Simple Summary
Little is known on the distribution of beta-lactam resistant Gram-negative bacteria in the gut of Madagascan animals. To increase respective knowledge, swabs from 49 animal stool droppings were collected in the Madagascan Tsimanapesotsa National Park and assessed by microbial culture, antimicrobial susceptibility testing, and sequence analysis. Third generation cephalosporine resistance, predominantly mediated by ampC-type beta-lactamases, was the most frequently observed beta-lactam resistance phenotype. Future studies should assess whether and to what extent an exchange of resistant isolates between humans and animals facilitates a local spread of resistant bacteria.
Abstract
Third generation cephalosporin-resistant (3GCR) Enterobacterales are known to be prevalent in Madagascar, with high colonization or infection rates in particular in Madagascan patients. Extended spectrum beta-lactamases (ESBLs) have been reported to be the predominant underlying resistance mechanism in human isolates. So far, little is known on antimicrobial resistance and its molecular determinants in Enterobacterales and other bacteria causing enteric colonization of Madagascan wild animals. To address this topic, swabs from 49 animal stool droppings were collected in the Madagascan Tsimanapesotsa National Park and assessed by cultural growth of bacterial microorganisms on elective media. In addition to 7 Acinetobacter spp., a total of 31 Enterobacterales growing on elective agar for Enterobacterales could be isolated and subjected to whole genome sequencing. Enterobacter spp. was the most frequently isolated genus, and AmpC-type beta-lactamases were the quantitatively dominating molecular resistance mechanism. In contrast, the blaCTX-M-15 gene, which has repeatedly been associated with 3GC-resistance in Madagascan Enterobacterales from humans, was detected in a single Escherichia coli isolate only. The identification of the fosfomycin-resistance gene fosA in a high proportion of isolates is concerning, as fosfomycin is increasingly used to treat infections caused by multidrug-resistant bacteria. In conclusion, the proof-of-principle assessment indicated a high colonization rate of resistant bacteria in stool droppings of Madagascan wild animals with a particular focus on 3GCR Enterobacterales. Future studies should confirm these preliminary results in a more systematic way and assess the molecular relationship of animal and human isolates to identify potential routes of transmission.
1. Introduction
Similar to other African countries, third generation cephalosporin (3GC) resistance in Enterobacterales has relevantly increased in the course of the recent decades, even in severe infections like bacteremia [1]. In other types of infections, like urogenital tract infections [2,3,4] and wound infections [5], considerable resistance rates were also recorded with an increasing tendency during the last decade, next to varying, up to two-digit proportions of asymptomatic enteric carriage in Madagascans [6,7,8]. Higher rates of enteric colonization with 3GC-resistant (3GCR) Enterobacterales after hospitalization than before suggest ready transmission and/or selection under antibiotic pressure in the local healthcare setting [9]. Increased resistance rates in Madagascan Enterobacterales are particularly worrisome, because they have been described to play a predominant role in local neonatal infections [6,10,11] and thus affect a very vulnerable population. High rates of >70% for the acquisition of 3GCR Enterobacterales have been demonstrated for exposed Madagascan newborns [12].
Most Madagascan studies describe extended-spectrum beta-lactamases (ESBLs) as the major cause of 3GC-resistance in human Enterobacterales isolates [6,13,14,15], even in genera like Enterobacter spp. [6,13,15], in which inducible ampC beta-lactamases might have been expected to play a more dominant role. In clinical isolates, blaCTX-M-15 and blaSHV-12 have been described to be the regionally most abundant ESBL genes [15,16,17]. Only individual studies suggested a relevant proportion of inducible ampC mechanisms to account for relevant colonizing rates with 3GCR Enterobacterales on the skin and mucous membranes of Madagascan patients [18]. Reports on carbapenem resistance, mostly blaNDM-gene-associated, in Madagascan Enterobacterales have been published as well [3,8,17,19], although carbapenem resistance is less frequent there in comparison to 3GC-resistance. In comparison to Enterobacterales, acquired carbapenem resistance in Madagascan bacterial isolates has been described to be more relevant in non-fermentative Gram-negative rod-shaped bacteria like Acinetobacter baumannii [20].
Although the regional potential of animal sources of 3GCR Enterobacterales has been well recognized, studies on animal colonization in Madagascar are still scarce [21]. In regional livestock-associated ESBL-positive Enterobacterales, blaCTX-M-1 has been demonstrated as a quantitatively relevant resistance-associated gene [22].
To contribute to the so far scarce information on the carriage of 3GCR Enterobacterales and other antibiotic-resistant bacteria in Madagascan animals next to locally prevalent genetic resistance determinants, we screened for resistant bacteria in animal stool droppings in Madagascar and subjected resistant Enterobacterales and Acinetobacter spp. to whole genome sequencing. In silico analyses of genome sequences was performed to determine the presence of antimicrobial resistance (AMR) genes, virulence-associated genes, and multilocus sequence types. Genomic characteristics were further used to assess the pathogenic and zoonotic potential of bacterial strains obtained from animals in Madagascar.
2. Materials and Methods
2.1. Sample Collection and Ethical Permit
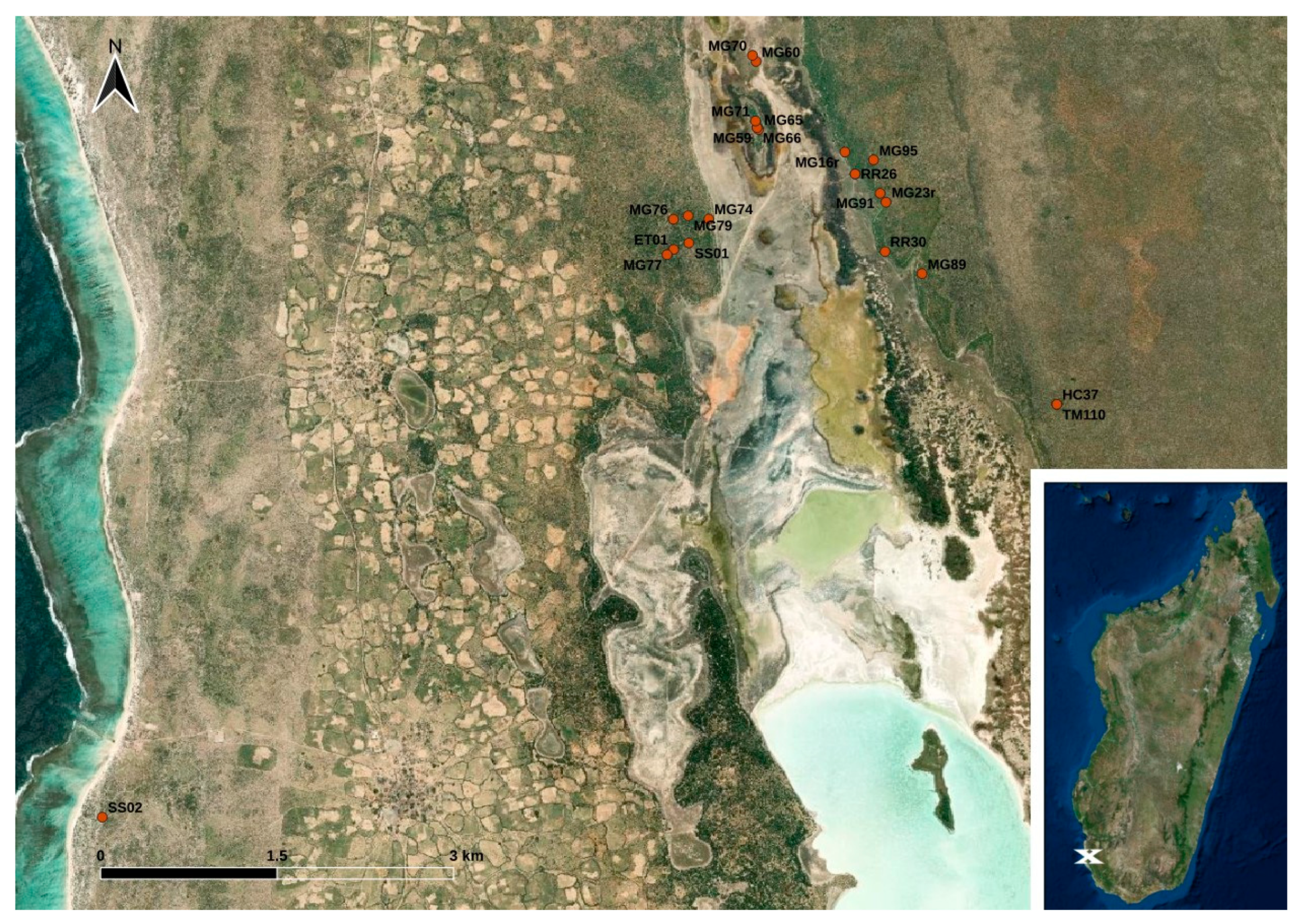
The sample collection took place in the coastal plain of south-west Madagascar close to the western border of the Tsimanampesotsa National Park. Within a three-months-interval from February to April 2017, animals were trapped with live traps (for details see Ehlers et al., 2019) [23] and brought to the research camp where fecal swabbings of a total of 49 stool droppings from Echinops telfairi (lesser hedgehog tenrec; n = 1), Macronycteris commersoni (Commerson’s roundleaf bat; n = 6), Microcebus griseorufus (reddish-gray mouse lemur; n = 32), Pyxis arachnoides (spider tortoise; n = 1), Rattus rattus (black rat; n = 6), Setifer setosus (greater hedgehog tenrec; n = 2), and Triaenops menamena (rufous trident bat; n = 1) were collected using GenoTube Livestock Swabs (Thermo Fisher Scientific, Waltham, MA, USA) enabling transport and storage at room temperature. The animals caught at the study site were subjected to sampling with no particular selection criteria; thus, the species distribution indirectly reflects their local abundance at the study site or at least their affinity to the applied live traps. Details on the locations of the sampling sites are shown in Figure 1. Information on the precise sampling dates and sampling coordinates is provided in Table S1. The swabs were stored in a refrigerator at temperatures between 7 °C and 20 °C (depending on the power supply) until they were shipped to Germany for further analysis.
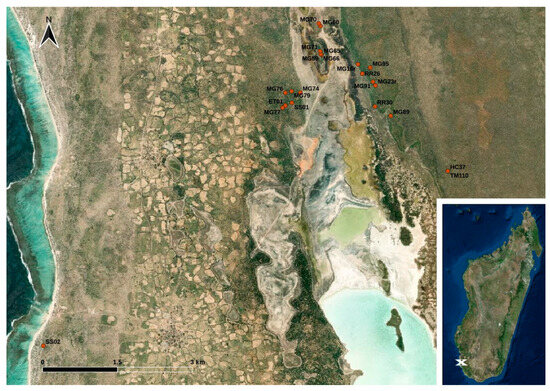
Figure 1.
Map of the study site located in south-west Madagascar. The dots indicate the trapping points of the animals (see Table S1) (designed with QGis based on Esri satellite imagery data (https://services.arcgisonline.com/ArcGIS/rest/services/World_Imagery/MapServer, accessed on 12 December 2023)).
During the sampling procedure, compliance with all applicable institutional and/or national guidelines for the care and use of animals was assured. Ethical clearance for the field work and permit for the sample shipment were provided by the ethics committee of the Institute of Zoology of Hamburg University before the initiation of this study and authorized by the Ministère de l’Environnement, de l’Ecologie, de la Mer et des Forêts (Research permits: N°136/16/- and N°002/17/MEEF/SG/DGF/DSAP/SCB.Re; export permit: N°345-17/MEEF/SG/DGF/DREEFAAND/SFR).
2.2. Screening for Antibiotic Resistant Bacteria and Phenotypic Assessment
At the Department A—Veterinary Medicine, Central Institute of the Bundeswehr Medical Service Kiel, Kronshagen, Germany, the samples were subjected to cultural growth on agar plates selecting for Enterobacterales (CHROMagar Orientation, Mast Group Ltd., Bootle, UK) and Acinetobacter spp. (CHROMagar Acinetobacter, Mast Group Ltd.). To verify culturable bacterial background, all samples were additionally streaked on sheep blood agar and Gassner agar (Merck KG, Darmstadt, Germany). The screening for Salmonella spp. was done after selective enrichment in Rappaport–Vasiliadis medium (Merck KG, Germany), followed by streaking on XLD and BPLS agar (Sigma Aldrich, Darmstadt, Germany). Suspicious isolates were subjected to biochemical differentiation. For each different colony morphology that was visible with the bare eye, a single colony was chosen for further assessments. Additional species identification was done by mass spectrometry (MALDI Biotyper Version 3.1, DBUpdate V10.0.0.0, MBT Compass Library Revision L (2020), Bruker Daltonik, Bremen, Germany) as well as slide agglutination of presumptive Salmonella spp. (Thermo Fisher Scientific, Karlsruhe, Germany). The taxonomic status of Enterobacter spp. was additionally assessed at the whole genome level by performing average nucleotide identity (ANI) calculations based on BLAST+ using ANIb implemented in JSpeciesWS (© 2014–2023 Ribocon GmbH—Version: 4.0.2, Bremen, Germany).
Antimicrobial susceptibility testing (AST) was performed with the VITEK®2 system (BioMérieux, Nürtingen, Germany) using card AST-GN38 for Enterobacterales spp. and card AST-GN97 for Acinetobacter spp. according to the standards described in the CLSI document M100 [24]. As veterinary breakpoints are only available for some of the tested antibiotics and only for animals and indications not addressed in this study, minimal inhibitory concentration (MIC) data were interpreted according to CLSI document M100 (33rd edition) following breakpoints and intrinsic resistance data provided for Enterobacterales and Acinetobacter species of the A. calcoaceticus–A. baumannii (Acb) complex [24]. MICs for ceftiofur were interpreted according to CLSI document VET01S in case of Enterobacterales spp. [25] and as proposed in a recent publication in the case of Acinetobacter spp. [26].
2.3. Whole Genome Sequencing and Bioinformatic Analysis
Selected Enterobacterales with phenotypical resistance against 3GCR, as well as Acinetobacter spp. grown on CHROMagar Acinetobacter were sent for whole genome sequencing to the Institute for Hygiene and Infectious Diseases of Animals, Justus Liebig University of Giessen, Germany, in order to identify molecular AMR determinants and clonal lineages. Isolates were sequenced on an Illumina MiSeq platform using paired-end sequencing. After quality control using default parameters, adapter-trimmed reads were assembled using SPAdes v3.13.1 (Bankevich et al., 2012) [27]. Draft genomes were annotated using Prokka v1.13 (Seemann, 2014) [28]. Multilocus sequence types of E. coli (Achtman seven gene MLST scheme), Enterobacter spp., Klebsiella spp., Salmonella spp., and A. baumannii (Pasteur scheme) were identified in silico using MLST 2.0 provided by the Center for Genomic Epidemiology (https://cge.food.dtu.dk/services/MLST/, accessed on 6 January 2024).
2.4. Determination of Virulence-Associated Genes
Virulence genotyping of isolates belonging to the species E. coli, Enterobacter spp., Salmonella enterica, and Klebsiella spp. was performed by using VirulenceFinder 2.0 (https://cge.food.dtu.dk/services/VirulenceFinder/, accessed on 12 December 2023) hosted by the Center for Genomic Epidemiology and by using BacWGSTdb 2.0 (http://bacdb.cn/BacWGSTdb/Tools_results_single.php, accessed on 12 December 2023). The presence of the kleboxymycin biosynthetic locus in Klebsiella spp. was determined by using MyDbFinder 2.0 (https://cge.food.dtu.dk/services/MyDbFinder/, accessed on 6 January 2024) and the kleboxymycin nucleotide sequence of K. oxytoca strain MH43-1 (GenBank: MF401554.1).
3. Results
3.1. Species Differentiation and Antimicrobial Susceptibility Testing
The conducted growth on the chosen media led to the identification of 41 bacterial isolates (Table 1). In short, growth on agar selecting for Enterobacterales allowed the isolation of 21 Enterobacter spp. from Rattus rattus (2 isolates/6 animals, 33.3%), Setifer setosus (1/2, 50%), Macronycteris commersoni (2/2, 100%), Triaenops menamena (1/1, 100%), and Microcebus griseorufus (14/32, 43.8%). The ANI values of the Enterobacter spp. genomes against reference genomes and the core genome-based phylogeny revealed the presence of E. hormaechei ssp. steigerwaltii (n = 11), E. hormaechei ssp. xiangfangensis (n = 7), E. hormaechei ssp. hormaechei (n = 2), E. cloacae ssp. cloacae (n = 1), and E. quasihormaechei (n = 1). Five Salmonella spp. isolates were further obtained from Setifer setosus (2/2, 100%), Echinops telfairi, (1/1, 100%), and Microcebus griseorufus (2/32, 6.3%); two Morganella morganii were isolated from Microcebus griseorufus (2/32, 6.3%); and one Escherichia coli, one Klebsiella oxytoca, and one Serratia marcescens were isolated from Microcebus griseorufus (1 each/32, 3.1%). From selective media for Acinetobacter spp., five A. radioresistens isolates were obtained from Microcebus griseorufus (4/32, 9.4%) and from Setifer setosus (1/2, 50%), and one each of A. baumannii and A. variabilis were isolated from Microcebus griseorufus (3.1%).

Table 1.
Association between obtained bacterial isolates and animal fecal samples.
Phenotypically obtained antimicrobial resistance data from the Enterobacterales spp. and Acinetobacter spp. are shown in Table 2 and Table 3.

Table 2.
Results of antimicrobial susceptibility testing of Enterobacterales isolates (MIC data are provided as mg/L).

Table 3.
Phenotypic resistance results (MIC value) of the Acinetobacter spp.
3.2. Determination of Antimicrobial Resistance Genes
All 33 isolated Enterobacterales as well as seven Acinetobacter spp. were subjected to whole genome sequence analysis. The phenotypically observed third generation cephalosporin-resistance in the assessed Enterobacter spp. was most likely associated with class C ACT-type β-lactamases, which may naturally occur in these bacteria. Among 21 ACT type β-lactamases producing isolates, five known (3 × ACT-15, n = 3; ACT-16, n = 6; ACT-17, n = 3; ACT-56, n = 2; and ACT-115, n = 1) and five novel variants (ACT-120 to ACT-124) were assigned. The E. quasihormaechei isolate MG79-1 revealed a blaACT-59-like gene variant which contained an internal stop codon, putatively encoding a non-functional enzyme. A novel variant of the AmpC β-lactamase family CMH was identified in E. cloacae ssp. cloacae strain MG59-6. Among 12 currently published CMH variants (CMH-1–CMH-12), the novel CMH-13 type showed highest amino acid nucleotide identity to CMH-3 (amino acid substitutions at positions 153 (Arg → Ser) and 218 (Asn → His).
Two novel DHA-type AmpC β-lactamases were observed in the two Morganella morganii isolates. Strain MG60-1 (IHIT45572) carried a new DHA variant (GenBank: WLO97155.1) with 99.21% amino acid identity to DHA-18 (NCBI Reference Sequence WP_063860101.1) that was originally identified in the species M. morganii. This new variant, termed DHA-31, differed from DHA-18 by three amino acids at positions 2 (Lys → Thr), 5 (Leu → Val), and 295 (Trp → Ser). The second novel DHA-variant (DHA-32) showed 99.47% amino acid identity to DHA-18 and showed the same amino acid changes at positions 2 and 5, while it was identical to the reference sequence at position 295. The only extended-spectrum β-lactamase gene observed in this study was blaCTX-M-15 that occurred in E. coli isolate MG59-3. The 3GC-suceptible K. oxytoca isolate carried a blaOXY-2-18-like gene, differing from the reference gene by a nucleotide change at position 213 (C to A), resulting in an amino acid change from aspartic acid to alanine. In accordance with the phenotypic AST results, isolates belonging to S. enterica and Serratia marcescens were negative for β-lactamase genes conferring extended cephalosporin resistance.
Intrinsic class-D beta lactamase genes blaOXA-813, blaOXA-815, and blaOXA-816 were identified among four A. radioresistens isolates, while one isolate carried a new oxacillinase gene of the OXA-23 family, which showed 98.53% amino acid identity to OXA-818 and was assigned as OXA-1221. A single A. baumannii isolate obtained in this study carried OXA-51-like gene blaOXA-91. Acquired blaOXA genes capable of conferring resistance to carbapenems were not determined among the Acinetobacter spp. isolates from this study.
Few additional antimicrobial resistance determinants were also recorded as detailed in Table 4. Nearly all Enterobacter spp. isolates as well as the two M. morganii isolates, S. marcescens isolate MG91-1, and K. oxytoca isolate MG86-2 carried the fosfomycin-resistance gene fosA. An evolutionary analysis (Figure 2) revealed the assignment of Enterobacter spp. FosA proteins to the FosA2 family, which is correlated with a chromosomal location of the fosA gene. FosA proteins of M. morganella and S. marcescens isolates were genetically highly related or identical to FosA reference proteins of the same species, not clearly labelled with an allele number. The FosA protein of K. oxytoca isolate MG86-2 revealed the highest similarity to FosA5 (88.5%) and FosA10 (88.5%). Also for the non-Enterobacter sp. isolates, there was no indication for a plasmid location of fosA.

Table 4.
Multilocus sequence types and genotypic resistance of Enterobacterales and other gram-negative species isolated in this study.
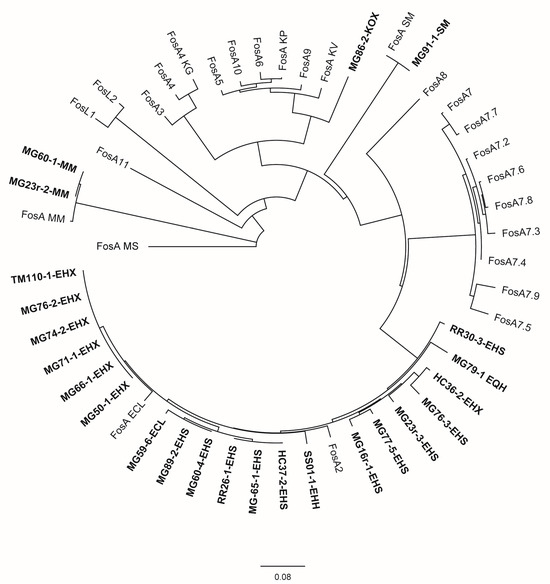
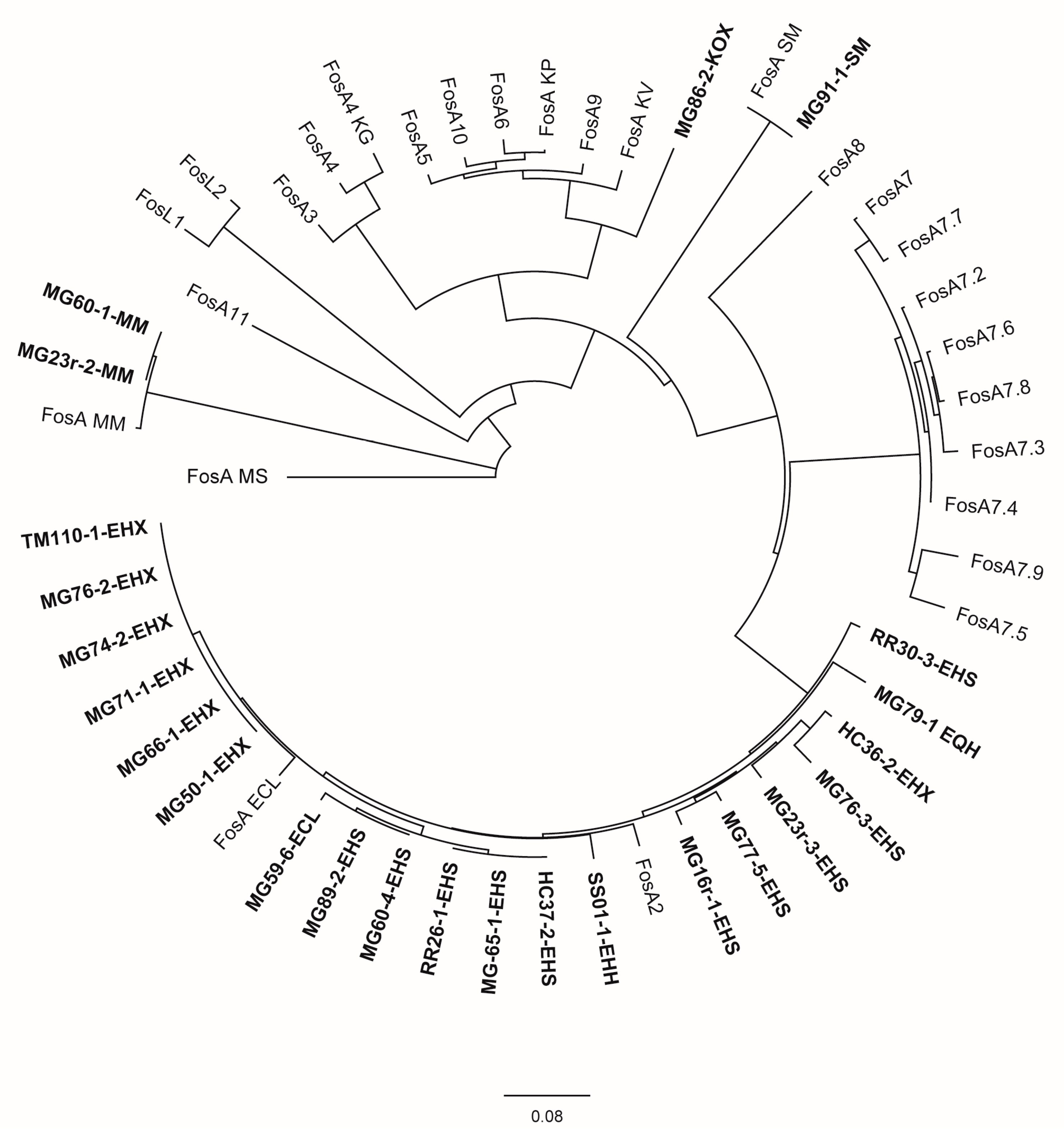
Figure 2.
Evolutionary analysis and phylogenetic tree of FosA/C2/L1-2 proteins available in GenBank for Enterobacterales and FosA proteins identified in this study inferred by applying the maximum likelihood method using Geneious Prime® 2023.2.1 GenBank Accession numbers of public sequences are given in parentheses below. Study isolates are written in bold. Isolate MG59-4-EHH was excluded from the analysis due to an interruption of the fosA gene by an IS911 element. ECL = E. cloacae ssp. cloacae, EHH = E. hormaechei ssp. hormaechei, EHX = E. hormaechei ssp. xiangfangensis, EHS = E. hormaechei ssp. steigerwaltii, EQH = E. quasihormaechei, KG = Kluyvera georgiana, KOX = K. oxytoca, KV = K. variicola, MM = M. morganii, MS = multispecies, SM = S. marcescens; FosA ECL (AWG41971.1), FosA KP (CDO16183.1), FosA KV (AWG41960.1), FosA MM (VDY35025.1), FosA MS (WP_154635598.1), FosA SM (QOW96986.1), FosA2 (WP_025205684.1), FosA3 (WP_014839980.1), FosA4 (WP_034169466.1), FosA4 KG (WP_064548962.1), FosA5 (WP_012579083.1), FosA6 (WP_069174570.1), FosA7 (WP_000941934.1), FosA7.2 (WP_000941935.1), FosA7.3 (WP_023231494.1), FosA7.4 (WP_023216493.1), FosA7.5 (WP_000941933.1), FosA7.6 (WP_061377147.1), FosA7.7 (WP_058653118.1), FosA7.8 (WP_079820715.1), FosA7.9 (WP_071684814.1), FosA8 (WP_063277905.1), FosA9 (WP_114473955.1), FosA10 (WP_004214174.1), FosA11 (QZL11398.1), FosL1 (WP_161667239.1), FosL2 (WP_188331883.1).
The chromosomally encoded aminoglycoside acetyltransferase aac(6)-Iaa resistance gene was present in all Salmonella enterica isolates. Only E. coli isolate MG59-3 showed an accumulation of AMR genes that are associated with resistance to broad-spectrum β-lactams (blaTEM-1B) aminoglycosides (aph(6)-Id, aph(3″)-Ib, and mdf(A)), sulfonamides (sul2), trimethoprim (dfrA12), quinolones (qnrS1), and tetracycline (tet(A)). All AMR genes except blaTEM-1B and mdf(A) could be located on an IncFIB(K) plasmid that showed 99.2% identity to plasmid pCFSAN061766 (CP042872.1), which was identified in an E. coli strain from raw milk cheese in Egypt in 2016.
3.3. Determination of Multilocus Sequence Types
Multilocus sequence types (STs) were determined for those species where MLST schemes are publicly available. A total of 15 different STs were identified among 22 Enterobacter spp. isolates. Three E. hormaechei isolates isolated from a rat, a Commerson’s leaf-nosed bat, and a grey-brown mouse lemur revealed the already known ST987. Other STs that have previously been defined were observed in three E. hormaechei isolates from grey-brown mouse lemurs (ST1439, ST1461, and ST2412, respectively). Among 11 novel STs (ST2689–ST2699) determined in this study, most appeared as singletons, whereas ST2692 was identified in all E. hormaechei ssp. xiangfangensis isolates that were obtained from five grey-brown mouse lemurs and from a bat (Triaenops menamena).
The CTX-M-15-positive E. coli strain obtained in this study was assigned to ST7588 and phylogenetic group A. The single K. oxytoca isolate belonged to ST19 (clonal complex 2) which represents a highly prevalent and clinically relevant clonal group among human patients [29]. The five Salmonella enterica isolates were phylogenetically diverse as they were assigned to three known STs, including ST414, ST516, and ST3780, predicting serovars Give, Glostrup, and Eastborne, respectively, and two novel STs, namely ST10918 (predicted serological profile according to Enterobase: II Z:k:k) and ST10919 (predicted serological profile: II O:55:none:-).
Following the Pasteur scheme, the only A. baumannii isolate obtained in this study was assigned to ST2306, a so far rare sequence type that had initially been identified in a white stork (Ciconia ciconia) isolate from Poland in 2016 (GenBank: JAOWYX000000000.1).
3.4. Determination of Virulence-Associated Genes (VAGs)
Based on its VAG profile, the CTX-M-15-positive E. coli isolate could not be delineated to a distinct intestinal or extraintestinal pathogenic pathotype but rather resembled a non-pathogenic strain. In the K. oxytoca isolate MG86-2, WGS revealed the presence of various virulence genes, including genes for allantoin metabolism (allA-allD, allR, and allS), type 3 fimbriae (mrkA-mrkC), and siderophores, such as enterobactin (entA-entC, entS fepA, fepD, and fepD) and yersiniabactin (ybtA, ybtE, ybtP, ybtQ, ybtS, ybtT, ybtU, ybtX, fuyA, irp1, and irp2). Notably, the genome of isolate MG86-2 also contained the kleboxymycin biosynthetic gene cluster (BCG) that comprises 12 genes (mfsX, uvrX, hmoX, adsX, icmX, dhbX, aroX, npsA, thdA, npsB, npsC, and marR) and encodes for a tricyclic pyrrolobenzodiazepine that is associated with cytotoxicity in antibiotic-associated hemorrhagic colitis in humans [30]. The 17.430-bp locus of strain MG86-2 revealed 94.92% nucleotide sequence identity and 100% query coverage with the gene cluster of the K. oxytoca MH43-1 strain reference sequence (GenBank accession number MF401554.1). VAGs suggesting a virulence potential were also identified in the A. baumannii isolate. Among others, strain MG95-1 harbored genes encoding for the biosynthesis, efflux, and uptake of the major Acinetobacter siderophore acinetobactin (basA-basD, basF-basH, basJ, bauB-bauF), biofilm-associated locus genes pgaABCD, phospholipase C (plc and plcD), and Csu pilus genes (csuABCDE).
The five Salmonella enterica isolates harbored genes for various fimbrial operons, including bcfABCDEFG, csgABCDEFG, and fimCDFHI. Iron-acquisition-related aerobactin genes iucABCD and iutA were only present in the two unknown serotypes that were assigned to novel STs. In addition, several genes encoding for type three secretion system (T3SS) and T3SS effector proteins are more or less present in the Salmonella isolates. A detailed list of virulence gene profiles for all species included in VAG analysis is provided in Supplementary Table S2.
4. Discussion
This study was performed to contribute to epidemiological knowledge in the field of AMR in wild animals by assessing the enteric carriage of antibiotic-resistant bacteria in Madagascan animals. Our investigation revealed a number of novel data relevant to the field of AMR.
High proportions of intestinal colonization with antibiotic-resistant bacteria, in particular with 3GCR Enterobacterales, were found by cultural growth from the swabs of the stool droppings of different animal species. The observed high colonization rates with Enterobacter spp. matched previous findings from Madagascan human individuals [6,13,15,18]. However, while human isolates have been frequently associated with ESBL-type beta-lactamases in previous studies [6,13,15] with few exemptions [18], we could not confirm this finding for the Enterobacter spp. isolated from the animal stool samples. As expected, due to the abundance of inducible ampC-type beta-lactamases in Enterobacter spp. [31], the associated genes were considered as the most likely reasons for phenotypically observed 3GC-resistance in the obtained isolates from this study. The molecular resistance profile matches more a recent finding with Enterobacter spp.-colonized patients from the Madagascan Antananarivo hospital [18]. The blaCTX-M-15 gene as the underlying mechanism of 3GC-resistance in the single assessed E. coli isolate is typical for the Madagascan setting and well in line with previous regional reports [15,16,17]. The opportunistic pathogen K. oxytoca is naturally resistant to amino- and carboxypenicillins owing to low-level production of chromosomal β-lactamases of the OXY group [32]. Overproduction of blaOXY-genes in K. oxytoca due to promoter-up mutations results in reduced susceptibility or even resistance to other β-lactams, such as penicillin-inhibitor combinations, cefuroxime and cefotaxime and appears in approximately 10–20% of clinical isolates [32]. Our wild animal isolate harbored an OXY-2-8-like β-lactamase without a mutation in the −10 hexamer chromosomal region (−10 region: (A)GATAGT), which is in line with its 3GC-susceptible phenotype. Intrinsic class-D beta lactamase genes blaOXA-813, blaOXA-815, and blaOXA-816 were identified among four A. radioresistens isolates, while one isolate carried a new oxacillinase gene of the OXA-23 family, which was defined as OXA-1221. A single A. baumannii isolate obtained in this study carried the OXA-51-like gene blaOXA-91. Acquired blaOXA genes were not determined.
Overall, the low abundance of further molecular antimicrobial resistance (AMR) determinants, as shown in Table 4, suggests the isolation of environmental isolates with low antimicrobial selection pressure due to exposure to antimicrobial drugs. However, the high percentage of isolates carrying a fosA gene, which confers resistance to fosfomycin, was not to be expected. This old antibiotic regained relevance in clinical practice for the treatment of complicated infections caused by multidrug-resistant bacteria [33]. Therefore, the emergence of fosA among rather naïve wild animal populations warrants further investigations, as it might have a significant impact on public health.
The CTX-M-15-producing E. coli strain MG59-3 from a grey-brown mouse lemur revealed a number of additional AMR genes that might confer resistance to aminoglycosides, sulfonamides, and tetracyclines. Although the strain also harbored the plasmid-mediated quinolone-resistance (PMQR) gene qnrS1, we could not determine phenotypic resistance to fluoroquinolones. FQ-resistance is mostly due to chromosomal mutations that alter the drug target enzymes DNA gyrase (topoisomerase II) and (gyrA and gyrB) and DNA topoisomerase IV (parC and parE) [34]. Our strain lacked mutations in these regions, but the presence of qnrS1 most likely conferred the observed increased MIC to enrofloxacin (1 mg/L). The multidrug-resistant phenotype of strain MG59-3 indicates that wild animals might acquire AMR bacteria and/or genes from sources that are under anthropogenic influence. The Enterobase strain database (https://enterobase.warwick.ac.uk/species/ecoli/search_strains; accessed on 21 December 2023) contains 21 E. coli strains from various sources (e.g., environment, livestock, and wild animals) and countries (e.g., Kenya, Pakistan, United Arab Emirates, and USA) that belong to the same sequence type as our strain, namely ST7588. Interestingly, one strain from an unspecified source originated from the Antananarivo area in Madagascar and showed an AMR gene profile that was highly similar to that of strain MG59-3. Like our strain, E. coli C32b187a harbored blaCTX-M-15, blaTEM-1B, aph(6)-Id, dfrA14, mdf(A), and tet(A), while it lacked aph(3″)-Ib, qnrS1, and sul2. Moreover, a core-genome-based MLST comparison performed with BacWGSTdb (http://bacdb.cn/BacWGSTdb/Tools.php; accessed on 21 December 2023) further revealed a high similarity (30 different alleles) of strain MG59-3 with two ST7588 E. coli strains isolated from cattle in the USA in 2016. One of these strains (KCJK6915; Acc.-No. SRNT01) even showed an identical AMR gene profile. Thus, although we observed MG59-3 as a single isolate, the phylogenetic grouping and AMR gene profile indicate a wider distribution of this clonal type, which might be due to transmission events taking place between environmental sources, livestock and wild animals.
We also detected one K. oxytoca isolate (MG86-2) from a bat. After K. pneumoniae, K. oxytoca is the second most common Klebsiella species causing diseases in humans, such as pneumonia, urinary tract infection and skin infections [35]. As an opportunistic pathogen, it can also act in the dysbiotic human intestinal microbiota causing antibiotic-associated hemorrhagic colitis (AAHC) [30]. The single K. oxytoca isolate obtained in this study could be assigned to ST19 that has been reported as highly prevalent among clinical isolates from the UK [29] and has recently been associated with AAHC in a human patient in Austria [36]. Of note, it harbored the biosynthetic gene cluster encoding for a tricyclic pyrrolobenzodiazepine, which has been associated with cytotoxicity in AAHC caused by K. oxyotoca [30], suggesting a novel source of this molecular determinant in wild animals in Madagascar.
The only A. baumannii isolate (MG95-1) obtained in this study was cultured from the feces of a grey-brown mouse lemur. It was assigned to ST2306, a sequence type that was originally detected in an isolate from a white stork (Ciconia ciconia) in Poland in 2016 and that was reported in a recent study in a multidrug-resistant A. baumannii clinical strain [37]. The detected actinetobactin virulence cluster is considered one of the most relevant mechanisms mediating virulence of Acinetobacter baumannii isolates [38]. Further, the isolate encoded factors critical for biofilm formation [39], including genes for CSU pili [40] as well as the cytolytic factor phospholipase C [41], stressing its potential etiological relevance in case of nosocomial transmission to susceptible hosts as well.
The lacking assignability of the majority (72.7%) of Enterobacter spp. isolates to known multilocus sequence types confirms that they are most likely different from typical hospital-associated clones or those associated with clinical diseases in humans. Except for β-lactamase genes, other AMR genes were only rarely detected, including the fosfomycin gene fosA that occurred in 95.5% of the isolates, suggesting the presence of a rather antimicrobial naïve population of Enterobacter spp. isolates among the wild animals studied. In contrast, at least three of the five Salmonella enterica isolates could be assigned to sequence types that have previously been observed in isolates from humans, livestock, and wild animals (ST516; SS01-3 from greater hedgehog tenrec), from wild animals and food (ST3780, SS02-5 from greater hedgehog tenrec), and from humans and animals (ST414, ET01-4 from lesser hedgehog tenrec). Salmonella Give ST516 was one of the dominant sequence types identified in a study from Lagos, Nigeria (12 from humans, 2 from poultry and cattle, 2 from sewage samples), underlining the worldwide distribution of this non-typhoidal Salmonella serovar [42]. While fluoroquinolone resistance was common in Salmonella Give in the previous African study [41], such resistance was not observed in our assessment. Of note, the detection of aminoglycoside resistance genes in the salmonellae isolates in our study, however, confirm the high phenotypic aminoglycoside resistance rate previously reported [42].
The novel ST10918 is a double locus variant of ST7327. The Enterobase database contains two human clinical isolates of ST7327 from the UK and Canada. Interestingly, the second novel ST detected in this study from a grey-brown mouse lemur is phylogenetically related to ST1122 (double locus variant). Only one ST1122 isolate is listed in the Enterobase database, and this was isolated from a wild animal, namely a reptile, from Madagascar in the year 1962. Although none of our wild animal isolates carried genes typically located on Salmonella virulence plasmids (spv genes), most isolates possessed VAGs specific for Salmonella pathogenicity islands (Table S2). Salmonella infections still represent an important public health issue worldwide and non-typhoidal Salmonella (NTS) have previously been associated with bloodstream infections and gastroenteritis, especially in children in Sub-Saharan Africa [42]. Next to genes for the type III secretion system, which is considered as a main element of salmonellae-associated virulence [43], fimbrial genes associated with persistence of salmonellae in the mammal intestine could be recorded [44].
Altogether, the proportion of detected AMR bacteria within the assessed animal stool samples is low to moderate and can thus be well explained by occasional contacts with the Madagascan human civilization that is much more severely affected by the resistance issue. In particular, only individual cases of ESBL-type resistance were detected in the animal stool samples of this study, while the ESBL mechanism has been described to account for high colonization rates with third generation cephalosporin-resistant Enterobacterales in both Madagascan people and livestock [6,13,14,15,16,17,21,22]. The considerably higher rates of colonization with ampC-positive Enterobacter spp. are well in line with observations in Madagascan patients and healthcare workers, in which this genus-resistance type-combination accounted for a major part of recorded 3GCR in isolated colonizing Enterobacterales [17]. In this regard, their regional abundance seems to be typical for Madagascar. Finally and with focus on the isolated Acinetobacter spp., recorded resistance profiles were close to the wild-type situation, and in particular, carbapenem resistance-mediating genes like those previously described for the Madagascan setting were not recorded. Altogether, the observed resistance profiles match the expectations from the literature quite well.
This study has a few limitations. The major limitations are the relatively low sample size, the more or less arbitrary sampling pattern, and the non-selective culturing approach. As such, the assessment provides only preliminary information and cannot replace future systematic analyses. The selective culturing of samples on media containing cefotaxime and ceftazidime might have increased the number of isolated AMR bacteria. Thus, the data cannot provide a real estimate for the distribution of ESBL- and AmpC-β-lactamase-producing bacteria among samples from the given animal population. Considering the still scarcely available epidemiological information on environmental isolates from the assessed region and with respect to the detailed molecular characterization provided for the obtained Enterobacterales and Acinetobacter isolates, the assessment may nevertheless serve as a useful proof-of-principle. However, it is still interesting to isolate bacterial species of clinical relevance from wild mammals, for which epidemiological reports from Madagascar are widely missing.
5. Conclusions
The presented proof-of-principle assessment indicated high enteric colonization rates of Madagascan mammals with resistant bacteria. In particular, 3GCR Enterobacterales with ampC-type beta-lactamases as the underlying resistance mechanism were found to be highly prevalent. In addition, the identification of the fosfomycin-resistance gene fosA in a significant number of isolates is a cause for serious concern and warrants further investigation. Due to the expected low antibiotic pressure, natural colonization is assumed, showing the general occurrence of antibiotic resistance genes in nature. Future systematic screening should be conducted to confirm the findings and further, molecular comparisons of colonizing resistant bacteria in animals and human individuals seem advisable to assess potential transmission routes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani14050741/s1, Table S1: Details on the sampling dates and exact locations of the swabbing of the stool droppings; Table S2: Virulence-associated genes identified among (A) Salmonella enterica, (B) E. coli, (C) K. oxytoca, and (D) A. baumannii.
Author Contributions
Conceptualization, U.S., J.E., H.F. and S.P.; methodology, U.S., J.E., T.S. and C.E.; software, S.A.W. and C.E.; validation, U.S. and C.E.; formal analysis, U.S. and C.E.; investigation, U.S., J.E. and C.E.; resources, U.S., R.R., T.S. and C.E.; data curation, U.S. and C.E.; writing—original draft preparation, H.F.; writing—review and editing, U.S., J.E., R.R., J.N., H.F., S.P. and C.E.; visualization, J.E.; supervision, R.R. and S.P.; project administration, S.P.; funding acquisition, S.P. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by internal grants of the Bernhard Nocht Institute for Tropical Medicine, Hamburg, Germany.
Institutional Review Board Statement
All applicable institutional and/or national guidelines for the care and use of animals were followed. The field work was approved by the ethics committee of the Institute of Zoology of Hamburg University before the initiation of this study and authorized by the Ministère de L’Environnement, de l’Ecologie, de la Mer et des Forêts. (Research permit: N°002/17/MEEF/SG/DGF/DSAP/SCB.Re; export permit: N°345-17/MEEF/SG/DGF/DREEFAAND/SFR).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Toy, T.; Pak, G.D.; Duc, T.P.; Campbell, J.I.; El Tayeb, M.A.; Von Kalckreuth, V.; Im, J.; Panzner, U.; Cruz Espinoza, L.M.; Eibach, D.; et al. Multicountry Distribution and Characterization of Extended-spectrum β-Lactamase-associated Gram-negative Bacteria from Bloodstream Infections in Sub-Saharan Africa. Clin. Infect. Dis. 2019, 69 (Suppl. 6), S449–S458. [Google Scholar] [CrossRef]
- Rasamiravaka, T.; Shaista Sheila, H.S.; Rakotomavojaona, T.; Rakoto-Alson, A.O.; Rasamindrakotroka, A. Changing profile and increasing antimicrobial resistance of uropathogenic bacteria in Madagascar. Med. Mal. Infect. 2015, 45, 173–176. [Google Scholar] [CrossRef]
- Rasamiravaka, T.; Batavisoa, E.; Ranaivosoa, M.K.; Rasamindrakotroka, A. Profile and antimicrobial resistance to newly available drugs of urinary tract pathogens among Malagasy pregnant women. Trop. Biomed. 2016, 33, 35–140. [Google Scholar]
- Randrianirina, F.; Soares, J.L.; Carod, J.F.; Ratsima, E.; Thonnier, V.; Combe, P.; Grosjean, P.; Talarmin, A. Antimicrobial resistance among uropathogens that cause community-acquired urinary tract infections in Antananarivo, Madagascar. J. Antimicrob. Chemother. 2007, 59, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.S.; Bebell, L.M.; Meney, C.; Valeri, L.; White, M.C. Epidemiology of antibiotic-resistant wound infections from six countries in Africa. BMJ Glob. Health 2018, 2 (Suppl. 4), e000475. [Google Scholar] [CrossRef]
- Herindrainy, P.; Randrianirina, F.; Ratovoson, R.; Ratsima Hariniana, E.; Buisson, Y.; Genel, N.; Decré, D.; Arlet, G.; Talarmin, A.; Richard, V. Rectal carriage of extended-spectrum beta-lactamase-producing gram-negative bacilli in community settings in Madagascar. PLoS ONE 2011, 6, e22738. [Google Scholar] [CrossRef]
- Randrianirina, F.; Ratsima, E.H.; Ramparany, L.; Randremanana, R.; Rakotonirina, H.C.; Andriamanantena, T.; Rakotomanana, F.; Rajatonirina, S.; Richard, V.; Talarmin, A. Antimicrobial resistance of bacterial enteropathogens isolated from stools in Madagascar. BMC Infect. Dis. 2014, 14, 104. [Google Scholar] [CrossRef]
- Chereau, F.; Herindrainy, P.; Garin, B.; Huynh, B.T.; Randrianirina, F.; Padget, M.; Piola, P.; Guillemot, D.; Delarocque-Astagneau, E. Colonization of extended-spectrum-β-lactamase- and NDM-1-producing Enterobacteriaceae among pregnant women in the community in a low-income country: A potential reservoir for transmission of multiresistant Enterobacteriaceae to neonates. Antimicrob. Agents Chemother. 2015, 59, 3652–3655. [Google Scholar] [CrossRef] [PubMed]
- Andriatahina, T.; Randrianirina, F.; Hariniana, E.R.; Talarmin, A.; Raobijaona, H.; Buisson, Y.; Richard, V. High prevalence of fecal carriage of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in a pediatric unit in Madagascar. BMC Infect. Dis. 2010, 10, 204. [Google Scholar] [CrossRef]
- Huynh, B.T.; Kermorvant-Duchemin, E.; Herindrainy, P.; Padget, M.; Rakotoarimanana, F.M.J.; Feno, H.; Hariniaina-Ratsima, E.; Raheliarivao, T.; Ndir, A.; Goyet, S.; et al. Bacterial Infections in Neonates, Madagascar, 2012–2014. Emerg. Infect. Dis. 2018, 24, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Huynh, B.T.; Kermorvant-Duchemin, E.; Chheang, R.; Randrianirina, F.; Seck, A.; Hariniaina Ratsima, E.; Andrianirina, Z.Z.; Diouf, J.B.; Abdou, A.Y.; Goyet, S.; et al. Severe bacterial neonatal infections in Madagascar, Senegal, and Cambodia: A multicentric community-based cohort study. PLoS Med. 2021, 18, e1003681. [Google Scholar] [CrossRef]
- Bonneault, M.; Andrianoelina, V.H.; Herindrainy, P.; Rabenandrasana, M.A.N.; Garin, B.; Breurec, S.; Delarocque-Astagneau, E.; Guillemot, D.; Andrianirina, Z.Z.; Collard, J.M.; et al. Transmission Routes of Extended-Spectrum Beta-Lactamase-Producing Enterobacteriaceae in a Neonatology Ward in Madagascar. Am. J. Trop. Med. Hyg. 2019, 100, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Ranosiarisoa, Z.N.; El Harrif, S.; Andrianirina, A.Z.; Duron, S.; Simon-Ghediri, M.J.; Ramparany, L.; Robinson, A.L.; Tsifiregna, R.; Randrianirina, F.; Ratsima, E.; et al. Epidemiology of Early-onset Bacterial Neonatal Infections in Madagascar. Pediatr. Infect. Dis. J. 2019, 38, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Randrianirina, F.; Vaillant, L.; Ramarokoto, C.E.; Rakotoarijaona, A.; Andriamanarivo, M.L.; Razafimahandry, H.C.; Randrianomenjanahary, J.; Raveloson, J.R.; Hariniana, E.R.; Carod, J.F.; et al. Antimicrobial resistance in pathogens causing nosocomial infections in surgery and intensive care units of two hospitals in Antananarivo, Madagascar. J. Infect. Dev. Ctries. 2010, 4, 74–82. [Google Scholar] [CrossRef]
- Naas, T.; Cuzon, G.; Robinson, A.L.; Andrianirina, Z.; Imbert, P.; Ratsima, E.; Ranosiarisoa, Z.N.; Nordmann, P.; Raymond, J. Neonatal infections with multidrug-resistant ESBL-producing E. cloacae and K. pneumoniae in Neonatal Units of two different Hospitals in Antananarivo, Madagascar. BMC Infect. Dis. 2016, 16, 275. [Google Scholar] [CrossRef]
- Rakotonirina, H.C.; Garin, B.; Randrianirina, F.; Richard, V.; Talarmin, A.; Arlet, G. Molecular characterization of multidrug-resistant extended-spectrum β-lactamase-producing Enterobacteriaceae isolated in Antananarivo, Madagascar. BMC Microbiol. 2013, 13, 85. [Google Scholar] [CrossRef] [PubMed]
- Rakotondrasoa, A.; Passet, V.; Herindrainy, P.; Garin, B.; Kermorvant-Duchemin, E.; Delarocque-Astagneau, E.; Guillemot, D.; Huynh, B.T.; Brisse, S.; Collard, J.M. Characterization of Klebsiella pneumoniae isolates from a mother-child cohort in Madagascar. J. Antimicrob. Chemother. 2020, 75, 1736–1746. [Google Scholar] [CrossRef]
- Micheel, V.; Hogan, B.; Rakotoarivelo, R.A.; Rakotozandrindrainy, R.; Razafimanatsoa, F.; Razafindrabe, T.; Rakotondrainiarivelo, J.P.; Crusius, S.; Poppert, S.; Schwarz, N.G.; et al. Identification of nasal colonization with β-lactamase-producing Enterobacteriaceae in patients, health care workers and students in Madagascar. Eur. J. Microbiol. Immunol. 2015, 5, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Osei Sekyere, J.; Reta, M.A.; Bernard Fourie, P. Risk factors for, and molecular epidemiology and clinical outcomes of, carbapenem- and polymyxin-resistant Gram-negative bacterial infections in pregnant women, infants, and toddlers: A systematic review and meta-analyses. Ann. N. Y. Acad. Sci. 2021, 1502, 54–71. [Google Scholar] [CrossRef]
- Andriamanantena, T.S.; Ratsima, E.; Rakotonirina, H.C.; Randrianirina, F.; Ramparany, L.; Carod, J.F.; Richard, V.; Talarmin, A. Dissemination of multidrug resistant Acinetobacter baumannii in various hospitals of Antananarivo Madagascar. Ann. Clin. Microbiol. Antimicrob. 2010, 9, 17. [Google Scholar] [CrossRef]
- Gay, N.; Belmonte, O.; Collard, J.M.; Halifa, M.; Issack, M.I.; Mindjae, S.; Palmyre, P.; Ibrahim, A.A.; Rasamoelina, H.; Flachet, L.; et al. Review of Antibiotic Resistance in the Indian Ocean Commission: A Human and Animal Health Issue. Front. Public Health 2017, 5, 162. [Google Scholar] [CrossRef]
- Gay, N.; Leclaire, A.; Laval, M.; Miltgen, G.; Jégo, M.; Stéphane, R.; Jaubert, J.; Belmonte, O.; Cardinale, E. Risk Factors of Extended-Spectrum β-Lactamase Producing Enterobacteriaceae Occurrence in Farms in Reunion, Madagascar and Mayotte Islands, 2016–2017. Vet. Sci. 2018, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, J.; Poppert, S.; Ratovonamana, R.Y.; Ganzhorn, J.U.; Tappe, D.; Krüger, A. Ectoparasites of endemic and domestic animals in southwest Madagascar. Acta Trop. 2019, 196, 83–92. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 33rd ed.; CLSI supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2023; ISBN 978-1-68440-160-3/978-1-68440-171-0. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 6th ed.; CLSI supplement VET01S; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2023; ISBN 978-1-68440-166-6/978-1-68440-167-3. [Google Scholar]
- Klotz, P.; Göttig, S.; Leidner, U.; Semmler, T.; Scheufen, S.; Ewers, C. Carbapenem-resistance and pathogenicity of bovine Acinetobacter indicus-like isolates. PLoS ONE 2017, 12, e0171986. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef] [PubMed]
- Shibu, P.; McCuaig, F.; McCartney, A.L.; Kujawska, M.; Hall, L.J.; Hoyles, L. Improved molecular characterization of the Klebsiella oxytoca complex reveals the prevalence of the kleboxymycin biosynthetic gene cluster. Microb. Genom. 2021, 7, 000592. [Google Scholar] [CrossRef]
- Tse, H.; Gu, Q.; Sze, K.H.; Chu, I.K.; Kao, R.Y.; Lee, K.C.; Lam, C.W.; Yang, D.; Tai, S.S.; Ke, Y.; et al. A tricyclic pyrrolobenzodiazepine produced by Klebsiella oxytoca is associated with cytotoxicity in antibiotic-associated hemorrhagic colitis. J. Biol. Chem. 2017, 292, 19503–19520. [Google Scholar] [CrossRef] [PubMed]
- Hanson, N.D.; Sanders, C.C. Regulation of inducible AmpC beta-lactamase expression among Enterobacteriaceae. Curr. Pharm. Des. 1999, 5, 881–894. [Google Scholar] [CrossRef]
- Izdebski, R.; Fiett, J.; Urbanowicz, P.; Baraniak, A.; Derde, L.P.; Bonten, M.J.; Carmeli, Y.; Goossens, H.; Hryniewicz, W.; Brun-Buisson, C.; et al. Phylogenetic lineages, clones and β-lactamases in an international collection of Klebsiella oxytoca isolates non-susceptible to expanded-spectrum cephalosporins. J. Antimicrob. Chemother. 2015, 70, 3230–3237. [Google Scholar]
- Mattioni Marchetti, V.; Hrabak, J.; Bitar, I. Fosfomycin resistance mechanisms in Enterobacterales: An increasing threat. Front. Cell. Inf. Microbiol. 2023, 13, 1178547. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, G.A. Mechanisms of resistance to quinolones. Clin. Infect. Dis. 2005, 41 (Suppl. 2), S120–S126. [Google Scholar] [CrossRef] [PubMed]
- Merla, C.; Rodrigues, C.; Passet, V.; Corbella, M.; Thorpe, H.A.; Kallonen, T.V.S.; Zong, Z.; Marone, P.; Bandi, C.; Sassera, D.; et al. Description of Klebsiella spallanzanii sp. nov. and of Klebsiella pasteurii sp. nov. Front. Microbiol. 2019, 10, 2360. [Google Scholar] [CrossRef] [PubMed]
- Herzog, K.A.; Schneditz, G.; Leitner, E.; Feierl, G.; Hoffmann, K.M.; Zollner-Schwetz, I.; Krause, R.; Gorkiewicz, G.; Zechner, E.L.; Högenauer, C. Genotypes of Klebsiella oxytoca isolates from patients with nosocomial pneumonia are distinct from those of isolates from patients with antibiotic-associated hemorrhagic colitis. J. Clin. Microbiol. 2014, 52, 1607–1616. [Google Scholar] [CrossRef]
- Righetto, G.M.; Lopes, J.L.S.; Bispo, P.J.M.; André, C.; Souza, J.M.; Andricopulo, A.D.; Beltramini, L.M.; Camargo, I.L.B.D.C. Antimicrobial Activity of an Fmoc-Plantaricin 149 Derivative Peptide against Multidrug-Resistant Bacteria. Antibiotics 2023, 12, 391. [Google Scholar] [CrossRef]
- Conde-Pérez, K.; Vázquez-Ucha, J.C.; Álvarez-Fraga, L.; Ageitos, L.; Rumbo-Feal, S.; Martínez-Guitián, M.; Trigo-Tasende, N.; Rodríguez, J.; Bou, G.; Jiménez, C.; et al. In-Depth Analysis of the Role of the Acinetobactin Cluster in the Virulence of Acinetobacter baumannii. Front. Microbiol. 2021, 12, 752070. [Google Scholar] [CrossRef]
- Choi, A.H.; Slamti, L.; Avci, F.Y.; Pier, G.B.; Maira-Litrán, T. The pgaABCD locus of Acinetobacter baumannii encodes the production of poly-beta-1-6-N-acetylglucosamine, which is critical for biofilm formation. J. Bacteriol. 2009, 191, 5953–5963. [Google Scholar] [CrossRef]
- Ahmad, I.; Nadeem, A.; Mushtaq, F.; Zlatkov, N.; Shahzad, M.; Zavialov, A.V.; Wai, S.N.; Uhlin, B.E. Csu pili dependent biofilm formation and virulence of Acinetobacter baumannii. npj Biofilms Microbiomes 2023, 9, 101. [Google Scholar] [CrossRef]
- Fiester, S.E.; Arivett, B.A.; Schmidt, R.E.; Beckett, A.C.; Ticak, T.; Carrier, M.V.; Ghosh, R.; Ohneck, E.J.; Metz, M.L.; Sellin Jeffries, M.K.; et al. Iron-Regulated Phospholipase C Activity Contributes to the Cytolytic Activity and Virulence of Acinetobacter baumannii. PLoS ONE 2016, 11, e0167068. [Google Scholar] [CrossRef]
- Akinyemi, K.O.; Fakorede, C.O.; Linde, J.; Methner, U.; Wareth, G.; Tomaso, H.; Neubauer, H. Whole genome sequencing of Salmonella enterica serovars isolated from humans, animals, and the environment in Lagos, Nigeria. BMC Microbiol. 2023, 23, 164. [Google Scholar] [CrossRef]
- Dos Santos, A.M.P.; Ferrari, R.G.; Conte-Junior, C.A. Type three secretion system in Salmonella Typhimurium: The key to infection. Genes Genom. 2020, 42, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Weening, E.H.; Barker, J.D.; Laarakker, M.C.; Humphries, A.D.; Tsolis, R.M.; Bäumler, A.J. The Salmonella enterica serotype Typhimurium lpf, bcf, stb, stc, std, and sth fimbrial operons are required for intestinal persistence in mice. Infect. Immun. 2005, 73, 3358–3366. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).