0.18 | 0.03 | 0.02 | 0.85 |
| IgG, mg/mL | 6.02 b | 6.09 ab | 6.23 ab | 6.32 ab | 6.50 a | 0.13 | 0.05 | <0.0001 | 0.13 | 0.36 | 0.21 | 0.71 | 0.01 | 0.004 | 0.70 |
| Item | Treatment | p-Values | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose Response Leaves (%0–25–50) 9 | Dose Response Root (%0–25–50) 9 | ||||||||||||||
| Control | MPEL 25 | MPEL 50 | MPER 25 | MPER 50 | SEM 8 | Treatment | Time | T × T | C vs. T | Linear | Quadratic | C vs. T | Linear | Quadratic | |
| RBC 2, 106/dL | 7.15 | 7.00 | 7.03 | 7.09 | 7.31 | 0.12 | 0.41 | <0.0001 | 0.43 | 0.43 | 0.54 | 0.61 | 0.71 | 0.34 | 0.35 |
| Hematocrit, % | 25.68 | 25.62 | 25.79 | 25.94 | 25.83 | 0.12 | 0.32 | <0.0001 | 0.64 | 0.86 | 0.52 | 0.45 | 0.19 | 0.42 | 0.22 |
| Hemoglobin, g/dL | 9.19 | 9.31 | 9.30 | 9.23 | 9.38 | 0.08 | 0.48 | <0.0001 | 0.68 | 0.21 | 0.31 | 0.47 | 0.30 | 0.14 | 0.60 |
| MCV 3, fL | 37.92 | 37.95 | 37.69 | 37.77 | 37.63 | 0.14 | 0.43 | <0.0001 | 0.90 | 0.55 | 0.25 | 0.43 | 0.18 | 0.13 | 0.99 |
| MCH 4, pg | 12.45 | 12.44 | 12.60 | 12.57 | 12.47 | 0.08 | 0.53 | <0.0001 | 0.93 | 0.46 | 0.19 | 0.42 | 0.55 | 0.93 | 0.30 |
| MCHC 5, g/dL | 31.01 | 31.15 | 31.13 | 31.17 | 31.08 | 0.17 | 0.96 | 0.04 | 0.10 | 0.53 | 0.63 | 0.66 | 0.56 | 0.73 | 0.57 |
| RDW 6, % | 41.17 | 41.27 | 41.20 | 41.03 | 41.24 | 0.15 | 0.83 | 0.03 | 0.75 | 0.76 | 0.92 | 0.65 | 0.87 | 0.73 | 0.35 |
| Platelet, 109/L | 420.52 | 420.45 | 423.68 | 419.70 | 419.60 | 2.82 | 0.84 | 0.86 | 0.38 | 0.68 | 0.46 | 0.65 | 0.80 | 0.82 | 0.91 |
| WBC 7, 109/L | 7.45 b | 7.74 ab | 7.92 a | 7.54 b | 7.96 a | 0.07 | <0.0001 | 0.005 | 0.47 | <0.0001 | <0.0001 | 0.51 | 0.003 | <0.0001 | 0.07 |
| Neutrophil, % | 49.58 | 49.49 | 49.37 | 49.60 | 49.06 | 0.24 | 0.53 | <0.0001 | 0.97 | 0.66 | 0.57 | 0.93 | 0.40 | 0.13 | 0.31 |
| Lymphocytes, % | 39.38 | 38.93 | 38.89 | 39.26 | 39.09 | 0.29 | 0.70 | <0.0001 | 0.20 | 0.24 | 0.28 | 0.61 | 0.59 | 0.48 | 0.88 |
| Monocytes, % | 9.37 | 9.18 | 9.59 | 9.50 | 9.54 | 0.17 | 0.38 | <0.0001 | 0.63 | 0.93 | 0.37 | 0.18 | 0.44 | 0.45 | 0.81 |
| Eosinophil, % | 0.89 | 0.89 | 1.07 | 1.08 | 1.11 | 0.08 | 0.08 | 0.19 | 0.21 | 0.70 | 0.23 | 0.19 | 0.04 | 0.07 | 0.30 |
| Basophils, % | 0.262 | 0.258 | 0.268 | 0.265 | 0.273 | 0.024 | 0.78 | 0.04 | 0.42 | 0.97 | 0.66 | 0.40 | 0.52 | 0.59 | 0.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uyarlar, C.; Rahman, A.; Ozcinar, U.; Cetingul, İ.S.; Gultepe, E.E.; Bayram, I. Effect of Myrtus communis L. Plant Extract as a Milk Supplement on the Performance, Selected Blood Parameters and Immune Response of Holstein Calves. Animals 2024, 14, 725. https://doi.org/10.3390/ani14050725
Uyarlar C, Rahman A, Ozcinar U, Cetingul İS, Gultepe EE, Bayram I. Effect of Myrtus communis L. Plant Extract as a Milk Supplement on the Performance, Selected Blood Parameters and Immune Response of Holstein Calves. Animals. 2024; 14(5):725. https://doi.org/10.3390/ani14050725
Chicago/Turabian StyleUyarlar, Cangir, Abdur Rahman, Umit Ozcinar, İbrahim Sadi Cetingul, Eyup Eren Gultepe, and Ismail Bayram. 2024. "Effect of Myrtus communis L. Plant Extract as a Milk Supplement on the Performance, Selected Blood Parameters and Immune Response of Holstein Calves" Animals 14, no. 5: 725. https://doi.org/10.3390/ani14050725







