Impact of Environmental Food Intake on the Gut Microbiota of Endangered Père David’s Deer: Primary Evidence for Population Reintroduction
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Sample Collection
2.3. Pretreatment of Residues in Fecal and Plant Samples
2.4. Microscopic Observation and Calculation
2.5. 16S rRNA High-Throughput Sequencing Analysis
2.6. Correlation between Diet and Gut Microbiota Composition
3. Results
3.1. Characteristics of Plant Cuticle Fragments
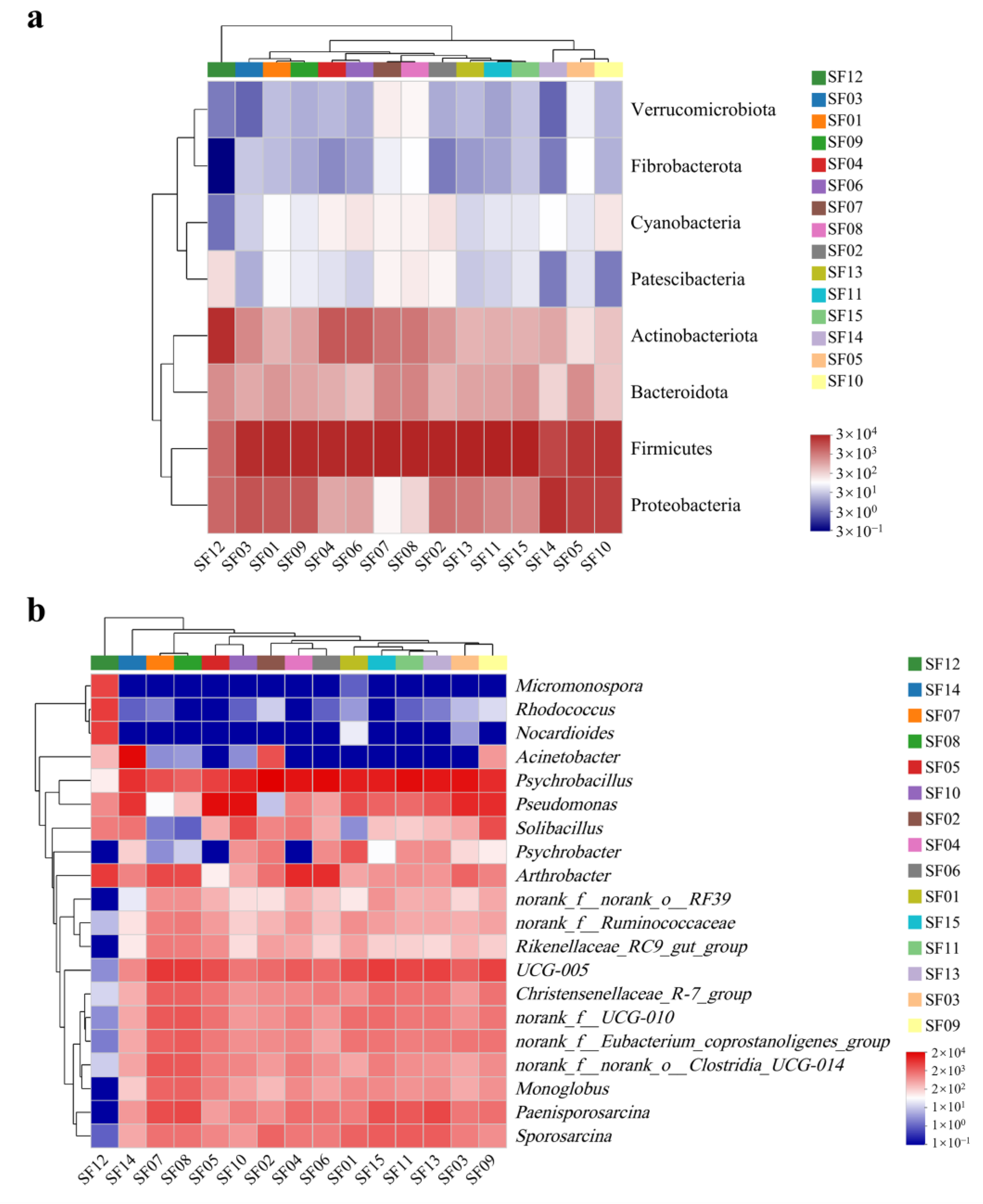
3.2. Composition of Fecal Residues from Père David’s Deer
3.3. Gut Microbiota Diversity
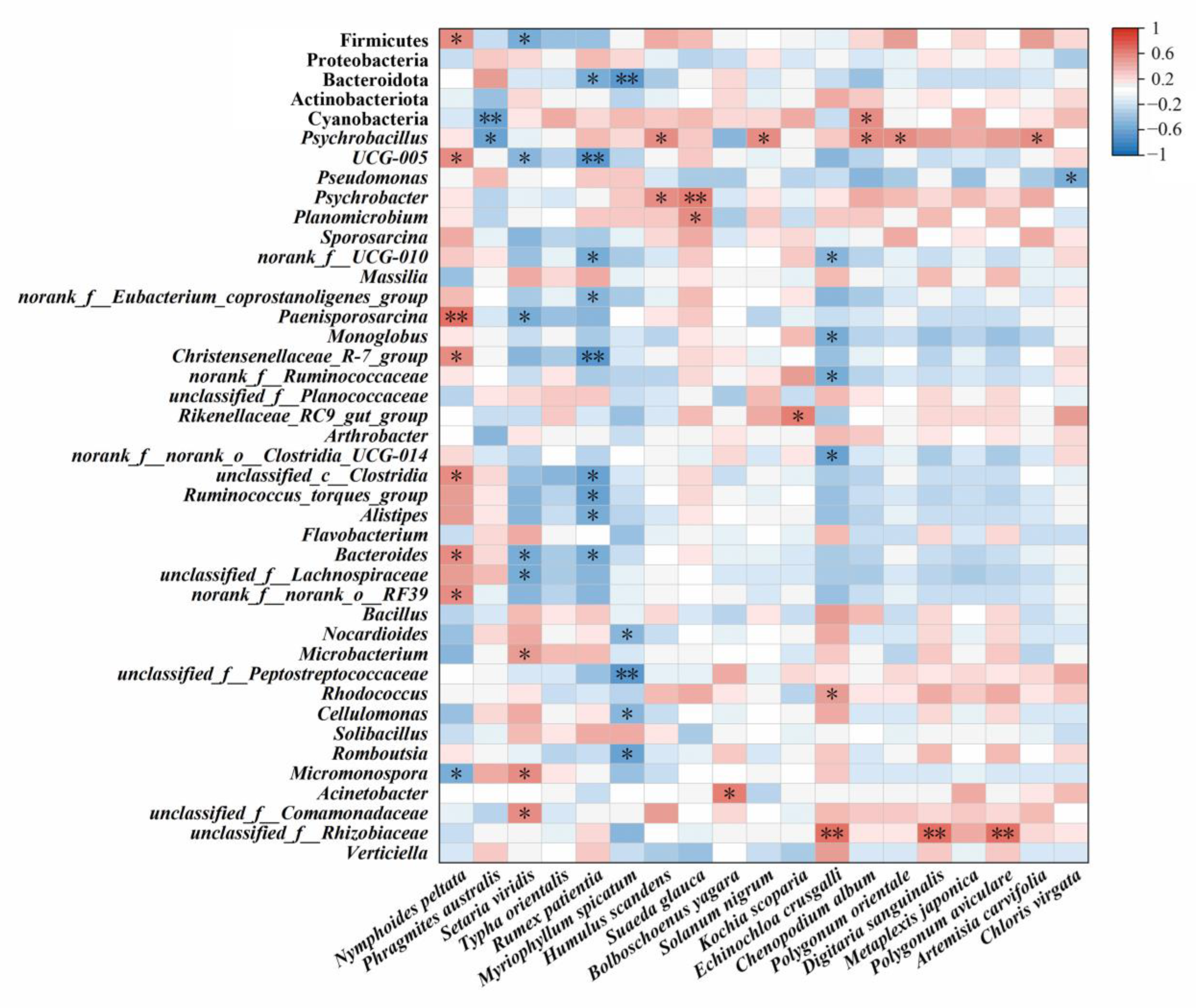
3.4. Potential Correlation between Food Composition and Gut Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seddon, P.J.; Armstrong, D.P.; Maloney, R.F. Developing the Science of Reintroduction Biology. Conserv. Biol. 2007, 21, 303–312. [Google Scholar] [CrossRef]
- Tang, J.; Wang, C.; Zhang, H.; Zhao, J.; Guo, W.; Mishra, S.; Kong, F.; Zeng, B.; Ning, R.; Li, D.; et al. Gut Microbiota in Reintroduction of Giant Panda. Ecol. Evol. 2020, 10, 1012–1028. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.L.; Ferry, B.; Holman, H.; Kovach, A.I. Monitoring a New England Cottontail Reintroduction with Noninvasive Genetic Sampling. Wildl. Soc. Bull. 2020, 44, 110–121. [Google Scholar] [CrossRef]
- Soorae, P.S. Global Conservation Translocation Perspectives: 2021. Case Studies from around the Globe, 7th ed.; IUCN SSC Conservation Translocation Specialist Group: Gland, Switzerland, 2021. [Google Scholar]
- Xue, D.; Zhang, Y.; Cheng, Z.; Zhong, Z.; Cao, M.; Fu, M.; Bai, J.; Yuan, X. Père David’s Deer (Elaphurus davidianus) in China: Population Dynamics and Challenges. J. Resour. Ecol. 2022, 13, 41–50. [Google Scholar] [CrossRef]
- Cheng, Z.; Tian, X.; Zhong, Z.; Li, P.; Sun, D.; Bai, J.; Meng, Y.; Zhang, S.; Zhang, Y.; Wang, L.; et al. Reintroduction, Distribution, Population Dynamics and Conservation of a Species Formerly Extinct in the Wild: A Review of Thirty-Five Years of Successful Milu (Elaphurus davidianus) Reintroduction in China. Glob. Ecol. Conserv. 2021, 31, e01860. [Google Scholar] [CrossRef]
- Ohtaishi, N.; Gao, Y. A Review of the Distribution of All Species of Deer (Tragulidae, Moschidae and Cervidae) in China. Mammal Rev. 1990, 20, 125–144. [Google Scholar] [CrossRef]
- Jiang, Z.; Harris, R.B.; Elaphurus davidianus. The IUCN Red List of Threatened Species 2016: E.T7121A22159785. Available online: https://www.iucnredlist.org/species/7121/22159785 (accessed on 6 October 2023).
- Zhang, H.; Li, P.; Wen, H.; Tian, G.; Chen, H.; Zhang, L.; Zhu, J. Population Status and Research Progress of Père David’s Deer (Elaphurus davidianus) in China. Pak. J. Zool. 2019, 51, 2359–2367. [Google Scholar] [CrossRef]
- White, T.H.; Collar, N.J.; Moorhouse, R.J.; Sanz, V.; Stolen, E.D.; Brightsmith, D.J. Psittacine Reintroductions: Common Denominators of Success. Biol. Conserv. 2012, 148, 106–115. [Google Scholar] [CrossRef]
- Jule, K.R.; Leaver, L.A.; Lea, S.E.G. The Effects of Captive Experience on Reintroduction Survival in Carnivores: A Review and Analysis. Biol. Conserv. 2008, 141, 355–363. [Google Scholar] [CrossRef]
- Custance, D.M.; Whiten, A.; Fredman, T. Social Learning and Primate Reintroduction. Int. J. Primatol. 2002, 23, 479–499. [Google Scholar] [CrossRef]
- Yao, R.; Xu, L.; Lu, G.; Zhu, L. Evaluation of the Function of Wild Animal Gut Microbiomes Using Next-Generation Sequencing and Bioinformatics and Its Relevance to Animal Conservation. Evol. Bioinform. 2019, 15, 1176934319848438. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, K.M.; Cromie, R.L.; Sainsbury, A.W.; Hilton, G.M.; Ewen, J.G.; Soorae, P.S.; Kock, R.A. Wildlife Health Outcomes and Opportunities in Conservation Translocations. Ecol. Solut. Evid. 2022, 3, e12164. [Google Scholar] [CrossRef]
- Cassini, M.H. Behavioral Mechanisms of Selection of Diet Components and Their Ecological Implications in Herbivorous Mammals. J. Mammal. 1994, 75, 733–740. [Google Scholar] [CrossRef]
- Matthews, J.K.; Ridley, A.; Kaplin, B.A.; Grueter, C.C. A Comparison of Fecal Sampling and Direct Feeding Observations for Quantifying the Diet of a Frugivorous Primate. Curr. Zool. 2020, 66, 333–343. [Google Scholar] [CrossRef]
- Boeker, E.L.; Scott, V.E.; Reynolds, H.G.; Donaldson, B.A. Seasonal Food Habits of Mule Deer in Southwestern New Mexico. J. Wildl. Manag. 1972, 36, 56. [Google Scholar] [CrossRef]
- Swan, G.J.F.; Bearhop, S.; Redpath, S.M.; Silk, M.J.; Goodwin, C.E.D.; Inger, R.; McDonald, R.A.; Freckleton, R. Evaluating Bayesian Stable Isotope Mixing Models of Wild Animal Diet and the Effects of Trophic Discrimination Factors and Informative Priors. Methods Ecol. Evol. 2019, 11, 139–149. [Google Scholar] [CrossRef]
- Wang, L.; Ding, J.; Yang, Z.; Chen, H.; Yao, R.; Dai, Q.; Ding, Y.; Zhu, L. Père David’s Deer Gut Microbiome Changes across Captive and Translocated Populations: Implications for Conservation. Evol. Appl. 2019, 12, 622–635. [Google Scholar] [CrossRef] [PubMed]
- Askarizadeh, D.; Heshmati, G.A.; Pessarakli, M.; Jouri, M.H. Survey of Evaluation Techniques for Studying Rangeland Grass Species Nutritional Values. J. Plant Nutr. 2011, 34, 2172–2182. [Google Scholar] [CrossRef]
- Wang, H.L.; Zhao, Y.; Wang, F.; Sun, X.J.; Zhu, J.-Q.; Zhang, Y.M.; Wei, S.; Chen, H. Diet Composition and Selection of Père David’s Deer in Hubei Shishou Milu National Nature Reserve, China. Ecol. Evol. 2023, 13, e9702. [Google Scholar] [CrossRef]
- Stevenson, R.D.; Woods, W.A., Jr. Condition Indices for Conservation: New Uses for Evolving Tools. Integr. Comp. Biol. 2006, 46, 1169–1190. [Google Scholar] [CrossRef]
- Szuba-Trznadel, A.; Hikawczuk, T.; Korzeniowska, M.; Fuchs, B. Effect of Different Amounts of Hybrid Barley in Diets on the Growth Performance and Selected Biochemical Parameters of Blood Serum Characterizing Health Status in Fattening Pigs. Animals 2020, 10, 1987. [Google Scholar] [CrossRef]
- Liddell, C.; Morgan, E.R.; Bull, K.; Ioannou, C.C. Response to Resources and Parasites Depends on Health Status in Extensively Grazed Sheep. Proc. Biol. Sci. 2020, 287, 20192905. [Google Scholar] [CrossRef] [PubMed]
- Deem, S.L.; Parker, P.G.; Cruz, M.B.; Merkel, J.; Hoeck, P.E. Comparison of Blood Values and Health Status of Floreana Mockingbirds (Mimus trifasciatus) on the Islands of Champion and Gardner-by-Floreana, Galápagos Islands. J. Wildl. Dis. 2011, 47, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.Y.; Mo, Q.Y.; Wu, H.; Zhao, D.P. How Do Living Conditions Affect the Gut Microbiota of Endangered Père David’s Deer (Elaphurus davidianus)? Initial Findings from the Warm Temperate Zone. Peer J. 2023, 11, e14897. [Google Scholar] [CrossRef]
- Paone, P.; Cani, P.D. Mucus Barrier, Mucins and Gut Microbiota: The Expected Slimy Partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, W.; Su, L.; Huang, M.; Lin, L.; Su, Q.; Li, G.; Ahmad, H.I.; Li, L.; Zhang, X.; et al. Impact of Host Intraspecies Genetic Variation, Diet, and Age on Bacterial and Fungal Intestinal Microbiota in Tigers. MicrobiologyOpen 2020, 9, e1050. [Google Scholar] [CrossRef]
- Zhen, J.; Ren, Y.; Zhang, H.; Yuan, X.; Wang, L.; Shen, H.; Liu, P.; Chen, Y. Effect of Different Dietary Regimes on the Gut Microbiota and Fecal Metabolites of Père David’s Deer. Animals 2022, 12, 584. [Google Scholar] [CrossRef] [PubMed]
- Institute of Botany, Chinese Academy of Sciences. Illustrated Book of Higher Plants of China; Science Press: Beijing, China, 2002. [Google Scholar]
- Svendsen, A.-S.L.; Nielsen, L.B.; Schmidt, J.B.; Bruhn, D.; Andersen, L.H.; Pertoldi, C. eDNA Metabarcoding- and Microscopic Analysis for Diet Determination in Waterfowl, a Comparative Study in Vejlerne, Denmark. Biology 2023, 12, 1272. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.Y.; Li, F.F.; Xu, T.; Cai, S.J.; Chu, W.Y.; Qiu, H.; Sha, S.; Cheng, G.Y.; Xu, Q.S. Bioaccumulation, Subcellular, and Molecular Localization and Damage to Physiology and Ultrastructure in Nymphoides peltata (Gmel.) O. Kuntze Exposed to Yttrium. Environ. Sci. Pollut. Res. Int. 2014, 21, 2935–2942. [Google Scholar] [CrossRef] [PubMed]
- Darbyshire, S.J.; Francis, A. The Biology of Invasive Alien Plants in Canada. 10. Nymphoides peltata (S. G. Gmel.) Kuntze. Can. J. Plant. Sci. 2008, 88, 811–829. [Google Scholar] [CrossRef]
- Ji, Y.F.; Wu, B.L.; Ding, Y.H.; Qin, P. Nutritional Components of Phragmites australis and Spartina alterniflora in Dafeng Freerange David’s Deer Habitat of Jiangsu Province, East China: A Comparative Analysis. Chin. J. Ecol. 2011, 30, 2240–2244. [Google Scholar] [CrossRef]
- Meng, Y.P.; Li, K.; Zhang, L.Y.; Yang, M.; Chen, Q. Measurement and Analysis on Feed Intake of Elaphurus davidianus in Beijing Milupark. Spec. Wild Econ. Anim. Plant Res. 2010, 32, 39–42. [Google Scholar] [CrossRef]
- Tazzoli, M.; Trocino, A.; Birolo, M.; Radaelli, G.; Xiccato, G. Optimizing Feed Efficiency and Nitrogen Excretion in Growing Rabbits by Increasing Dietary Energy with High-Starch, High-Soluble Fibre, Low-Insoluble Fibre Supply at Low Protein Levels. Livest. Sci. 2015, 172, 59–68. [Google Scholar] [CrossRef]
- Belovsky, G.E. Diet Optimization in a Generalist Herbivore: The Moose. Theor. Popul. Biol. 1978, 14, 105–134. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.Y.; An, Y.T.; Sun, D.M.; Shen, H.; Lu, L.L.; Yuan, B.D. Vegetation Community Characters under Varying Levels of Foraging Pressure by Père David’s Deer’s (Elaphurus davidianus) at Dafeng National Nature Reserve. Chin. J. Wildl. 2018, 39, 49–53. [Google Scholar] [CrossRef]
- Yang, G.; Yang, Q.; Wang, Y.; Yang, G.; Gan, W.; Lu, Q. Analysis of Yield and Nutritional Components of Different Forages in Longling County. China Dairy Cattle 2021, 35–40. [Google Scholar] [CrossRef]
- Jiang, X.; Song, X.; Chen, Y.; Zhang, W. Research on Biogas Production Potential of Aquatic Plants. Renew. Energy 2014, 69, 97–102. [Google Scholar] [CrossRef]
- Sun, C.H.; Liu, H.Y.; Liu, B.; Yuan, B.D.; Lu, C.H. Analysis of the Gut Microbiome of Wild and Captive Père David’s Deer. Front. Microbiol. 2019, 10, 2331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Shi, M.; Fan, M.; Xu, S.; Li, Y.; Zhang, T.; Cha, M.; Liu, Y.; Guo, X.; Chen, Q.; et al. Comparative Analysis of Gut Microbiota Changes in Père David’s Deer Populations in Beijing Milu Park and Shishou, Hubei Province in China. Front. Microbiol. 2018, 9, 1258. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Gao, H.; Qin, W.; Song, P.; Wang, H.; Zhang, J.; Liu, D.; Wang, D.; Zhang, T. Marked Seasonal Variation in Structure and Function of Gut Microbiota in Forest and Alpine Musk Deer. Front. Microbiol. 2021, 12, 699797. [Google Scholar] [CrossRef]
- Zhu, Z.; Sun, Y.; Zhu, F.; Liu, Z.; Pan, R.; Teng, L.; Guo, S. Seasonal Variation and Sexual Dimorphism of the Microbiota in Wild Blue Sheep (Pseudois nayaur). Front. Microbiol. 2020, 11, 1260. [Google Scholar] [CrossRef] [PubMed]
- Kaakoush, N.O. Insights into the Role of Erysipelotrichaceae in the Human Host. Front. Cell Infect. Microbiol. 2015, 5, 84. [Google Scholar] [CrossRef] [PubMed]
- Li, R.W.; Connor, E.E.; Li, C.; Baldwin, R.L., VI; Sparks, M.E. Characterization of the Rumen Microbiota of Pre-Ruminant Calves Using Metagenomic Tools. Environ. Microbiol. 2012, 14, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, G.; Shafer, A.B.A.; Wei, Y.; Zhou, J.; Lin, S.; Wu, H.; Zhou, M.; Hu, D.; Liu, S. Comparative Analysis of the Gut Microbial Communities in Forest and Alpine Musk Deer Using High-Throughput Sequencing. Front. Microbiol. 2017, 8, 572. [Google Scholar] [CrossRef] [PubMed]
- Konikoff, T.; Gophna, U. Oscillospira: A Central, Enigmatic Component of the Human Gut Microbiota. Trends Microbiol. 2016, 24, 523–524. [Google Scholar] [CrossRef]
- Li, Y.; Shi, M.; Zhang, T.; Hu, X.; Zhang, B.; Xu, S.; Ding, J.; Hu, D.; Liu, S. Dynamic Changes in Intestinal Microbiota in Young Forest Musk Deer During Weaning. Peer J. 2020, 8, e8923. [Google Scholar] [CrossRef]
- Hassan, F.U.; Guo, Y.; Li, M.; Tang, Z.; Peng, L.; Liang, X.; Yang, C. Effect of Methionine Supplementation on Rumen Microbiota, Fermentation, and Amino Acid Metabolism in in Vitro Cultures Containing Nitrate. Microorganisms 2021, 9, 1717. [Google Scholar] [CrossRef]
- Guan, Y.; Yang, H.; Han, S.; Feng, L.; Wang, T.; Ge, J. Comparison of the Gut Microbiota Composition between Wild and Captive Sika Deer (Cervus nippon hortulorum) from Feces by High-Throughput Sequencing. AMB Express 2017, 7, 212. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Jiang, W.; Tian, Z.; Wu, H.; Ning, H.; Yan, G.; Zhang, Z.; Li, Z.; Dong, F.; Sun, Y.; et al. Fecal g. Streptococcus and g. Eubacterium_coprostanoligenes_group Combined with Sphingosine to Modulate the Serum Dyslipidemia in High-Fat Diet Mice. Clin. Nutr. 2021, 40, 4234–4245. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, C.; Wu, H.; Meng, Q.; Zhou, Z. Small Intestine Microbiome and Metabolome of High and Low Residual Feed Intake Angus Heifers. Front. Microbiol. 2022, 13, 862151. [Google Scholar] [CrossRef]
- Kim, C.C.; Healey, G.R.; Kelly, W.J.; Patchett, M.L.; Jordens, Z.; Tannock, G.W.; Sims, I.M.; Bell, T.J.; Hedderley, D.; Henrissat, B.; et al. Genomic Insights from Monoglobus pectinilyticus: A Pectin-Degrading Specialist Bacterium in the Human Colon. ISME J. 2019, 13, 1437–1456. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Hold, G.L.; Flint, H.J. The Gut Microbiota, Bacterial Metabolites and Colorectal Cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sun, Y.; Shi, Z.; Liu, Z.; Zhao, C.; Lu, T.; Gao, H.; Zhu, F.; Chen, R.; Zhang, J.; et al. Gut Microbiota of Wild and Captive Alpine Musk Deer (Moschus chrysogaster). Front. Microbiol. 2019, 10, 3156. [Google Scholar] [CrossRef] [PubMed]
- You, Z.; Deng, J.; Liu, J.; Fu, J.; Xiong, H.; Luo, W.; Xiong, J. Seasonal Variations in the Composition and Diversity of Gut Microbiota in White-Lipped Deer (Cervus albirostris). Peer J. 2022, 10, e13753. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Gao, H.; Song, P.; Liang, C.; Jiang, F.; Xu, B.; Liu, D.; Zhang, T. Captivity Shifts Gut Microbiota Communities in White-Lipped Deer (Cervus albirostris). Animals 2022, 12, 431. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Dzakpasu, M.; Wang, X.; Zhang, L.; Ngo, H.H.; Guo, W.; Zhao, Y. Molecular Characterization of Long-Term Impacts of Macrophytes Harvest Management in Constructed Wetlands. Bioresour. Technol. 2018, 268, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial Signature of Dysbiosis in Gut Microbiota. Trends. Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
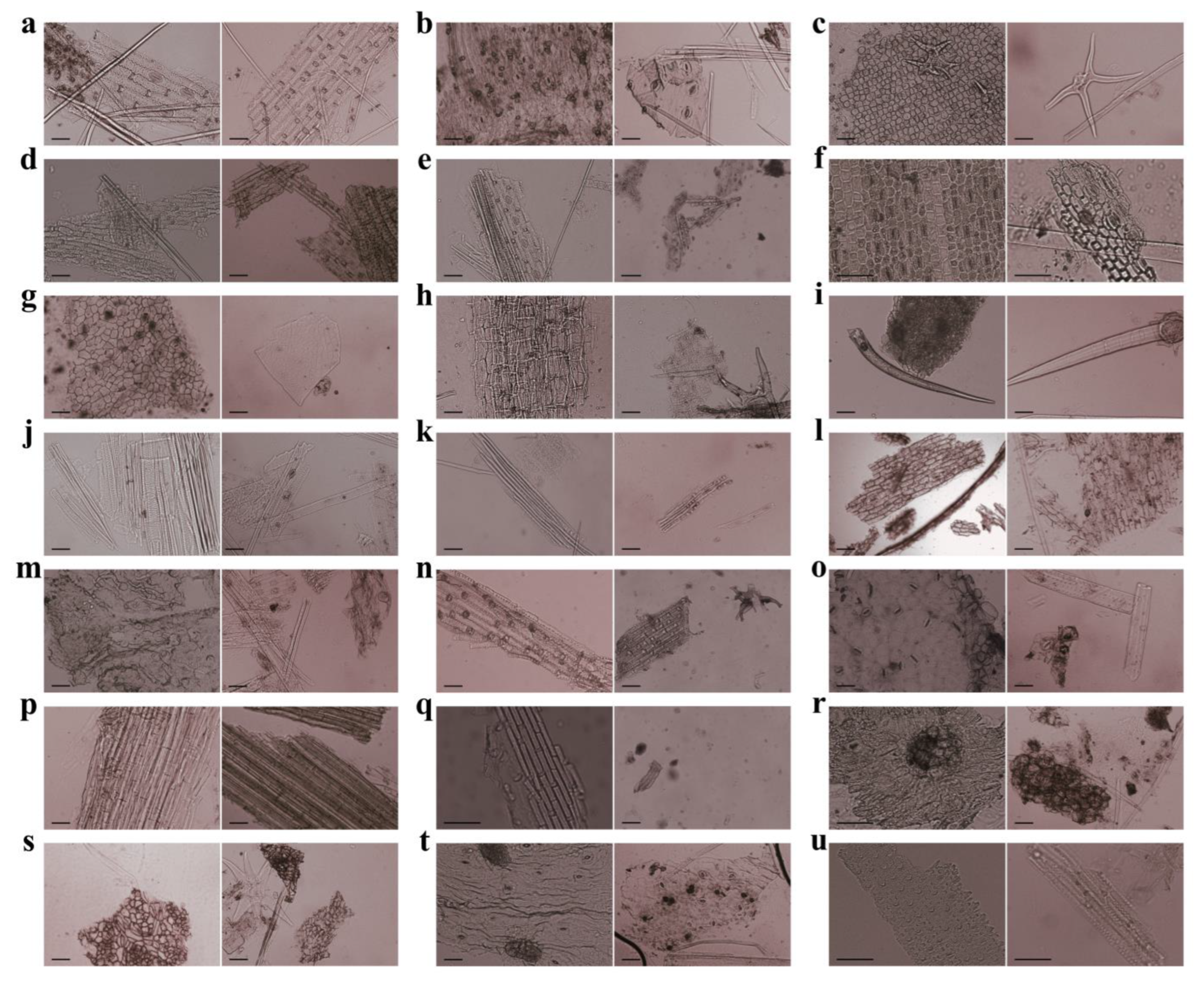


| Rank | Species | Family | RD (%) | Type |
|---|---|---|---|---|
| 1 | Nymphoides peltata | Gentianaceae | 35.14 ± 13.30 | Main |
| 2 | Phragmites australis | Poaceae | 26.62 ± 12.84 | Main |
| 3 | Setaria viridis | Poaceae | 12.11 ± 5.03 | Main |
| 4 | Typha orientalis | Typhaceae | 10.85 ± 4.26 | Main |
| 5 | Rumex patientia | Polygonaceae | 2.55 ± 2.64 | Common |
| 6 | Myriophyllum spicatum | Haloragidaceae | 2.38 ± 2.88 | Common |
| 7 | Humulus scandens | Moraceae | 2.38 ± 2.09 | Common |
| 8 | Suaeda glauca | Chenopodiaceae | 2.16 ± 2.90 | Common |
| 9 | Bolboschoenus yagara | Cyperaceae | 1.95 ± 3.34 | Common |
| 10 | Solanum nigrum | Solanaceae | 1.71 ± 2.08 | Common |
| 11 | Kochia scoparia | Chenopodiaceae | 0.77 ± 1.50 | Occasional |
| 12 | Echinochloa crus-galli | Poaceae | 0.34 ± 0.56 | Occasional |
| 13 | Chenopodium album | Chenopodiaceae | 0.28 ± 0.76 | Occasional |
| 14 | Polygonum orientale | Polygonaceae | 0.19 ± 0.64 | Occasional |
| 15 | Digitaria sanguinalis | Poaceae | 0.17 ± 0.55 | Occasional |
| 16 | Metaplexis japonica | Asclepiadaceae | 0.16 ± 0.60 | Occasional |
| 17 | Polygonum aviculare | Polygonaceae | 0.11 ± 0.33 | Occasional |
| 18 | Artemisia carvifolia | Asteraceae | 0.09 ± 0.23 | Occasional |
| 19 | Lysimachia barystachys | Poaceae | 0.06 ± 0.15 | Occasional |
| Species | P. australis | S. viridis | T. orientalis |
|---|---|---|---|
| Crude protein | 2.84 | 11.89 | 10.8 |
| Crude oil | 1.44 | 1.49 | 2.9 |
| Neutral detergent fiber (NDF) | 77.78 | 65.58 | - |
| Acid detergent fiber (ADF) | 45.9 | 40.91 | - |
| Acid detergent lignin (ADL) | - | 4.61 | 17.1 |
| Cellulose content (ADF—ADL) | - | - | 35.6 |
| Hemicellulose content (NDF—ADF) | - | - | 19.9 |
| Reference | [38] | [39] | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, Q.; Yao, H.; Wu, H.; Zhao, D. Impact of Environmental Food Intake on the Gut Microbiota of Endangered Père David’s Deer: Primary Evidence for Population Reintroduction. Animals 2024, 14, 728. https://doi.org/10.3390/ani14050728
Mo Q, Yao H, Wu H, Zhao D. Impact of Environmental Food Intake on the Gut Microbiota of Endangered Père David’s Deer: Primary Evidence for Population Reintroduction. Animals. 2024; 14(5):728. https://doi.org/10.3390/ani14050728
Chicago/Turabian StyleMo, Qiying, Hongyu Yao, Hong Wu, and Dapeng Zhao. 2024. "Impact of Environmental Food Intake on the Gut Microbiota of Endangered Père David’s Deer: Primary Evidence for Population Reintroduction" Animals 14, no. 5: 728. https://doi.org/10.3390/ani14050728





