Repeated Cross-Sectional Survey of Ectoparasites in Sheep from Central Tunisia: Does Low Prevalence Indicate Good Hygiene or Resistance to Ectoparasites?
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
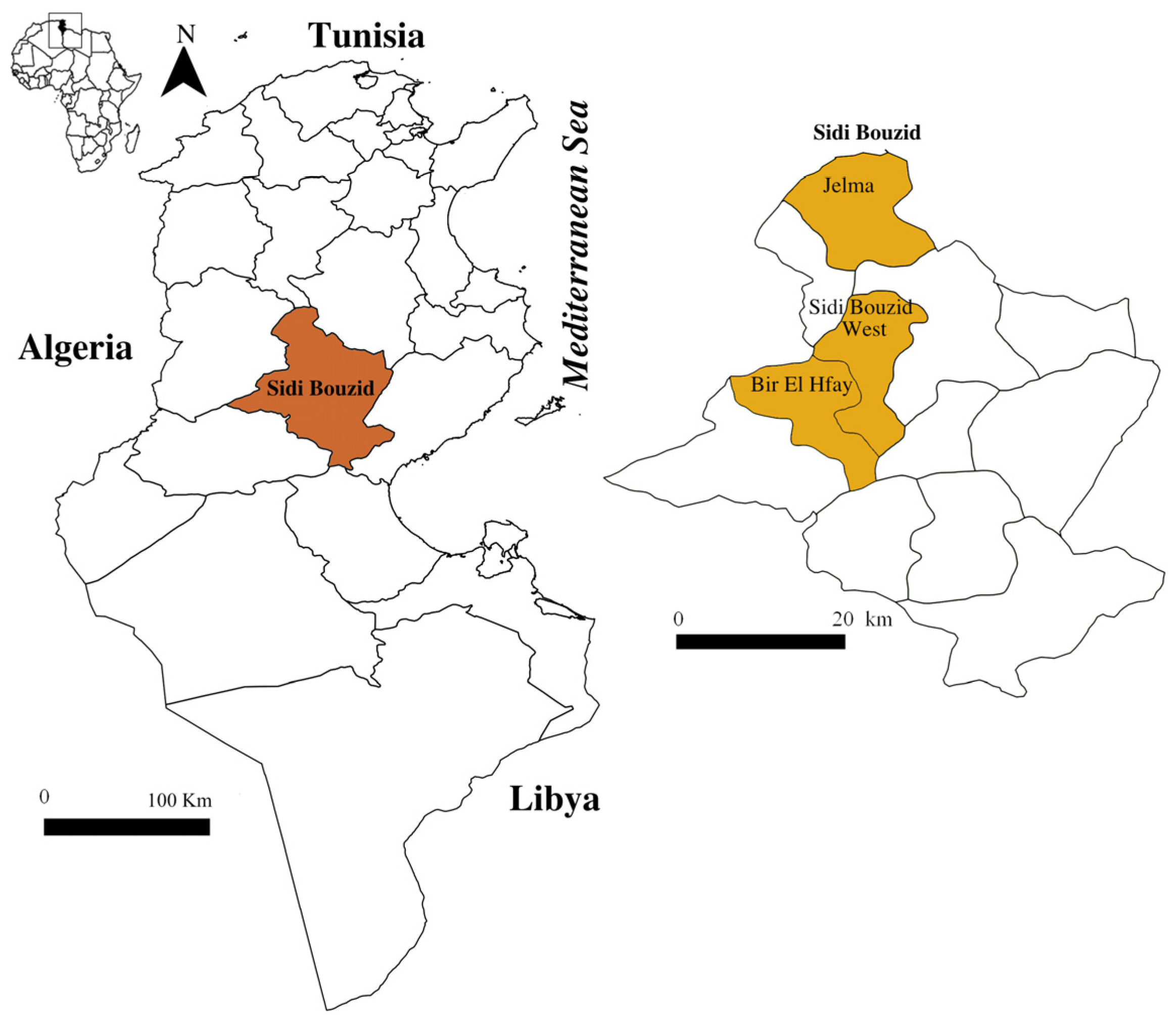
2.1. Study Area
2.2. Animals and Samples
2.3. Parasitological Parameters and Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tunisian Ministry of Agriculture Tunisia in Figures. INS. 2021. Available online: https://www.ins.tn/en/publication/tunisia-figures-2021 (accessed on 15 December 2023).
- Kohan Textile Journal—Middle East and Africa Textile News. Textile Industry in Tunisia. 2019. Available online: https://kohantextilejournal.com/textile-industry-in-tunisia-2/ (accessed on 13 August 2018).
- Mousli, S. Tunisie: Les Difficultés du Secteur du Cuir et de la Chaussure. Available online: http://kapitalis.com/tunisie/2018/02/13/tunisie-les-difficultes-du-secteur-du-cuir-et-de-la-chaussure/ (accessed on 13 August 2018).
- Sassi-Zaidy, Y.B.; Maretto, F.; Zanetti, E.; Hajji, G.M.; Charfi-Cheikrouha, F.; Cassandro, M. Genetic Structure and Variability within and among Populations of the Fat-Tailed Barbarine Sheep Breed Using Microsatellites Markers. Afr. J. Biotechnol. 2014, 13, 44–54. [Google Scholar] [CrossRef][Green Version]
- Megdiche, S. The Tunisian Barbary Sheep: A Look at the Morphostructural Characteristics of Purebred Ewes Reared under Arid Conditions. J. Saudi Soc. Agric. Sci. 2022, 21, 160–170. [Google Scholar] [CrossRef]
- Rekik, M.; Aloulou, R.; Ben Hamouda, M. Small ruminant breeds of Tunisia. In Characterisation of Small Ruminant Breeds in West Asia and North Africa; Rekik, M.; Aloulou, R.; Ben Hamouda, M. International Centre for Agricultural Research in the Dry Areas (ICARDA): Aleppo, Syria, 2005; Volume 2, pp. 91–140. [Google Scholar]
- Akkari, H.; Gharbi, M.; Darghouth, M.A. Dynamics of Infestation of Tracers Lambs by Gastrointestinal Helminths under a Traditional Management System in the North of Tunisia. Parasite 2012, 19, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Lahmar, S.; Trifi, M.; Naceur, S.B.; Bouchhima, T.; Lahouar, N.; Lamouchi, I.; Maâmouri, N.; Selmi, R.; Dhibi, M.; Torgerson, P.R. Cystic Echinococcosis in Slaughtered Domestic Ruminants from Tunisia. J. Helminthol. 2012, 87, 318–325. [Google Scholar] [CrossRef] [PubMed]
- M’ghirbi, Y.; Hurtado, A.; Bouattour, A. Theileria and Babesia Parasites in Ticks in Tunisia. Transbound. Emerg. Dis. 2010, 57, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Akerejola, O.O.; van Veen, T.W.S.; Njoku, C.O. Ovine and Caprine Diseases in Nigeria: A Review of Economic Losses. Bull. Anim. Health Prod. Afr. 1979, 27, 65–70. [Google Scholar]
- Chanie, M.; Negash, T.; Sirak, A. Ectoparasites Are the Major Causes of Various Types of Skin Lesions in Small Ruminants in Ethiopia. Trop. Anim. Health Prod. 2010, 42, 1103–1109. [Google Scholar] [CrossRef]
- Cortinas, R.; Jones, C.J. Ectoparasites of Cattle and Small Ruminants. Vet. Clin. N. Am. Food Anim. Pract. 2006, 22, 673–693. [Google Scholar] [CrossRef]
- Sonenshine, D.E.; Roe, R.M.; Sonenshine, D.E.; Roe, R.M. (Eds.) Biology of Ticks, 2nd ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 2014; Volume 2, ISBN 978-0-19-974406-0. [Google Scholar]
- Walker, A.R.; Bouattour, A.; Camicas, J.L.; Estrada-Pena, A.; Horac, I.G.; Latif, A.A.; Pegram, R.G.; Preston, P. Ticks of Domestic Animals in Africa: A Guide to Identification of Species; Bioscience Reports: Edinburgh, UK, 2003. [Google Scholar]
- Bouattour, A.; Darghouth, M.A.; Daoud, A. Distribution and Ecology of Ticks (Acari: Ixodidae) Infesting Livestock in Tunisia: An Overview of Eighth Years Field Collections. Parassitologia 1999, 41 (Suppl. S1), 5–10. [Google Scholar]
- Elati, K.; Hamdi, D.; Jdidi, M.; Rekik, M.; Gharbi, M. Differences in Tick Infestation of Tunisian Sheep Breeds. Vet. Parasitol. Reg. Stud. Rep. 2018, 13, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Elati, K.; Ayadi, A.A.; Khbou, M.K.; Jdidi, M.; Rekik, M.; Gharbi, M. Dynamique des populations de tiques infestant les ovins dans les steppes arides de Tunisie. Rev. D’élevage Méd. Vét. Pays Trop. 2018, 71, 131–135. [Google Scholar] [CrossRef]
- Liu, D.; Wang, Y.-Z.; Zhang, H.; Liu, Z.-Q.; Wureli, H.; Wang, S.-W.; Tu, C.-C.; Chen, C.-F. First Report of Rickettsia raoultii and R. slovaca in Melophagus ovinus, the Sheep Ked. Parasites Vectors 2016, 9, 600. [Google Scholar] [CrossRef][Green Version]
- Werszko, J.; Asman, M.; Witecka, J.; Steiner-Bogdaszewska, Ż.; Szewczyk, T.; Kuryło, G.; Wilamowski, K.; Karbowiak, G. The Role of Sheep Ked (Melophagus ovinus) as Potential Vector of Protozoa and Bacterial Pathogens. Sci. Rep. 2021, 11, 15468. [Google Scholar] [CrossRef]
- van den Broek, A.H.; Huntley, J.F. Sheep Scab: The Disease, Pathogenesis and Control. J. Comp. Pathol. 2003, 128, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Nixon, E.J.; Brooks-Pollock, E.; Wall, R. Sheep Scab Spatial Distribution: The Roles of Transmission Pathways. Parasites Vectors 2021, 14, 344. [Google Scholar] [CrossRef]
- Obasaju, M.F.; Otesile, E.B. Ctenocephalides Canis Infestation of Sheep and Goats. Trop. Anim. Health Prod. 1980, 12, 116–118. [Google Scholar] [CrossRef]
- Zouari, S.; Khrouf, F.; M’ghirbi, Y.; Bouattour, A. First Molecular Detection and Characterization of Zoonotic Bartonella Species in Fleas Infesting Domestic Animals in Tunisia. Parasites Vectors 2017, 10, 436. [Google Scholar] [CrossRef]
- McNair, C.M. Ectoparasites of Medical and Veterinary Importance: Drug Resistance and the Need for Alternative Control Methods. J. Pharm. Pharmacol. 2015, 67, 351–363. [Google Scholar] [CrossRef]
- Sparagano, O.A.E.; Giangaspero, A. 17—Parasitism in Egg Production Systems: The Role of the Red Mite (Dermanyssus gallinae). In Improving the Safety and Quality of Eggs and Egg Products; Nys, Y., Bain, M., Van Immerseel, F., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2011; pp. 394–414. ISBN 978-1-84569-754-9. [Google Scholar]
- Vudriko, P.; Okwee-Acai, J.; Tayebwa, D.S.; Byaruhanga, J.; Kakooza, S.; Wampande, E.; Omara, R.; Muhindo, J.B.; Tweyongyere, R.; Owiny, D.O.; et al. Emergence of Multi-Acaricide Resistant Rhipicephalus Ticks and Its Implication on Chemical Tick Control in Uganda. Parasites Vectors 2016, 9, 4. [Google Scholar] [CrossRef]
- Guerrero, F.D.; Lovis, L.; Martins, J.R. Acaricide Resistance Mechanisms in Rhipicephalus (Boophilus) microplus. Rev. Bras. Parasitol. Vet. 2012, 21, 1–6. [Google Scholar] [CrossRef]
- Gaur, R.S.; Sangwan, A.K.; Sangwan, N.; Kumar, S. Acaricide Resistance in Rhipicephalus (Boophilus) microplus and Hyalomma anatolicum Collected from Haryana and Rajasthan States of India. Exp. Appl. Acarol. 2016, 69, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Heath, A.; Levot, G.W. Parasiticide Resistance in Flies, Lice and Ticks in New Zealand and Australia: Mechanisms, Prevalence and Prevention. N. Z. Vet. J. 2015, 63, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Cloete, S.W.P.; Thutwa, K.; Scholtz, A.J.; Cloete, J.J.E.; Dzama, K.; Gilmour, A.R.; van Wyk, J.B. Breed Effects and Heterosis for Weight Traits and Tick Count in a Cross between an Indigenous Fat-Tailed and a Commercial Sheep Breed. Trop. Anim. Health Prod. 2021, 53, 165. [Google Scholar] [CrossRef]
- Shyma, K.P.; Gupta, J.P.; Singh, V. Breeding Strategies for Tick Resistance in Tropical Cattle: A Sustainable Approach for Tick Control. J. Parasit. Dis. 2015, 39, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bakheit, M.A.; Latif, A.A. The Innate Resistance of Kenana Cattle to Tropical Theileriosis (Theileria annulata Infection) in the Sudan. Ann. N. Y. Acad. Sci. 2002, 969, 159–163. [Google Scholar] [CrossRef]
- Ibelli, A.M.G.; Ribeiro, A.R.B.; Giglioti, R.; Regitano, L.C.A.; Alencar, M.M.; Chagas, A.C.S.; Paço, A.L.; Oliveira, H.N.; Duarte, J.M.S.; Oliveira, M.C.S. Resistance of Cattle of Various Genetic Groups to the Tick Rhipicephalus microplus and the Relationship with Coat Traits. Vet. Parasitol. 2012, 186, 425–430. [Google Scholar] [CrossRef]
- Tabor, A.E.; Ali, A.; Rehman, G.; Rocha Garcia, G.; Zangirolamo, A.F.; Malardo, T.; Jonsson, N.N. Cattle Tick Rhipicephalus microplus-Host Interface: A Review of Resistant and Susceptible Host Responses. Front. Cell. Infect. Microbiol. 2017, 7, 506. [Google Scholar] [CrossRef]
- Keleman Saxena, A.; Cadima Fuentes, X.; Gonzales Herbas, R.; Humphries, D.L. Indigenous Food Systems and Climate Change: Impacts of Climatic Shifts on the Production and Processing of Native and Traditional Crops in the Bolivian Andes. Front. Public Health 2016, 4, 20. [Google Scholar] [CrossRef]
- Giorgi, F. Climate Change Hot-Spots. Geophys. Res. Lett. 2006, 33. [Google Scholar] [CrossRef]
- Das, R.; Sailo, L.; Verma, N.; Bharti, P.; Saikia, J.; Imtiwati; Kumar, R. Impact of Heat Stress on Health and Performance of Dairy Animals: A Review. Vet. World 2016, 9, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Elbers, A.R.W.; Koenraadt, C.J.M.; Meiswinkel, R. Mosquitoes and Culicoides Biting Midges: Vector Range and the Influence of Climate Change. Rev.-Off. Int. Epizoot. 2015, 34, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Khamassi Khbou, M.; Rouatbi, M.; Romdhane, R.; Sassi, L.; Jdidi, M.; Haile, A.; Rekik, M.; Gharbi, M. Tick Infestation and Piroplasm Infection in Barbarine and Queue Fine de l’Ouest Autochthonous Sheep Breeds in Tunisia, North Africa. Animals 2021, 11, 839. [Google Scholar] [CrossRef] [PubMed]
- Tunisian Ministry of Agriculture. Results of the Survey on the Follow-Up Agricultural Season 2015–2016: Livestock; National Institute of Statistics: Tunis, Tunisia, 2016.
- Bouattour, A. Dichotomous identification keys of ticks (Acari: Ixodidae), livestock parasites in North Africa. Arch. Inst. Pasteur. Tunis 2002, 79, 43–50. [Google Scholar]
- Wall, R.L.; Shearer, D. Veterinary Ectoparasites: Biology, Pathology and Control; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 978-0-470-68022-3. [Google Scholar]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology Meets Ecology on Its Own Terms: Margolis et al. Revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D. Méthodes Statistiques à L’usage des Médecins et des Biologistes; Flammarion Médecine-Sciences: Paris, France, 1993; ISBN 978-2-257-10326-0. [Google Scholar]
- Bakken, J.S.; Dumler, J.S. Human Granulocytic Anaplasmosis. Infect. Dis. Clin. 2015, 29, 341–355. [Google Scholar] [CrossRef]
- Spernovasilis, N.; Markaki, I.; Papadakis, M.; Mazonakis, N.; Ierodiakonou, D. Mediterranean Spotted Fever: Current Knowledge and Recent Advances. Trop. Med. Infect. Dis. 2021, 6, 172. [Google Scholar] [CrossRef]
- Bishop, R.P.; Odongo, D.; Ahmed, J.; Mwamuye, M.; Fry, L.M.; Knowles, D.P.; Nanteza, A.; Lubega, G.; Gwakisa, P.; Clausen, P.-H.; et al. A Review of Recent Research on Theileria parva: Implications for the Infection and Treatment Vaccination Method for Control of East Coast Fever. Transbound. Emerg. Dis. 2020, 67, 56–67. [Google Scholar] [CrossRef]
- Friedhoff, K.T. Tick-Born Disease of Sheep and Goats Caused by Babesia, Theileria or Anaplasma spp. Parasitology 1997, 39, 99–109. [Google Scholar]
- Salifou, S.; Hessa, C.C.; Pangui, L.J. Enquête Préliminaire Sur Les Acariens et Les Insectes Parasites Des Petits Ruminants Dans Les Régions de l’Atlantique et Du Littoral (Sud-Bénin). Revue Méd. Vét. 2004, 155, 343–346. [Google Scholar]
- Yakhchali, M.; Hosseine, A. Prevalence and Ectoparasites Fauna of Sheep and Goats Flocks in Urmia Suburb, Iran. Vet. Arch. 2006, 76, 431–442. [Google Scholar]
- Tesfaye, D.; Assefa, M.; Demissie, T.; Taye, M. Ectoparasites of Small Ruminants Presented at Bahir Dar Veterinary Clinic, Northwest Ethiopia. Afr. J. Agric. Res. 2012, 7, 4669–4674. [Google Scholar] [CrossRef]
- Seyoum, Z.; Tadesse, T.; Addisu, A. Ectoparasites Prevalence in Small Ruminants in and around Sekela, Amhara Regional State, Northwest Ethiopia. J. Vet. Med. 2015, 2015, 216085. [Google Scholar] [CrossRef] [PubMed]
- Ahmed Ali, B.; Abdulqader Naqid, I.; Kadir Zangana, I. Distribution of Ectoparasites Infested Sheep and Goats in Duhok Province, North Iraq. Basrah J. Vet. Res. 2013, 12, 54–64. [Google Scholar] [CrossRef]
- Kumsa, B.; Beyecha, K.; Geloye, M. Ectoparasites of Sheep in Three Agro-Ecological Zones in Central Oromia, Ethiopia. Onderstepoort J. Vet. Res. 2012, 79, E1–E7. [Google Scholar] [CrossRef] [PubMed]
- Yasine, A.; Kumsa, B.; Hailu, Y.; Ayana, D. Mites of Sheep and Goats in Oromia Zone of Amhara Region, North Eastern Ethiopia: Species, Prevalence and Farmers Awareness. BMC Vet. Res. 2015, 11, 122. [Google Scholar] [CrossRef] [PubMed]
- Yacob, H.T.; Yalew, T.A.; Dinka, A.A. Part I: Ectoparasite Prevalences in Sheep and in Goats in and around Wolaita Soddo, Southern Ethiopia. Rev. De Méd. Vét. 2008, 159, 8–9. [Google Scholar]
- López-Pérez, A.M.; Pesapane, R.; Clifford, D.L.; Backus, L.; Foley, P.; Voll, A.; Silva, R.B.; Foley, J. Host Species and Environment Drivers of Ectoparasite Community of Rodents in a Mojave Desert Wetlands. PLoS ONE 2022, 17, e0269160. [Google Scholar] [CrossRef]
- Lutermann, H.; Fagir, D.M.; Bennett, N.C. Complex Interactions within the Ectoparasite Community of the Eastern Rock Sengi (Elephantulus myurus). Int. J. Parasitol. Parasites Wildl. 2015, 4, 148–158. [Google Scholar] [CrossRef][Green Version]
- Linardi, P.M.; Krasnov, B.R. Patterns of Diversity and Abundance of Fleas and Mites in the Neotropics: Host-Related, Parasite-Related and Environment-Related Factors. Med. Vet. Entomol. 2013, 27, 49–58. [Google Scholar] [CrossRef]
- Cuisance, D.; Barré, N.; de Deken, R. Ectoparasites of animals: Methods of ecological, biological, genetic and mechanical control. Rev. Sci. Tech. 1994, 13, 1305–1356. [Google Scholar] [CrossRef]
- Rjeibi, M.R.; Gharbi, M.; Mhadhbi, M.; Mabrouk, W.; Ayari, B.; Nasfi, I.; Jedidi, M.; Sassi, L.; Rekik, M.; Darghouth, M.A. Prevalence of Piroplasms in Small Ruminants in North-West Tunisia and the First Genetic Characterisation of Babesia ovis in Africa. Parasite 2014, 21, 23. [Google Scholar] [CrossRef]
- Bouattour, A. Les Tiques de Tunisie: Rôle de Hyalomma detritum Dans La Transmission de Theileria annulata. Ph.D. Thesis, Faculté des Sciences de Tunis, Tunis, Tunisia, 2001; 247p. [Google Scholar]
- Foughali, A.A.; Jedidi, M.; Dhibi, M.; Mhadhbi, M.; Sassi, L.; Berber, A.; Bitam, I.; Gharbi, M. Infection by Haemopathogens and Tick Infestation of Sheep during Summer Season in Constantine Region, Northeast Algeria. Vet. Med. Sci. 2021, 7, 1769–1777. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, Z.; Chavshin, A.R.; Telmadarraiy, Z.; Edalat, H.; Dabiri, F.; Vatandoost, H.; Zarei, Z.; Beik-Mohammadi, M. Ticks (Acari: Ixodidae) of Livestock and Their Seasonal Activities, Northwest of Iran. Asian Pac. J. Trop. Dis. 2014, 4, S754–S757. [Google Scholar] [CrossRef]
- ElGhali, A.; Hassan, S.M. Life Cycle of the Camel Tick Hyalomma dromedarii (Acari: Ixodidae) under Field Conditions in Northern Sudan. Vet. Parasitol. 2010, 174, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Onyiche, T.E.; MacLeod, E.T. Hard Ticks (Acari: Ixodidae) and Tick-Borne Diseases of Sheep and Goats in Africa: A Review. Ticks Tick-Borne Dis. 2023, 14, 102232. [Google Scholar] [CrossRef] [PubMed]
- Fourie, L.J.; Meintjes, T.; Kok, D.J.; Horak, I.G. The Growth of Sheep Scab Lesions in Relation to Sheep Breed and Time of the Year. Exp. Appl. Acarol. 2002, 27, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F. Biology and Ecology of the Brown Dog Tick, Rhipicephalus sanguineus. Parasites Vectors 2010, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; Farkas, R.; Jaenson, T.G.T.; Koenen, F.; Madder, M.; Pascucci, I.; Salman, M.; Tarrés-Call, J.; Jongejan, F. Association of Environmental Traits with the Geographic Ranges of Ticks (Acari: Ixodidae) of Medical and Veterinary Importance in the Western Palearctic. A Digital Data Set. Exp. Appl. Acarol. 2013, 59, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Jore, S.; Vanwambeke, S.O.; Viljugrein, H.; Isaksen, K.; Kristoffersen, A.B.; Woldehiwet, Z.; Johansen, B.; Brun, E.; Brun-Hansen, H.; Westermann, S.; et al. Climate and Environmental Change Drives Ixodes ricinus Geographical Expansion at the Northern Range Margin. Parasites Vectors 2014, 7, 11. [Google Scholar] [CrossRef] [PubMed]
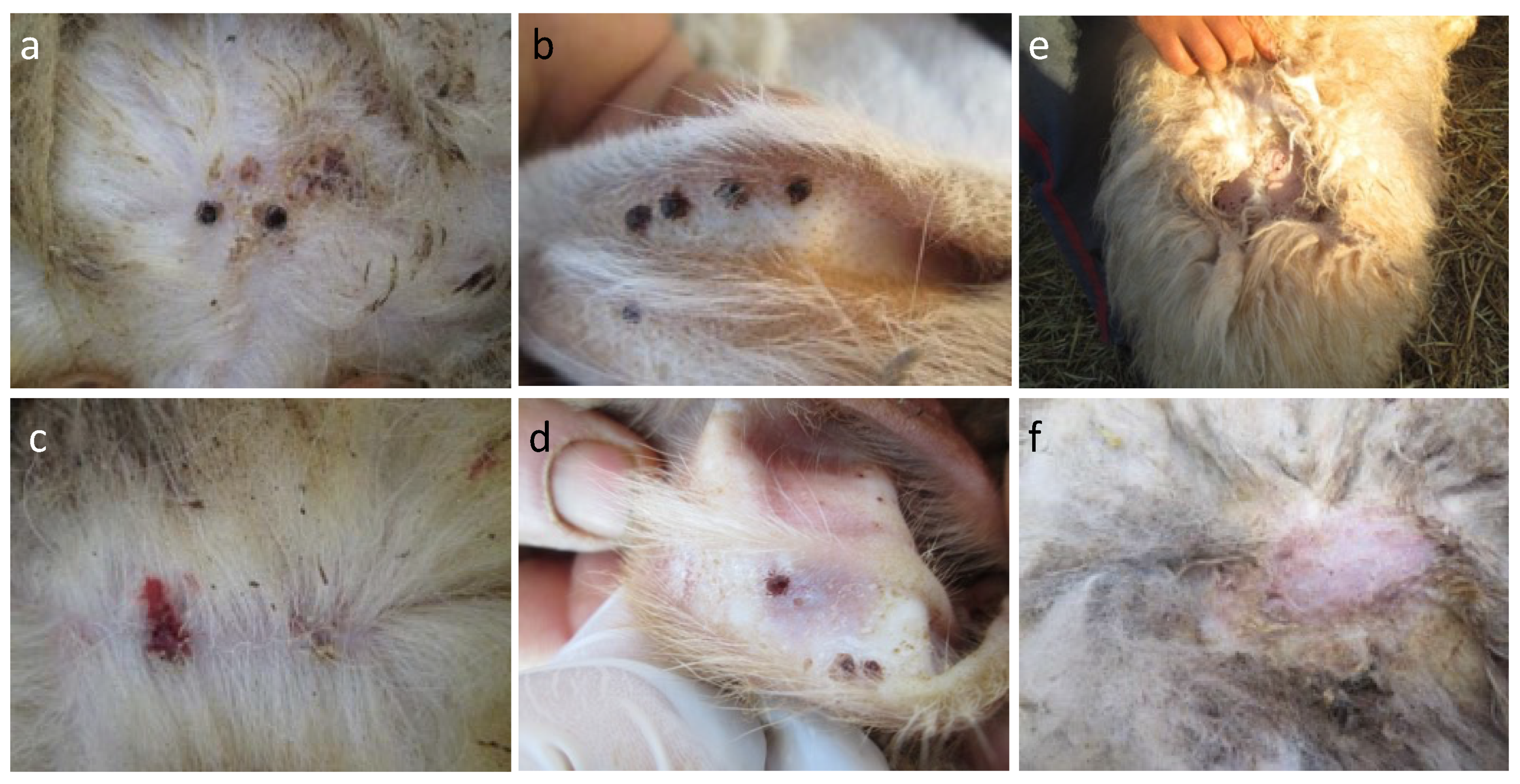
| Farm | Production System | Sheep Breed | Sympatric Animals | Antiparasitic Treatments | Ectoparasites Found |
|---|---|---|---|---|---|
| 1 | Semi-intensive | Barbarine | Goat and poultry |
| Psoroptes ovis Ticks |
| 2 | Intensive for fattening flocks and extensive for the original flock | QFO | Goat, cattle, poultry and dogs |
| Psoroptes ovis Ctenocephalides canis |
| 3 | Extensive | Barbarine | Dogs |
| Ticks Psoroptes ovis |
| 4 | Extensive | QFO | Poultry |
| Ticks |
| 5 | Extensive | Barbarine and QFO | Goats |
| Ticks |
| 6 | Extensive | QFO | Goats |
| 0 |
| Infested/Examined (Infestation Prevalence in % ± Standard Error) | ||||
|---|---|---|---|---|
| Parameters | Psoroptes ovis | Ticks | Ctenocephalides canis | Overall |
| Farm | ||||
| 1 | 1/236 (0.4 ± 0.8) | 1/236 (0.4 ± 0.8) * | 0/236 (0) | 2/236 (0.9 ± 1.2) * |
| 2 | 1/260 (0.3 ± 0.8) | 0/260 (0) | 1/260 (0.4 ± 0.8) | 3/260 (1.1 ± 1.3) |
| 3 | 4/127 (3.2 ± 3) | 60/127 (47.2 ± 8.7) | 0/127 (0) | 64/127 (50.3 ± 8.7) |
| 4 | 0/208 (0) | 4/208 (1.9 ± 1.9) | 0/208 (0) | 4/208 (1.9 ± 1.9) |
| 5 | 0/144 (0) | 1/144 (0.7 ± 1.4) | 0/144 (0) | 1/144 (0.7 ± 1.4) |
| 6 | 0/268 (0) | 0/268 (0) | 0/268 (0) | 0/268 (0) |
| Gender | ||||
| Female | 5/736 (5 ± 0.7) | 42/736 (5.7 ± 1.7) | 0/736 (0) | 47/736 (6.4 ± 1.8) |
| Male | 1/507 (1 ± 0.2) | 24/507 (4.7 ± 1.9) | 1/507 (0.2 ± 0.4) | 27/507 (5.3 ± 2) |
| Breed | ||||
| Barbarine | 5/363 (5 ± 1.4) | 61/363 (16.8 ± 3.9) * | 0/363(0) | 67/363 (18.4 ± 4) * |
| Queue Fine de l’Ouest (QFO) | 1/880 (1 ± 0.1) | 5/880 (0.6 ± 0.5) | 1/880 (0.1 ± 0.2) | 7/880 (0.8 ± 0.6) |
| Age (years) | ||||
| [0–1] | 2/871 (0.2 ± 0.3) | 37/871 (4.3 ± 1.3) | 1/871 (0.1 ± 0.2) | 41/871 (4.7 ± 1.4) |
| [1–2] | 2/108 (1.9 ± 2.5) | 6/108 (5.6 ± 4.3) | 0/108 (0) | 8/108 (7.4 ± 2.5) |
| [2–3] | 2/130 (1.5 ± 2.1) | 10/130 (7.7 ± 4.6) | 0/130 (0) | 12/130 (9.2 ± 2.5) |
| [3–4] | 0/109 (0) | 11/109 (10.1 ± 5.7) | 0/109 (0) | 11/109 (10.1 ± 2.9) |
| [4–5] | 0/20 (0) | 2/20 (10 ± 13.2) | 0/20 (0) | 2/20 (10 ± 13.2) |
| [5–10] | 0/5 (0) | 0/5 (0) | 0/5 (0) | 0/5 (0) |
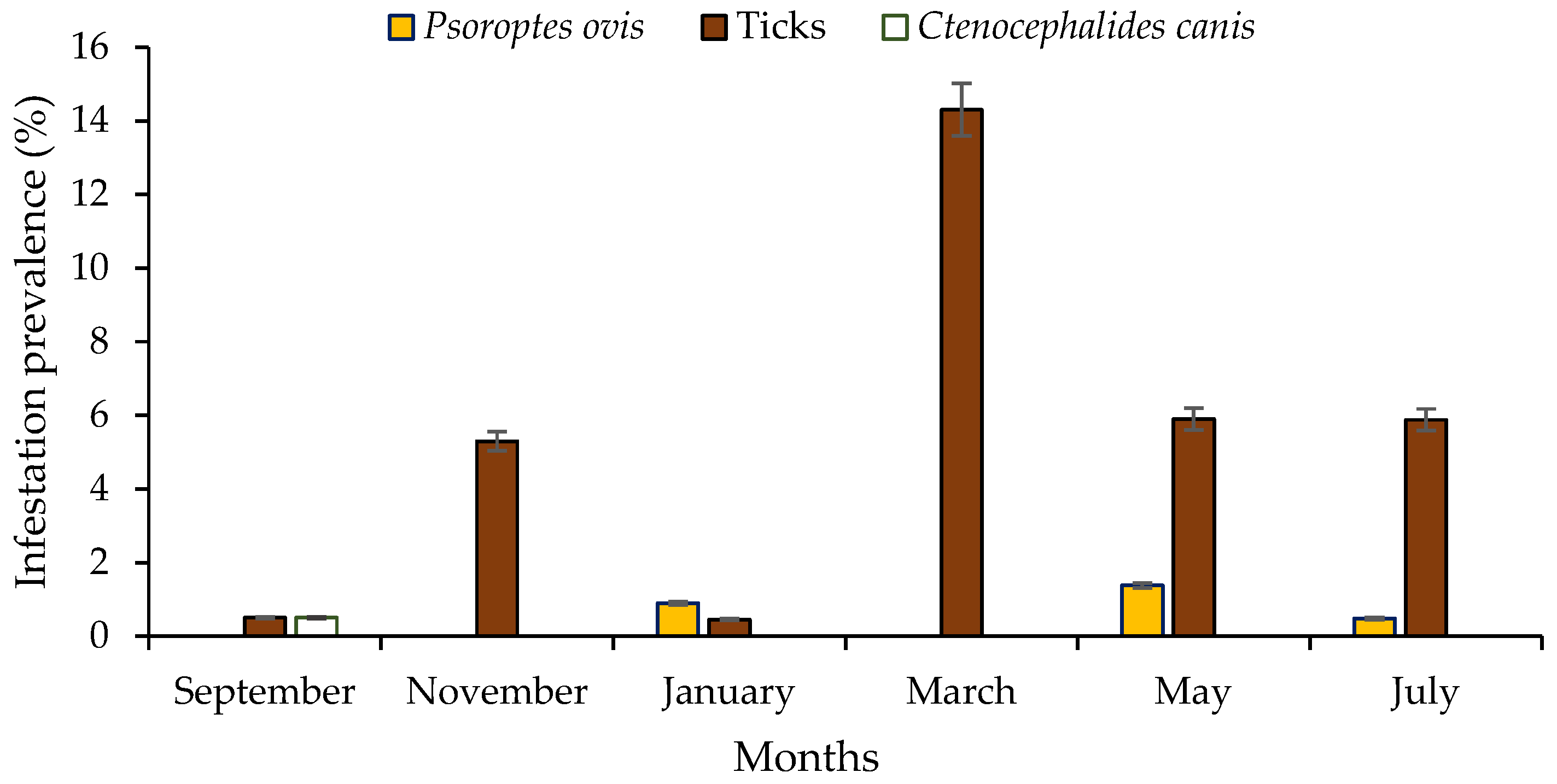
| Overall | 6/1243 (0.48 ± 0.2) | 66/1243 (5.3 ± 0.6) | 1/1243 (0.08 ± 0.2) | 74/1243 (5.95 ± 0.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elati, K.; Daly, N.; Dhibi, M.; Laaribi, H.; Rekik, M.; Gharbi, M. Repeated Cross-Sectional Survey of Ectoparasites in Sheep from Central Tunisia: Does Low Prevalence Indicate Good Hygiene or Resistance to Ectoparasites? Animals 2024, 14, 801. https://doi.org/10.3390/ani14050801
Elati K, Daly N, Dhibi M, Laaribi H, Rekik M, Gharbi M. Repeated Cross-Sectional Survey of Ectoparasites in Sheep from Central Tunisia: Does Low Prevalence Indicate Good Hygiene or Resistance to Ectoparasites? Animals. 2024; 14(5):801. https://doi.org/10.3390/ani14050801
Chicago/Turabian StyleElati, Khawla, Nesrine Daly, Mokhtar Dhibi, Hela Laaribi, Mourad Rekik, and Mohamed Gharbi. 2024. "Repeated Cross-Sectional Survey of Ectoparasites in Sheep from Central Tunisia: Does Low Prevalence Indicate Good Hygiene or Resistance to Ectoparasites?" Animals 14, no. 5: 801. https://doi.org/10.3390/ani14050801
APA StyleElati, K., Daly, N., Dhibi, M., Laaribi, H., Rekik, M., & Gharbi, M. (2024). Repeated Cross-Sectional Survey of Ectoparasites in Sheep from Central Tunisia: Does Low Prevalence Indicate Good Hygiene or Resistance to Ectoparasites? Animals, 14(5), 801. https://doi.org/10.3390/ani14050801





