Simple Summary
Bovine respiratory disease (BRD) is a globally prevalent multifactorial infection primarily caused by viral, bacterial, and Mycoplasma coinfections. Vaccines exist for individuals or several pathogens, but there are currently no live and marker combined vaccines commercially available. Based on our previous study, we developed an attenuated and marker M. bovis–BoHV-1 combined vaccine for the first time and determined the optimal antigen ratio of the combined vaccine. In this work, we exhibited different vaccine doses to further determine the optimal immunization dose using a rabbit model. The findings indicated that the 2:2 immunization dose group showed the best performance in humoral and cellular immune responses. Also, it had a nearly standard lung tissue structure and was able to provide the best protective efficacy in facing the challenge with both pathogens. The results of this study determined the optimal immune dose for the combined vaccine. Combined with our previous research, these findings lay the foundation for further bovine experiments and actual clinical applications of the attenuated and marker M. bovis–BoHV-1 combined vaccine.
Abstract
Bovine respiratory disease (BRD) is one of the most common diseases in the cattle industry; it is a globally prevalent multifactorial infection primarily caused by viral and bacterial coinfections. In China, Mycoplasma bovis (M. bovis) and bovine herpesvirus type 1 (BoHV-1) are the most notable pathogens associated with BRD. Our previous study attempted to combine the two vaccines and conducted a preliminary investigation of their optimal antigenic ratios. Based on this premise, the research extended its investigation by administering varying vaccine doses in a rabbit model to identify the most effective immunization dosage. After immunization, all rabbits in other immunization dose groups had a normal rectal temperature without obvious clinical symptoms. Furthermore, assays performed on the samples collected from immunized rabbits indicated that there were increased humoral and cellular immunological reactions. Moreover, the histological analysis of the lungs showed that immunized rabbits had more intact lung tissue than their unimmunized counterparts after the challenge. Additionally, there appears to be a positive correlation between the protective efficacy and the immunization dose. In conclusion, the different immunization doses of the attenuated and marker M. bovis HB150 and BoHV-1 gG-/tk- combined vaccine were clinically safe in rabbits; the mix of 2.0 × 108 CFU of M. bovis HB150 and 2.0 × 106 TCID50 BoHV-1 gG-/tk- strain was most promising due to its highest humoral and cellular immune responses and a more complete morphology of the lung tissue compared with others. These findings determined the optimal immunization dose of the attenuated and marker M. bovis HB150 and BoHV-1 gG-/tk- combined vaccine, laying a foundation for its clinical application.
1. Introduction
Bovine respiratory disease (BRD) is a widespread cause of morbidity, mortality, and economic loss in beef and dairy cattle farming [1]. BRD may also be responsible for up to 70% of morbidity and mortality in U.S. feedlot cattle [2,3], and BRD is recognized as the primary disease affecting cattle in over 90% of feedlots across the United States, with an estimated annual cost ranging from 1 to 3 billion USD [4,5]. In US feedlots, it costs $23.6 to treat each cattle with respiratory diseases, and a recent study estimated the cost per BRD-affected calf to be as high as $42.15 [6]. In Australia, the recorded rates of illness and death for BRD were 18% and 2.1%, respectively, with an average financial loss of $1076.17 per death [7]. BRD is a multifactorial disease, and its etiology involves bacterial and viral coinfections with high re-infection rates [8]; also, the susceptibility of cattle to this disease can be influenced by a complex interaction between stress, management practices, physiological conditions, and the host immune response [1,9,10]. Moreover, immunosuppression caused by viral infections is also significantly associated with the risk of severe secondary bacterial infections.
Common pathogens associated with BRD include bovine herpesvirus type 1 (BoHV-1) and bovine viral diarrhea virus, Mycoplasma bovis (M. bovis), Mannheimia haemolytica, and Pasteurella multocida [11]. In China, M. bovis and BoHV-1 are the pathogens most frequently associated with BRD [12]. M. bovis was initially identified in 2008 among recently imported beef cattle suffering from pneumonia, and its prevalence has become widespread [13]. BoHV-1 was initially detected in 1981 in an imported cow and quickly disseminated nationwide. Approximately 40% of cattle in China were found to be infected with BoHV-1 [14]. In Inner Mongolia and Henan Province, the nucleic acid positivity rate of BoHV-1 could reach 7% to 15% and 24.83%, respectively [9,14]. Vaccines exist for individuals or several pathogens; Bimeda Biochemicals and Boehringer Ingelheim Animal Health have developed live vaccines to target specific pathogens of BRD, such as M. bovis and BoHV-1. Nonetheless, there are currently no live and marker combined vaccines commercially available.
In our previous study, we developed an attenuated and marker M. bovis–BoHV-1 combined vaccine for the first time, and the results showed that the combined vaccine provided sufficient and effective protection for rabbits when facing the challenge with both pathogens. Moreover, the protective effect was best at a 1:1 ratio of the two antigens in all vaccinated groups when challenged with M. bovis HB0801 or BoHV-1 HB06 [12,15,16]. However, its protective efficacy has not been quantitatively evaluated. To enhance the immunoprotective rate of the attenuated and marker M. bovis–BoHV-1 combined vaccine, different vaccine doses were developed to further determine the optimal immunization dose using a rabbit model in this study and evaluated the vaccine’s protective efficacy by quantitative scores. Our study provides a solid foundation for clinically applying the attenuated and marker M. bovis–BoHV-1 combined vaccine.
2. Materials and Methods
2.1. Cells and Viruses
The wild-type BoHV-1 strain HB06 (GenBank accession number: AJ004801.1), along with the BoHV-1 gG-/tk- strain and M. bovis strains HB0801 (GenBank accession number: CP002058.1) and HB150, was preserved at the State Key Laboratory of Agricultural Microbiology. Madin–Darby bovine kidney cells (MDBK) were obtained from the China Institute of Veterinary Drug Control.
2.2. Culture of M. bovis and BoHV-1
M. bovis and BoHV-1 were cultured as previously described. Briefly, M. bovis HB0801 and HB150 strains were cultured in a complete PPLO medium at 37 °C in a 5% CO2 incubator for 40–48 h. BoHV-1 HB06 and gG-/tk- strains were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (Inner Mongolia Opcel Biotechnology Co., Ltd.) using MDBK cells at 37 °C in a 5% CO2 incubator.
The M. bovis HB150 and BoHV-1 gG-/tk- initial doses were 1.0 × 108 CFU and 1.0 × 106 TCID50, respectively. The ratios of 1:1, 2:2, and 3:3 were prepared as follows: 1.0 × 108 CFU M. bovis HB150 with 1.0 × 106 TCID50 BoHV-1 gG-/tk-, 2.0 × 108 CFU M. bovis HB150 with 2.0 × 106 TCID50 BoHV-1 gG-/tk-, and 3.0 × 108 CFU M. bovis HB150 with 3.0 × 106 TCID50 BoHV-1 gG-/tk-.
2.3. Animal Experiments
A group of 27 Japanese white rabbits, with an average weight of 1.5 kg each, were found to be seronegative for M. bovis, BoHV-1, Pasteurella, and Manniella hemolysticus and were divided into 9 groups. All rabbits were housed in isolation to prevent cross-infection. According to the antigen dose, groups 1 and 2 were immunized with 1.0 × 108 CFU M. bovis HB150 combined with 1.0 × 106 TCID50 BoHV-1 gG-/tk- strain (presented as 1:1); groups 3 and 4 were immunized with 2.0 × 108 CFU M. bovis HB150 mixed with 2.0 × 106 TCID50 BoHV-1 gG-/tk- strain (presented as 2:2); groups 5 and 6 were immunized with 3.0 × 108 CFU M. bovis HB150 mixed with 3.0 × 106 TCID50 BoHV-1 gG-/tk- strain (presented as 3:3), respectively; groups 7 and 8 were inoculated with PPLO complete medium and DMEM, and group 9 served as a control. The design of the study is outlined in detail in Table 1. All experimental groups were then challenged with 1.0 × 109 CFU M. bovis HB0801 strain or 4.0 × 107 TCID50 BoHV-1 HB06 strain 28 days after immunization. Nasal swabs were collected daily for 14 days after immunization and challenge, and blood samples were collected weekly until the experiment’s end. Samples were stored at −80 °C. Animals were euthanized at the end of the investigation, and lung tissue samples were collected for further experiments.

Table 1.
Animal immunization and challenge information.
2.4. Clinical Evaluation and Sample Collection
Rectal temperature and clinical observation were recorded for 14 days following immunization and the challenge. A thermometer was inserted approximately 4 cm into the rectum until the temperature stopped changing. The rectal temperature of rabbits was measured in the morning and afternoon, and the average value was recorded.
After immunization and challenge, nasal swabs were obtained daily for 14 days. The samples were thoroughly mixed by vortexing in tubes with 1 mL of sterile PBS, then filtered through a 0.45 µm filter, and preserved at −20 °C for subsequent PCR/RT-PCR analysis and M. bovis quantification. Blood samples were collected weekly for antibody and cytokine detection until the end of the experiment. Lung samples were collected 28 days after the challenge. The tissue samples were preserved in a 4% paraformaldehyde solution for 48 h and subsequently encased in paraffin. The sections were subjected to hematoxylin–eosin staining and later underwent histopathological evaluation.
2.5. Virus and Bacteria Shedding
The total nucleic acids of nasal swabs were extracted and amplified by PCR/RT-PCR to detect the shedding of M. bovis HB150 using the uvrC gene and BoHV-1 gG-/tk- using glycoprotein G gG and thymidine kinase tk genes, as previously described under the following reaction conditions: M. bovis uvrC gene PCR reaction conditions: 95 °C for 3 min, 95 °C for 15 s, 55 °C for 15 s, 72 °C for 30 s, 35 cycles; 72 °C for 5 min; BoHV-1 gG PCR reaction conditions: 95 °C for 3 min, 95 °C for 15 s, 60.5 °C for 15 s, 72 °C for 40 s, 35 cycles, 72 °C for 5 min; BoHV-1 tk PCR reaction conditions: 95 °C for 3 min; 95 °C for 15 s, 60.5 °C for 15 s, 72 °C for 30 s, 35 cycles, 72 °C for 5 min. The primers used in this study are listed in Table 2.

Table 2.
Detailed primer sequences.
To measure the shedding of M. bovis HB0801 post-challenge, the nasal swabs subjected to treatment were progressively diluted by a factor of 10. Subsequently, 100 μL from each dilution was added to a PPLO liquid medium and incubated at 37 °C in a 5% CO2 incubator for 3–5 days or until no further change in the medium color occurred. The highest dilution showing a color change was 1 CCU/mL. The counting of M. bovis HB0801 shedding was then determined based on the dilution factor. BoHV-1 HB06 shedding after the challenge was detected from the DNA extracted from nasal swabs used for RT-PCR using the envelope glycoprotein B gB gene. The program for BoHV-1 gB gene was as follows: 95 °C for 30 s, 95 °C for 10 s, 60 °C for 20 s, 40 cycles; 95 °C for 15 s, 60 °C for 20 s, 95 °C for 15 s.
2.6. Serum Antibody of M. bovis
Serum antibodies against M. bovis were detected using competitive ELISA, following established protocols. Briefly, the test serum, positive control, and negative control sera, all diluted fourfold, were added to plates coated with M. bovis p579 protein and HRP-labeled monoclonal antibodies. The plates were then incubated at 37 °C for 60 min. After washing, 100 µL of chromogenic substrate solution was added and incubated at room temperature, shielded from light, for 10 min. The OD450 nm was promptly measured upon halting the reaction. The blocking rate (P.I. value) was calculated as follows:
Blocking rate = (1 − S/N) × 100%, S = sample OD450 nm; N = mean OD450 nm of negative control serum. Conditions for the establishment of the test: 0.65 < OD450 nm negative control < 2.0, PIpositive control > 60%. PIsample ≥ 41% means positive; PIsample < 41% means negative.
2.7. Neutralization Assay
Inactivated serum (56 °C for 30 min) was serially diluted in a 96-well cell culture plate and then incubated with 100 TCID50 BoHV-1 HB06 virus at 37 °C in a 5% CO2 incubator for 1 h. The serum–virus mixture was then transferred to a 96-well cell culture plate containing MDBK cells and cultured in a 5% incubator at 37 °C for three days. Neutralizing antibody titers, the highest serum dilutions that inhibit BoHV-1 infection, were calculated using the Reed–Muench method.
2.8. Detection of Cytokines and ELISA Antibodies
Commercial ELISA kits (Jiangsu Meimian Industrial Co., Ltd., Yancheng, China) were used to detect changes in serum cytokine and antibody levels, including IL-1β, IL-6, TNF-α, IFN-γ, sIgA, and IgG.
2.9. Calculation of Protective Efficacy
The immunoprotective rate of the attenuated and marker M. bovis–BoHV-1 combined vaccine after challenging with M. bovis HB0801 and BoHV-1 HB06 was calculated using the following equation:
SPC, SVAC, and SNC represent the mean of the sum of clinical symptom scores and lung pathological changes in the rabbits in the non-immune challenge group, immune challenge group, and control group, respectively.
2.10. Statistical Analysis
Student’s t-test and one-way ANOVA were used to detect significant differences between groups, where p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), or p < 0.0001 (****) were considered to be statistical differences. * represents there exist a difference, but the difference is not significant; **, *** and **** represent the significant degree of the differences, respectively. Error bars indicate the standard error of the mean.
3. Results
3.1. Clinical Signs
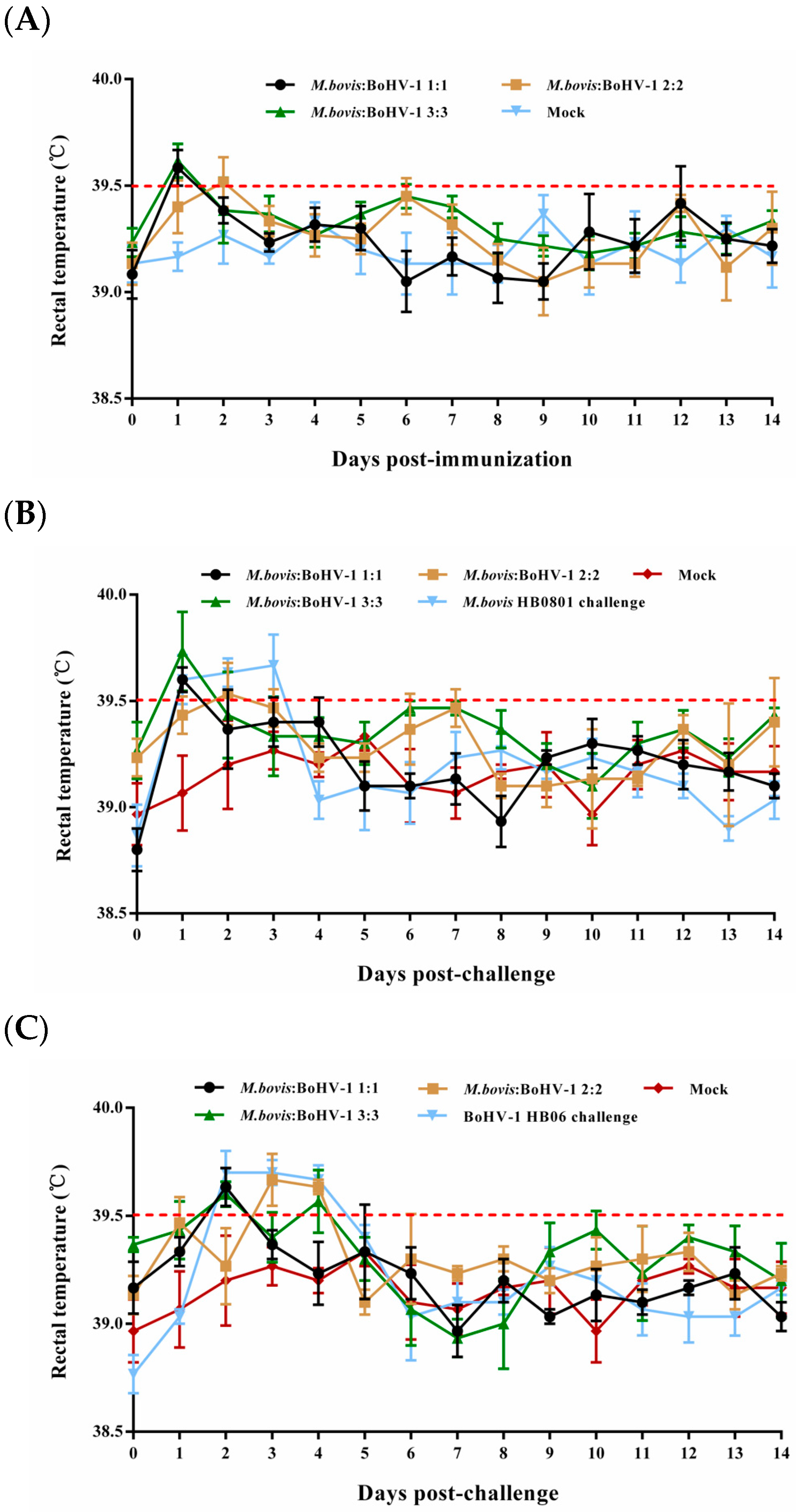
Most rabbits in the immunized groups had normal rectal temperature without obvious respiratory signs during the entire observation period; only some underwent slight rectal temperature elevation one or two days after immunization and returned to normal (Figure 1A). In the unimmunized but challenged groups, the rectal temperatures of the rabbits surpassed 39.5 °C on days 1–3 and 2–4 following the challenge with BoHV-1 HB06 and M. bovis HB0801, respectively (Figure 1B,C). All animals in these two groups showed clinical signs such as coughing, nasal secretion, and ocular secretions. These results confirmed that rabbits were successfully challenged with M. bovis HB0801 and BoHV-1 HB06 and that the developed vaccine was safe.
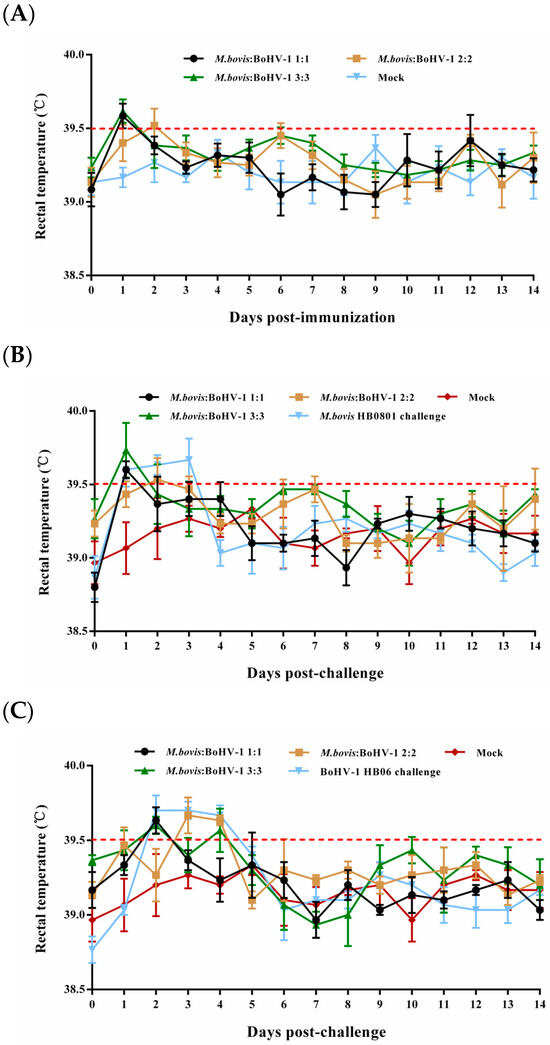
Figure 1.
Illustrates the rectal temperature of experimental rabbits before (A) and after challenge with M. bovis HB0801 (B) and BoHV-1 HB06 (C). Each data point represents the average rectal temperature of all rabbits in the experimental group on that day, and the red dotted line represents the upper limit of normal rectal temperature of rabbits.
3.2. Detection of M. bovis and BoHV-1 Shedding
3.2.1. M. bovis HB150 and BoHV-1 gG-/tk- Shedding after Immunization
Assays performed on the samples collected from groups immunized with an immunization dose 1:1 showed that rabbits stopped shedding M. bovis HB150 on the 5th day after immunization, and the 2:2 and 3:3 groups stopped shedding on the 6th and 7th days, respectively. In addition, the analysis showed that in the 1:1 group, over 50% of the animals exhibited continuous shedding on day 3 post-immunization. The condition lasted one more day in the 2:2 and 3:3 immunization groups (Table 3).

Table 3.
Shedding of M. bovis HB150 and BoHV-1 gG-/tk- after immunization.
When the collected nasal swabs were collected and analyzed, we found that no shedding of BoHV-1 gG-/tk- was detected in the 2:2 group on day 8 post-immunization, and the 1:1 and 3:3 groups stopped shedding 9 days post-immunization. The control group consistently showed no signs of viral shedding during observation. On day 5 post-immunization, more than 50% of animals in all immunized groups continued to shed (Table 3).
Therefore, among all mixed immunization groups, the 3:3 mixed immunization group exhibited the highest and most sustained levels of shedding for both pathogens.
3.2.2. M. bovis HB0801 and BoHV-1 HB06 Shedding after Challenge
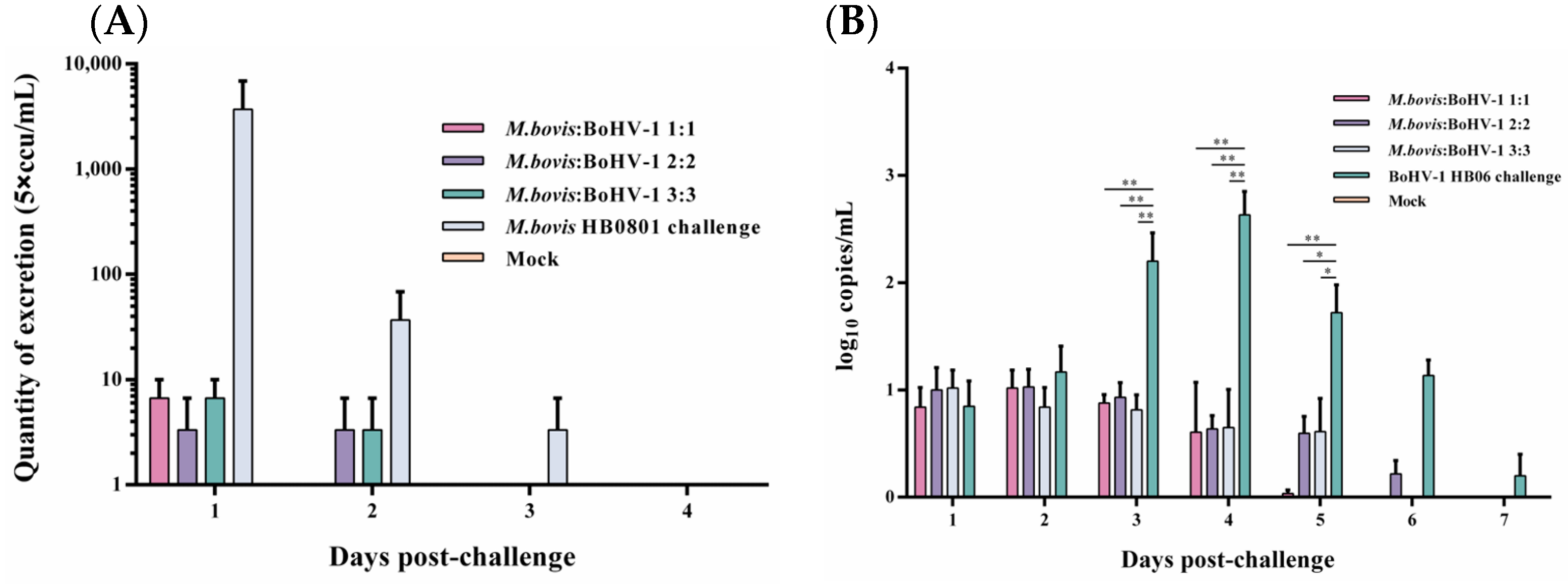
Following the challenge with M. bovis HB0801, nasal swab samples were collected and analyzed everyday, and the conclusion was that only a small quantity of M. bovis HB0801 shedding was observed in the 1:1 group on the initial day, and no shedding was detected on the second day post-challenge. During the initial 1–2 days, all mixed-vaccine immunization groups displayed a decrease in M. bovis HB0801 shedding compared to the non-immune-challenged group. Despite this, no statistically significant differences were observed between the vaccinated and non-immune-challenged groups during this period (Figure 2A).
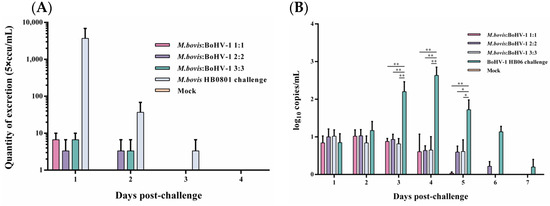
Figure 2.
Detection of M. bovis HB0801 (A) and BoHV-1 HB06 (B) shedding in nasal swabs after challenge.
BoHV-1 HB06 shedding after the challenge was measured using RT-PCR. When we processed and analyzed the nasal swabs samples collected daily, we found that high titers of BoHV-1 HB06 were detected in the non-immune-challenged group from day 3 to day 6 and peaked on day 4 after the challenge. In contrast, in different immunization dose groups, all immunized groups exhibited lower BoHV-1 HB06 shedding compared to the nonimmune-challenged group on days 3–5 after the challenge (p < 0.05). Importantly, no significant difference was found between immunized groups during the entire observation period (Figure 2B). Ultimately, the 1:1 group exhibited the lowest shedding compared to the other mixed immunization groups following the challenge with either M. bovis HB0801 or BoHV-1 HB06.
3.3. Antibody Response
3.3.1. M. bovis Serum Antibody Levels
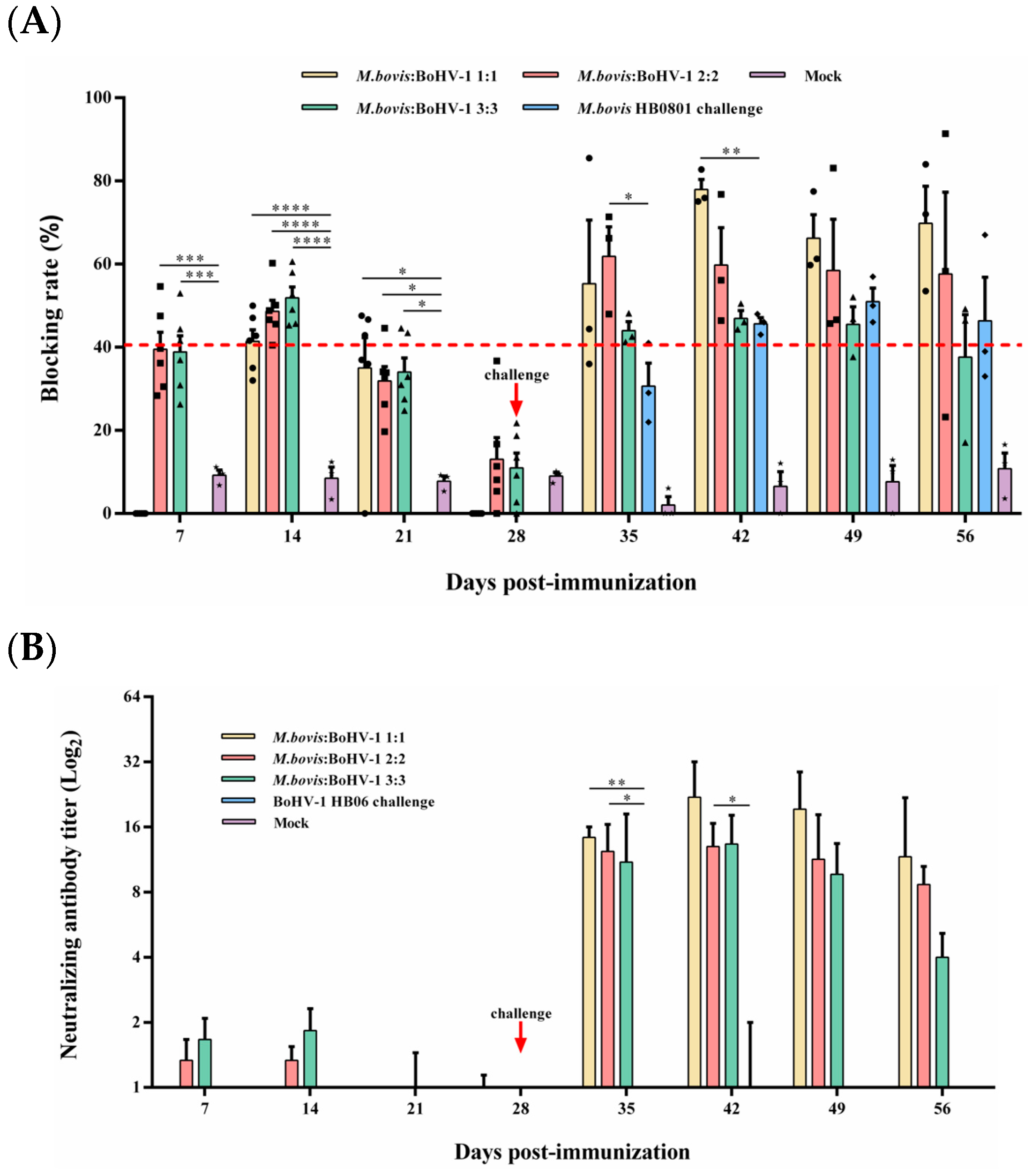
Competitive ELISA was used to measure the level of M. bovis serum antibodies. The antibody level of the 2:2 and 3:3 groups was significantly higher than that of the control from days 7 to 21 after immunization (p < 0.05), and the blocking rate reached 48.6% and 51.9% at day 14 after immunization, respectively. From day 7 to 14 post-challenge, the 2:2 and 1:1 groups displayed substantially higher antibody titer compared to the non-immunized but challenged groups, respectively (p < 0.05) (Figure 3A); at 14 days post-challenge, the 1:1 group had the highest blocking rate during the entire observation period, which could reach 77.9%.
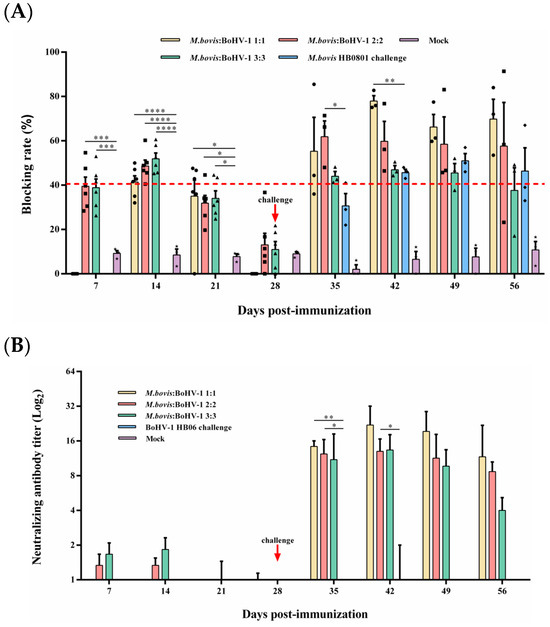
Figure 3.
Humoral immune responses induced by M. bovis–BoHV-1 combined vaccine. Weekly serum samples were collected for analysis. (A) M. bovis serum antibody and (B) BoHV-1 serum neutralizing antibody.
3.3.2. BoHV-1-Neutralizing Antibody Response
BoHV-1-neutralizing antibodies in rabbits were quantified using a serum neutralization assay. All immunized groups found low levels of neutralization titer until 7 days post-challenge; at this time, the 1:1 and 2:2 groups induced significantly higher neutralization titer than the non-immunized but challenged group (p < 0.05). At 14 days after the challenge, the 1:1 group caused the highest levels of neutralizing antibody titer in all experimental groups during the observation period, which reached 1:22. Rabbits in the control group did not produce any neutralizing antibodies throughout the experimental period (Figure 3B). In summary, the 1:1 mixed-immunization group can induce the highest and most sustained M. bovis and BoHV-1 antibody titers among all immunization groups.
3.4. M. bovis–BoHV-1 Combined Vaccine Induces Cellular Immunity in Rabbits
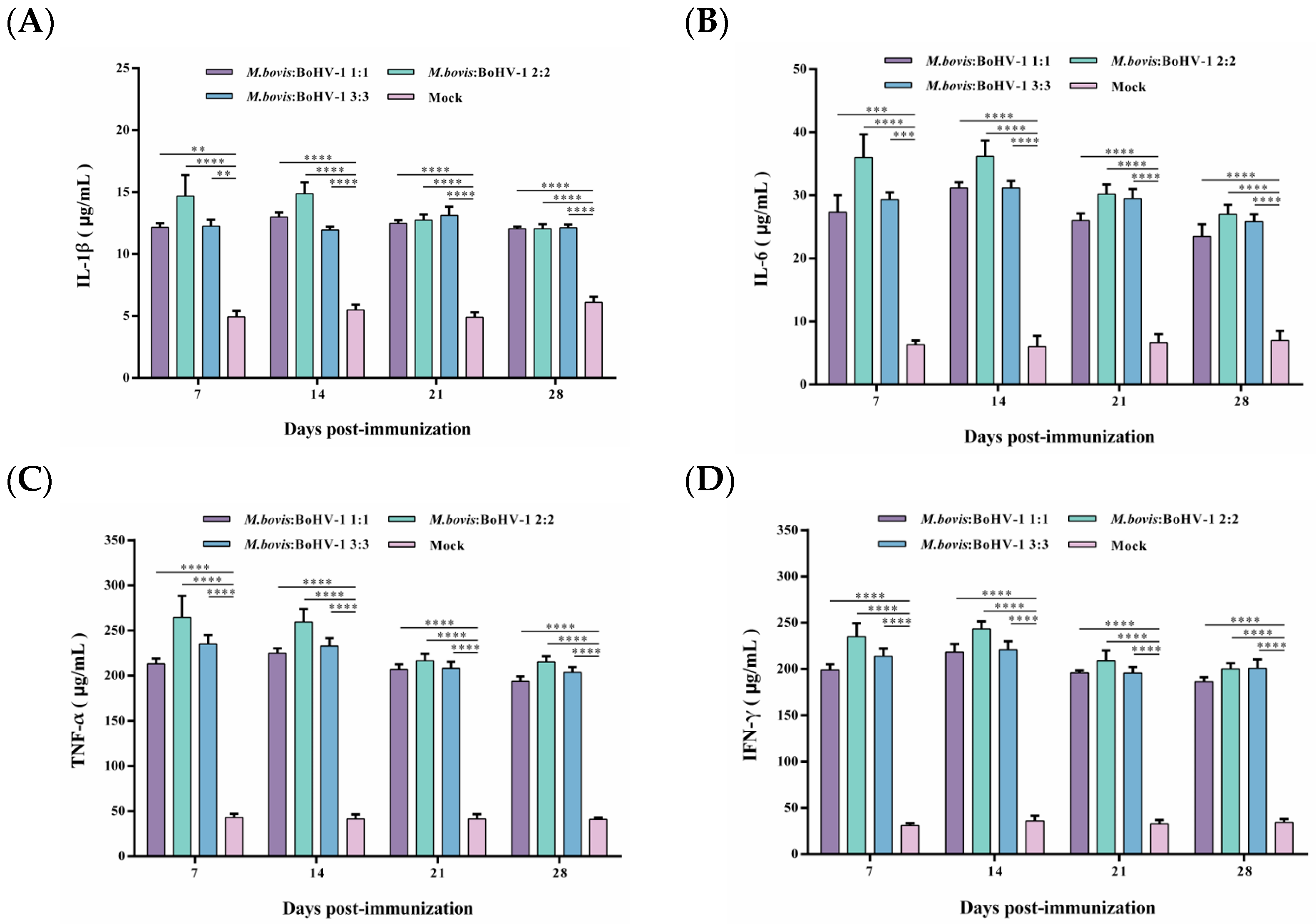
After vaccination, the TNF-α and IFN-γ levels were significantly higher in all immunized groups, and levels of IL-1β and IL-6 slightly increased. Still, the levels of these cytokines in the immunized groups were considerably higher than those in the control group (p < 0.01). Notably, the 2:2 group showed levels as high as 250 μg/mL and 200 μg/mL of TNF-α and IFN-γ from 7 to 14 days post-immunization, respectively, inducing the most robust cellular immune response of all groups. Thus, after immunization, the M. bovis–BoHV-1 combined vaccine represented by the 2:2 group induced a mixed Th1/Th2 response biased toward Th1 in rabbits (Figure 4A–D).
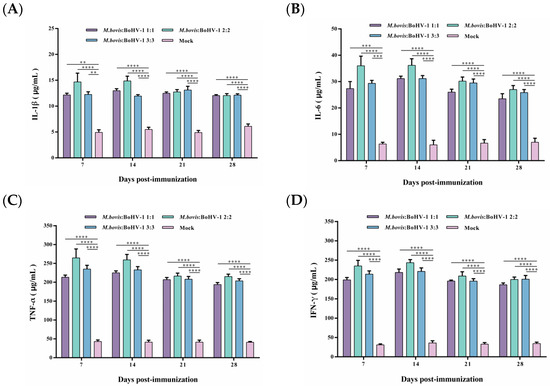
Figure 4.
Displays the production of IL-1β (A), IL-6 (B), TNF-α (C), and IFN-γ (D) post-immunization. Commercial ELISA kits were utilized to detect these cytokines. The results are presented as means ± SEM.
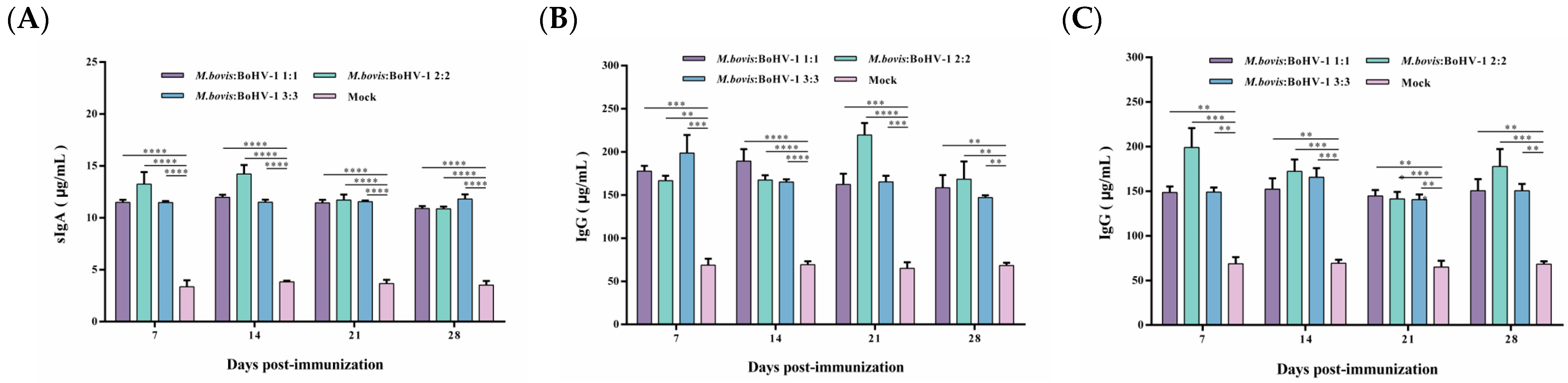
3.5. sIgA and IgG Titers in Rabbits
To evaluate the vaccine-induced systemic immune response, serum IgA and IgG antibody levels were measured. The vaccine can produce sIgA antibodies after immunization, with the highest antibody levels induced in the 2:2 group, indicating that the vaccine can cause a mucosal immune response. The sIgA antibody levels in all vaccinated groups were significantly higher than those of the control during the whole observation period (p < 0.0001) (Figure 5A).
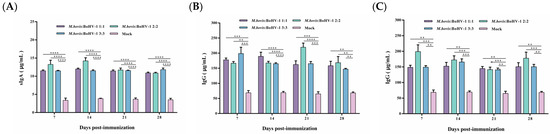
Figure 5.
Post-immunization sIgA (A) and post-challenge IgG antibody levels after M. bovis HB0801 (B) and BoHV-1 HB06 (C) challenge were monitored.
IgG plays a key role in the secondary immune response. Here, vaccinated rabbits rapidly produced high levels of IgG antibodies after the challenge. IgG antibody levels dramatically increased in the immunized groups challenged by M. bovis HB0801, with the most pronounced elevation to 220 μg/mL in the 2:2 group at 21 days post-challenge. During the whole observation period, the IgG levels in all vaccinated groups were significantly higher than those in the control group (p < 0.01) (Figure 5B,C). Following exposure to BoHV-1 HB06, the immunized groups exhibited elevated levels of IgG antibodies. Notably, the IgG levels induced in all vaccinated groups were significantly higher than the control group during the experimental period (p < 0.01). Overall, the 2:2 group induced the highest levels of sIgA and IgG antibodies after immunization and challenge, respectively.
3.6. Evaluation of Micro Pathological Injury
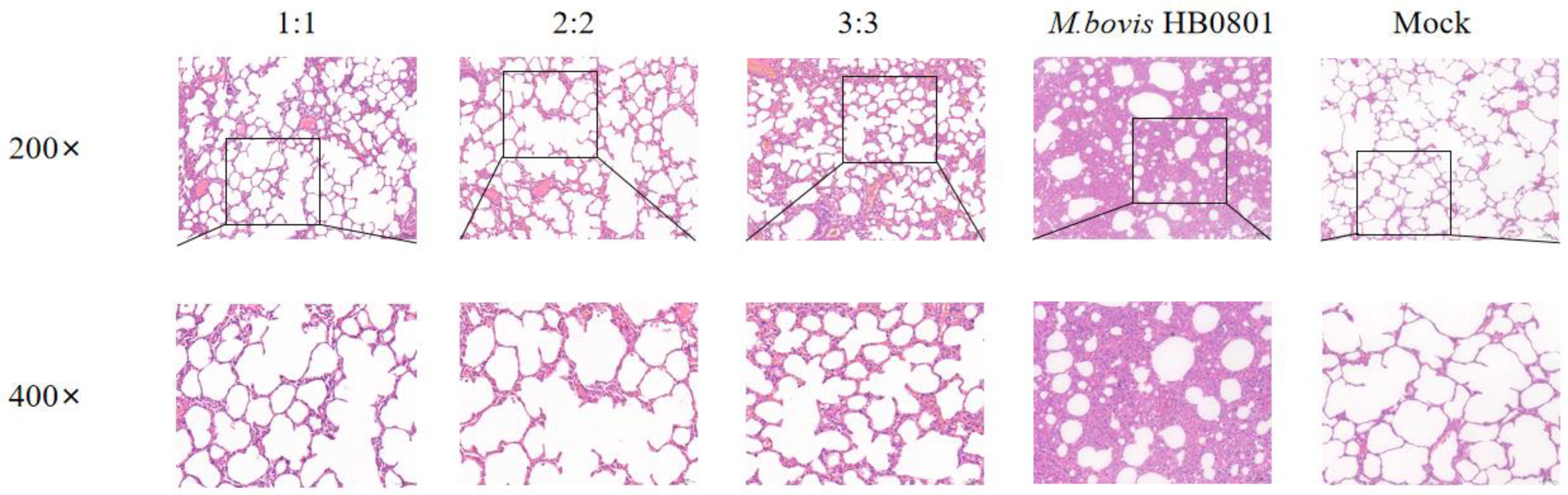
After M. bovis HB0801 challenge, the alveolar structure was relatively complete, with no apparent pathological alterations in the alveolar wall and alveolar cavity. Only a small amount of plasmacytic exudation with inflammatory cells was found in the alveolar cavity in immunized groups, and there were varying degrees of alveolar wall hyperplasia but little disruption of the structure; especially in the 3:3 group, after observing the lung structure in this experimental group, we found that the alveolar structure was slightly damaged. In contrast, after analysis of the lung structure of unvaccinated rabbits challenged with M. bovis, HB0801 presented the most severe lesions and loss of normal lung tissue structure, interstitial hyperplasia, and inflammatory infiltrates; the alveoli exhibited a size reduction or fused to form larger alveolar cavities, leading to a decrease in the overall number of alveoli (Figure 6).
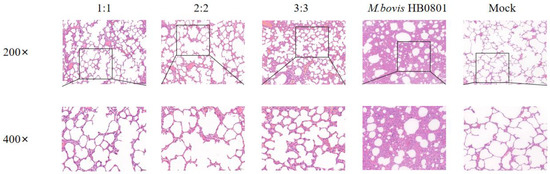
Figure 6.
Histopathological images of lung tissues after M. bovis HB0801 challenge tainted by H&E. The scale sizes are 100 μm (top) and 50 μm (bottom), respectively. The figure below is an enlargement of part of the area of the upper figure.
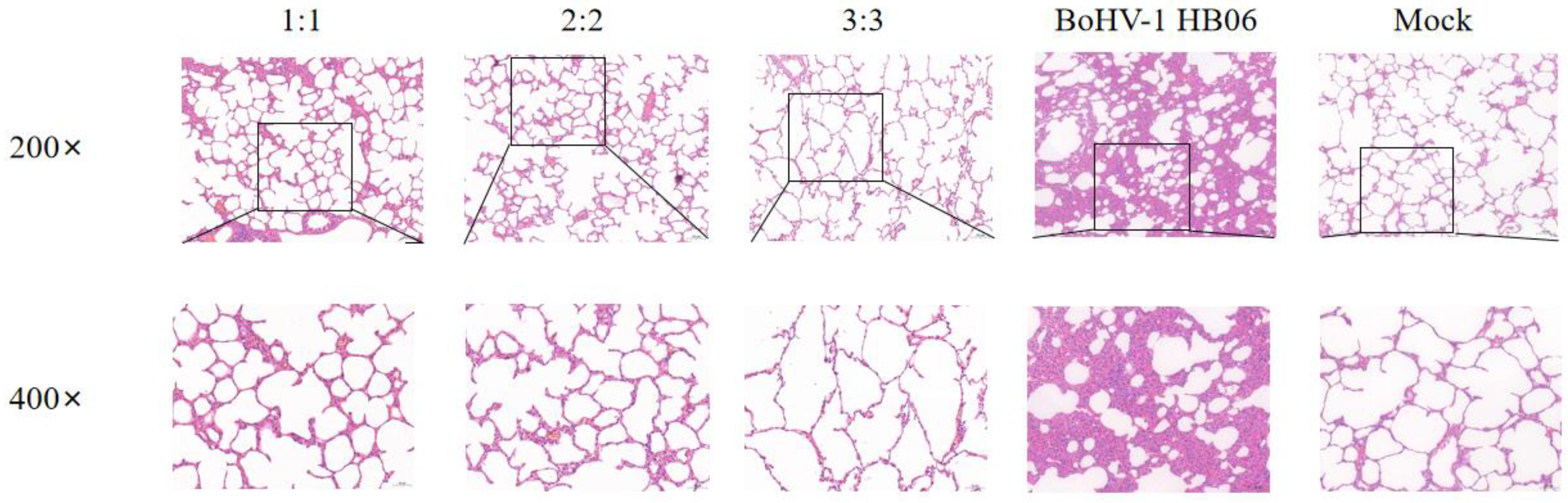
In the groups challenged with BoHV-1 HB06, the alveolar structures in all vaccinated groups mainly stayed intact, showing only a minor thickening of the alveolar wall and a small presence of plasmacytic infiltration in the alveolar cavity. Only the alveoli in the 3:3 group fused into larger alveolar cavities after the challenge. In contrast, the unimmunized but challenged group experienced severe lesions, and the alveolar structure was destroyed with interstitial hyperplasia with inflammatory cell exudation (Figure 7). After exposure to M. bovis HB0801 and BoHV-1 HB06, the tissue from the control group was sampled and analyzed, and the results displayed a regular alveolar structure, showing clean alveolar cavities and smooth alveolar walls, without any signs of inflammatory exudate. The lung tissue morphology in immunized rabbits was more intact than in unimmunized rabbits. Ultimately, the vaccine provided sufficient protection, particularly in the 2:2 group.
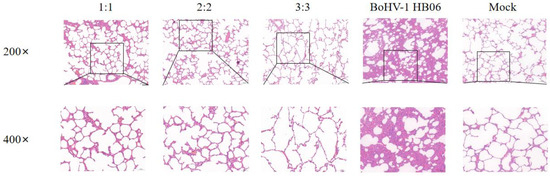
Figure 7.
Histopathological images of lung tissues after BoHV-1 HB06 challenge tainted by H&E. The scale sizes are 100 μm (top) and 50 μm (bottom), respectively. The figure below is an enlargement of part of the area of the upper figure.
3.7. Calculation of Protective Rate of the Attenuated and Marker M. bovis–BoHV-1 Combined Vaccine after Challenge
As described in the Materials and Methods Section, we calculated the protective rate after M. bovis HB0801 and BoHV-1 HB06 challenge. As demonstrated in Table 4 and Table 5, the 2:2 immunization dose group provided the best protection when challenged with M. bovis HB0801, and its protection rate reached 75.7%. When experimental groups were challenged with BoHV-1 HB06, the 2:2 immunization dose group had the highest protection rate, as high as 87.6%. When facing the challenge of two pathogens, the protection rate of the 2:2 immunization dose group was higher than that of the 1:1 group by 3.9% and 3.7%, respectively, showing the best immunoprotection efficacy.

Table 4.
Protective rate after M. bovis HB0801 challenge.

Table 5.
Protective rate after BoHV-1 HB06 challenge.
4. Discussion
Bovine respiratory disease (BRD) is considered to be among the most complex mammalian diseases. It involves stress immunosuppression, one or more viral/bacterial infections, and ultimately causes bronchopneumonia. The term bovine respiratory disease can encompass a range of pneumonic diseases, from acute fatal respiratory disease to chronic long-term respiratory disease, in which chronic, necrotizing pneumonia is commonly associated with M. bovis, and M. bovis is also one of the most common microbial pathogens in cases of lethal BRD [17]. Animal performance and economic efficiency decrease significantly as BRD treatments increase. Furthermore, animals treated with more than one BRD may lack the initial therapeutic effect and develop more severe BRD cases [7]. Although respiratory mucosal epithelial cells are not generally included in the immune system, they play a key role in the first line of defense against infection [10]. Viral pathogens such as BoHV-1 cause ciliary dysfunction, and the temporary immunosuppression induced by it makes animals more susceptible to secondary bacterial infections, leading to BRD. We hypothesized that the coexistence of M. bovis with bovine respiratory viruses such as BoHV-1 leads to changes in the respiratory micro-ecological environment. Under these conditions, the virulence of pathogenic microorganisms in vivo also increases.
Vaccination is the most direct and effective way to prevent and control BRD, but challenges remain in vaccine development because BRD is multipathogenic. Therefore, combined and multivalent vaccines must be developed against different pathogens. Several monovalent or multivalent vaccines are currently available to prevent BRD [18,19,20]. Modified live virus (MLV) vaccines induce robust and long-lasting humoral and cellular immunity and only require a smaller dose to provide protection [21,22]. Thus, the MLV vaccine provides better clinical protection against BRD. However, combined live and marker vaccines are not yet available.
In a previous study, we developed an attenuated and marker M. bovis–BoHV-1 combined vaccine for the first time. We conducted a comprehensive evaluation of the vaccine using a rabbit model, and the results confirmed its clinical safety and robust protective efficacy [12]. In this study, we further investigated the optimal immunizing dose of the combined vaccine.
Identifying pathogen shedding during the challenging period is pivotal for diagnosing disease [23]. For M. bovis, the 1:1 dose of the vaccinated group could effectively inhibit the M. bovis HB0801 shedding on the second day after the challenge, and the other doses of the immunized group subsequently failed to detect the shed of M. bovis HB0801 by day 3. As for pathogen shedding in the nasal cavity after BoHV-1 challenge, the experimental groups with 1:1 and 3:3 immunization doses displayed deficient shedding levels on the sixth day after challenge, and the vaccinated group with the 2:2 immunization dose had undetectable BoHV-1 shedding on the seventh day after challenge. Our rabbit model results are consistent with previous studies. Following a 2- to 4-day incubation period post-challenge with BoHV-1, viral replication occurs in the mucosal and tonsillar tissues, resulting in clinical manifestations in cattle and subsequent virus shedding within 3 to 10 days post-infection [24]. Subsequently, we assessed antibody titers against BoHV-1 and M. bovis and serum-specific IgA and IgG antibody levels. The vaccine-induced antibody response is one of the most important immunological factors against infection. It has also been revealed that gene-deletion vaccines can produce high titers of antibodies after BoHV-1 challenge [25]. sIgA is the most critical antibody for local mucosal immunity. It determines the resistance of the respiratory mucosa to pathogens, whereas systemic humoral immunity depends on IgG and plays an essential role in anti-infective immunity. As expected, when these rabbits vaccinated with different immunizing doses were challenged, high titers of antibodies against both pathogens and high levels of serum-specific antibodies were produced simultaneously, indicating that the attenuated and marker M. bovis–BoHV-1 combined vaccine induced a specific humoral immune response along with an effective mucosal immune response. Among all rabbits, the immunized group with a 2:2 immunization dose had the best overall performance, consistent with our speculation that the vaccine’s protective efficacy is positively correlated with the immunization dose. However, the results showed no statistical difference between the 2:2 and the 1:1 immunization dose group.
Moreover, we measured the expression of vaccine-induced cytokines to assess the cellular immune response associated with protection. The homeostatic balance between cytokines plays a key role in the pathogenesis of viral and bacterial infections, including BRD [8,10]. TNF-α is a pro-inflammatory cytokine that can recruit leukocytes to areas of infection [26]. It stands out as one of the key inflammatory mediators essential in initiating and pathogenesis of pulmonary fibrosis [27]. IFN-γ has antiviral effects necessary for innate and adaptive immune responses [28]. Furthermore, the level of IFN-γ secretion was positively correlated with the reduction in clinical signs in infected animals [29]. The production of Th1-type cytokines such as TNF-α and IFN-γ combat intracellular pathogens, and these cytokines also modulate other small-molecule proteins of the immune system through potent antiviral activity and promotion of other immune effector functions [30]. In this study, rabbits immunized with different doses of the vaccine produced high levels of IL-1β, IL-6, TNF-α, and IFN-γ, most notably in TNF-α and IFN-γ. Our attenuated and marker M. bovis–BoHV-1 combined vaccine can induce a Th1-biased cellular immune response in animals after immunization. The 2:2 group produced the best immunoprotected response among all immunized groups, but no significant difference existed between all experimental groups.
5. Conclusions
In conclusion, the optimal immunization dose of the attenuated and marker M. bovis–BoHV-1 combined vaccine was determined in this study; the 2:2 immunization dose group showed the best performance in humoral and cellular immune responses, although there was no significant difference between it and the other immunization dose groups. In addition, from the results of lung histopathological sections and the quantification of immunoprotective rate, the 2:2 vaccinated groups had a nearly standard lung tissue structure and were able to provide the best protective efficacy in facing the challenge with both pathogens. So, we considered that the optimal immunization dose of the vaccine is 2.0 × 108 CFU M. bovis HB150 with 2.0 × 106 TCID50 BoHV-1 gG-/tk-. One problem was that the 3:3 immunization dose group was not as immunoprotective as the other experimental groups. Our argument stems from the premise that the immunization doses, calibrated according to bovine standards, may have led to an overdose in rabbits. The 3:3 immunization dose group did not produce excellent protective efficacy precisely because of the side effects of overdose immunization. Further bovine experiments are needed to determine the actual clinical application of our study.
Author Contributions
Conceptualization, S.Z., L.Y., A.G. and Y.C.; methodology, S.Z., G.L., W.W. and I.S.; Software, S.Z.; Validation, G.L., W.W. and L.Y.; Investigation, S.Z., G.L., W.W. and I.S.; Resources, S.Z. and G.L.; Writing—original draft preparation, S.Z. and I.S.; Writing—review and editing, A.G. and Y.C.; Supervision, A.G. and Y.C.; Project administration, L.Y., A.G. and Y.C.; Funding acquisition, A.G. and Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (#2023YFD1802502), the Key Research and Development Program of the Ningxia Hui Autonomous Region (#2023BCF01038), and the China Agriculture Research System of MOF and MARA (CARS-37).
Institutional Review Board Statement
The research adhered to the principles of the Declaration of Helsinki and received approval from the Animal Experiment Ethics Committee of Huazhong Agricultural University. The study was conducted strictly within the Guidelines for the Care and Use of Laboratory Animals of Wuhan, Hubei, China. Huazhong Agricultural University Ethics Approval Number: HZAURAB-2024-0001.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
Li Yang is employed by the Wuhan Keqian Biology Co., Ltd., Wuhan, China. The remaining authors declare that they have no conflicts of interest.
References
- Nicola, I.; Cerutti, F.; Grego, E.; Bertone, I.; Gianella, P.; D’Angelo, A.; Peletto, S.; Bellino, C. Characterization of the upper and lower respiratory tract microbiota in Piedmontese calves. Microbiome 2017, 5, 152. [Google Scholar] [CrossRef]
- Loneragan, G.H.; Dargatz, D.A.; Morley, P.S.; Smith, M.A. Trends in mortality ratios among cattle in US feedlots. J. Am. Vet. Med. Assoc. 2001, 219, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Edwards, T.A. Control Methods for Bovine Respiratory Disease for Feedlot Cattle. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Dudek, K.; Szacawa, E. Infections: Occurrence, Pathogenesis, Diagnosis and Control, Including Prevention and Therapy. Pathogens 2020, 9, 994. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, N.N.; Inzana, T.J.; Rajagopalan, P. Bovine Airway Models: Approaches for Investigating Bovine Respiratory Disease. ACS Infect. Dis. 2023, 9, 1168–1179. [Google Scholar] [CrossRef] [PubMed]
- Dubrovsky, S.A.; Van Eenennaam, A.L.; Aly, S.S.; Karle, B.M.; Rossitto, P.V.; Overton, M.W.; Lehenbauer, T.W.; Fadel, J.G. Preweaning cost of bovine respiratory disease (BRD) and cost-benefit of implementation of preventative measures in calves on California dairies: The BRD 10K study. J. Dairy Sci. 2020, 103, 1583–1597. [Google Scholar] [CrossRef] [PubMed]
- Blakebrough-Hall, C.; McMeniman, J.P.; González, L.A. An evaluation of the economic effects of bovine respiratory disease on animal performance, carcass traits, and economic outcomes in feedlot cattle defined using four BRD diagnosis methods. J. Anim. Sci. 2020, 98, skaa005. [Google Scholar] [CrossRef]
- Ellis, J.A. The immunology of the bovine respiratory disease complex. Vet. Clin. N. Am.-Food Anim. Pract. 2001, 17, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Zhang, J.H.; Chen, X.D.; Wei, X.; Wu, C.X.; Cui, Q.; Hao, Y.Q. Investigation of viral pathogens in cattle with bovine respiratory disease complex in Inner Mongolia, China. Microb. Pathog. 2021, 153, 104594. [Google Scholar] [CrossRef]
- McGill, J.L.; Sacco, R.E. The Immunology of Bovine Respiratory Disease Recent Advancements. Vet. Clin. N. Am.-Food Anim. Pract. 2020, 36, 333–348. [Google Scholar] [CrossRef]
- Klima, C.L.; Zaheer, R.; Cook, S.R.; Booker, C.W.; Hendrick, S.; Alexander, T.W.; McAllister, T.A. Pathogens of Bovine Respiratory Disease in North American Feedlots Conferring Multidrug Resistance via Integrative Conjugative Elements. J. Clin. Microbiol. 2014, 52, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, Y.S.; Liu, G.X.; Wang, C.; Ji, Y.; Chen, J.G.; Hu, C.M.; Chen, X.; Guo, A.Z.; Chen, Y.Y.; et al. The Safety and Protective Efficacy Evaluation of an Attenuated-BoHV-1 Bivalent Vaccine in Rabbits. Vaccines 2023, 11, 1698. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.J.; Guo, A.Z.; Cui, P.; Chen, Y.Y.; Mustafa, R.; Ba, X.L.; Hu, C.M.; Bai, Z.D.; Chen, X.; Shi, L.; et al. Comparative Geno-Plasticity Analysis of Mycoplasma bovis HB0801 (Chinese Isolate). PLoS ONE 2012, 7, e38239. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Wang, X.; Qi, Y.P.; Wen, X.B.; Li, C.X.; Liu, X.B.; Ni, H.B. Meta-analysis of prevalence of bovine herpes virus 1 in cattle in Mainland China. Acta Trop. 2018, 187, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Han, X.X.; Chen, Y.Y.; Mustafa, R.; Qi, J.J.; Chen, X.; Hu, C.M.; Chen, H.C.; Guo, A.Z. Attenuated Mycoplasma bovis strains provide protection against virulent infection in calves. Vaccine 2014, 32, 3107–3114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.M.; Fu, S.L.; Deng, M.L.; Xie, Q.; Xu, H.Y.; Liu, Z.F.; Hu, C.M.; Chen, H.C.; Guo, A.Z. Attenuation of bovine herpesvirus type 1 by deletion of its glycoprotein G and tk genes and protection against virulent viral challenge. Vaccine 2011, 29, 8943–8950. [Google Scholar] [CrossRef] [PubMed]
- Booker, C.W.; Abutarbush, S.M.; Morley, P.S.; Jim, G.K.; Pittman, T.J.; Schunicht, O.C.; Perrett, T.; Wildman, B.K.; Fenton, R.K.; Guichon, P.T.; et al. Microbiological and histopathological findings in cases of fatal bovine respiratory disease of feedlot cattle in western Canada. Can. Vet. J.-Rev. Vet. Can. 2008, 49, 473–481. [Google Scholar]
- Ellis, J.A.; Gow, S.P.; Goji, N.; Jones, C.; Workman, A.; Henderson, G.; Rhodes, C.; Alaniz, G.; Meinert, T.R.; Tucker, C.M. Efficacy of a combination viral vaccine for protection of cattle against experimental infection with field isolates of bovine herpesvirus-1. JAVMA-J. Am. Vet. Med. Assoc. 2009, 235, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Brock, K.V.; Widel, P.; Watz, P.; Walz, H.L. Onset of protection from experimental infection with type 2 bovine miral diarrhea virus following vaccination with a modified-live vaccine. Vet. Ther. 2007, 8, 88–96. [Google Scholar] [PubMed]
- Palomares, R.A.; Givens, D.; Wright, J.C.; Walz, P.H.; Brock, K.V. Evaluation of the onset of protection induced by a modified-live virus vaccine in calves challenge inoculated with type 1b bovine viral diarrhea virus. Am. J. Vet. Res. 2012, 73, 567–574. [Google Scholar] [CrossRef]
- Ellis, J.; Gow, S.; Bolton, M.; Burdett, W.; Nordstrom, S. Inhibition of priming for bovine respiratory syncytial virus-specific protective immune responses following parenteral vaccination of passively immune calves. Can. Vet. J.-Rev. Vet. Can. 2014, 55, 1180–1185. [Google Scholar]
- Mahan, S.M.; Sobecki, B.; Johnson, J.; Oien, N.L.; Meinert, T.R.; Verhelle, S.; Mattern, S.J.; Bowersock, T.L.; Leyh, R.D. Efficacy of intranasal vaccination with a multivalent vaccine containing temperature-sensitive modified-live bovine herpesvirus type 1 for protection of seronegative and seropositive calves against respiratory disease. JAVMA-J. Am. Vet. Med. Assoc. 2016, 248, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.C.; Shen, H.Y.; Cheng, L.T.; Liu, S.S.; Chu, C.Y. Effectiveness of a BHV-1/BEFV bivalent vaccine against bovine herpesvirus type 1 infection in cattle. Res. Vet. Sci. 2016, 109, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Salt, J.S.; Thevasagayam, S.J.; Wiseman, A.; Peters, A.R. Efficacy of a quadrivalent vaccine against respiratory diseases caused by BHV-1, PI3V, BVDV and BRSV in experimentally infected calves. Vet. J. 2007, 174, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Belknap, E.B.; Walters, L.M.; Kelling, C.; Ayers, V.K.; Norris, J.; McMillen, J.; Hayhow, C.; Cochran, M.; Reddy, D.N.; Wright, J.; et al. Immunogenicity and protective efficacy of a gE, gG and US2 gene-deleted bovine herpesvirus-1 (BHV-1) vaccine. Vaccine 1999, 17, 2297–2305. [Google Scholar] [CrossRef] [PubMed]
- Gondaira, S.; Higuchi, H.; Iwano, H.; Nakajima, K.; Kawai, K.; Hashiguchi, S.; Konnai, S.; Nagahata, H. Cytokine mRNA profiling and the proliferative response of bovine peripheral blood mononuclear cells to Mycoplasma bovis. Vet. Immunol. Immunopathol. 2015, 165, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Bringardner, B.D.; Baran, C.P.; Eubank, T.D.; Marsh, C.B. The role of inflammation in the pathogenesis of idiopathic pulmonary fibrosis. Antioxid. Redox Signal. 2008, 10, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-γ: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef]
- Risalde, M.A.; Molina, V.; Sánchez-Cordón, P.J.; Pedrera, M.; Panadero, R.; Romero-Palomo, F.; Gómez-Villamandos, J.C. Response of proinflammatory and anti-inflammatory cytokines in calves with subclinical bovine viral diarrhea challenged with bovine herpesvirus-1. Vet. Immunol. Immunopathol. 2011, 144, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Ban, Y.; Wei, F.; Ma, X.J. Regulation of Interleukin-12 Production in Antigen-Presenting Cells. Adv. Exp. Med. Biol. 2016, 941, 117–138. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).