Bovine Peripheral Blood-Derived Mesenchymal Stem Cells (PB-MSCs) and Spermatogonial Stem Cells (SSCs) Display Contrasting Expression Patterns of Pluripotency and Germ Cell Markers under the Effect of Sertoli Cell Conditioned Medium
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Experimental Design
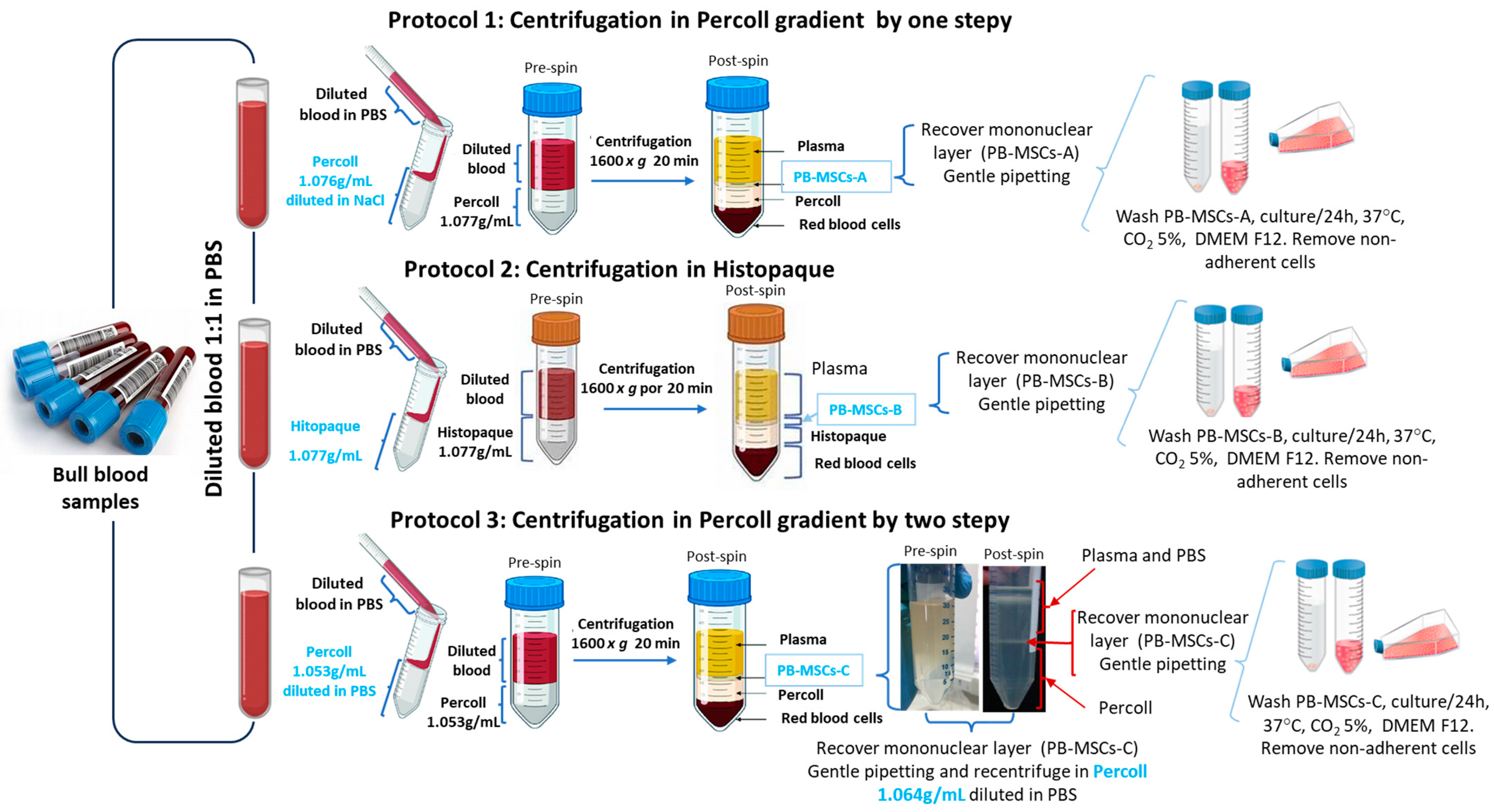
2.3. Isolation, Purification, and Characterization of Bull PB-MSCs
2.4. Isolation, Purification, and Characterization of Bull SCs
2.5. Isolation, Purification, and Characterization of Bull SSCs
2.6. Culture of PB-MSCs and SSCs under SCs/CM Conditions
2.7. Q-PCR Analysis
2.8. Immunofluorescence Analysis
2.9. Statistical Analysis
3. Results
3.1. Morphology of PB-MSCs, SSCs, and SCs Derived from Bull Tissues Isolated by Different Cell Isolation Protocols
3.2. Analysis of Cell-Specific Marker Expression in PB-MSCs, SSCs, and SCs Isolated by Different Protocols
3.3. Effect of SCs/CM on In Vitro Differentiation of PB-MSCs-B and SSCs-A into GCs
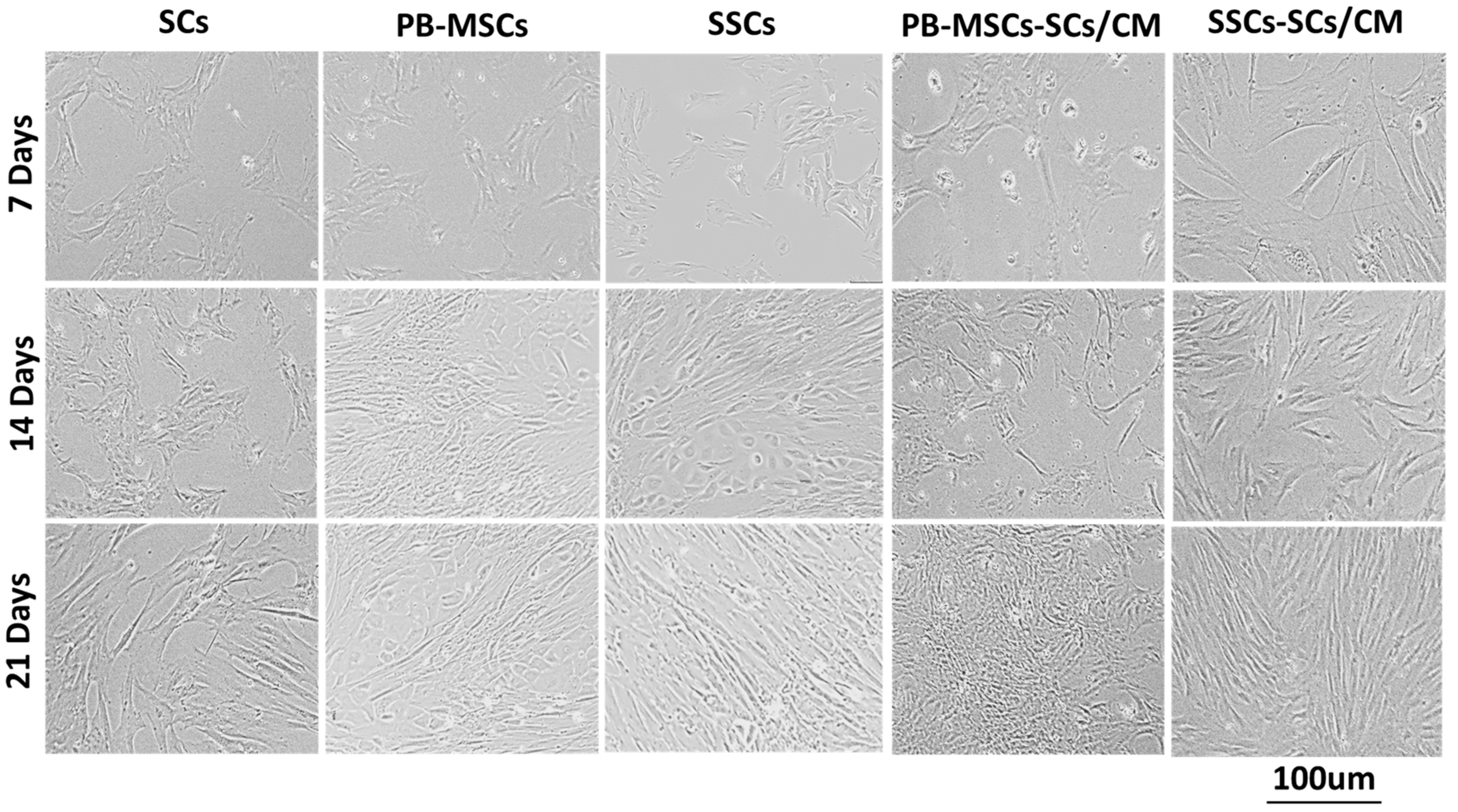
3.3.1. Morphology of PB-MSCs, SCs, and SSCs Derived from Bull Tissues and Cultured with SCs/CM
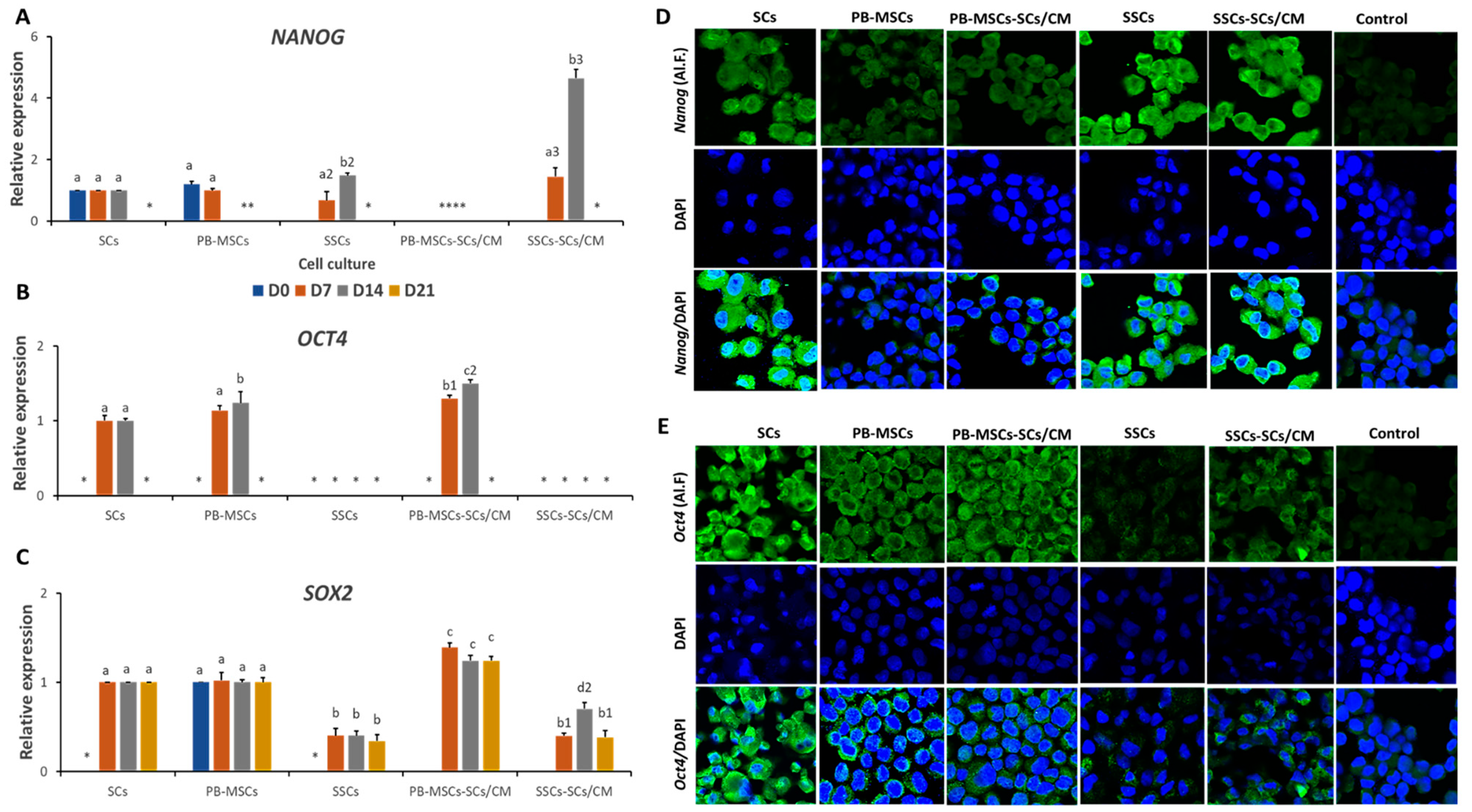
3.3.2. Expression of Pluripotency Markers in PB-MSCs or SSCs Cultured with SCs/CM for 21 Days
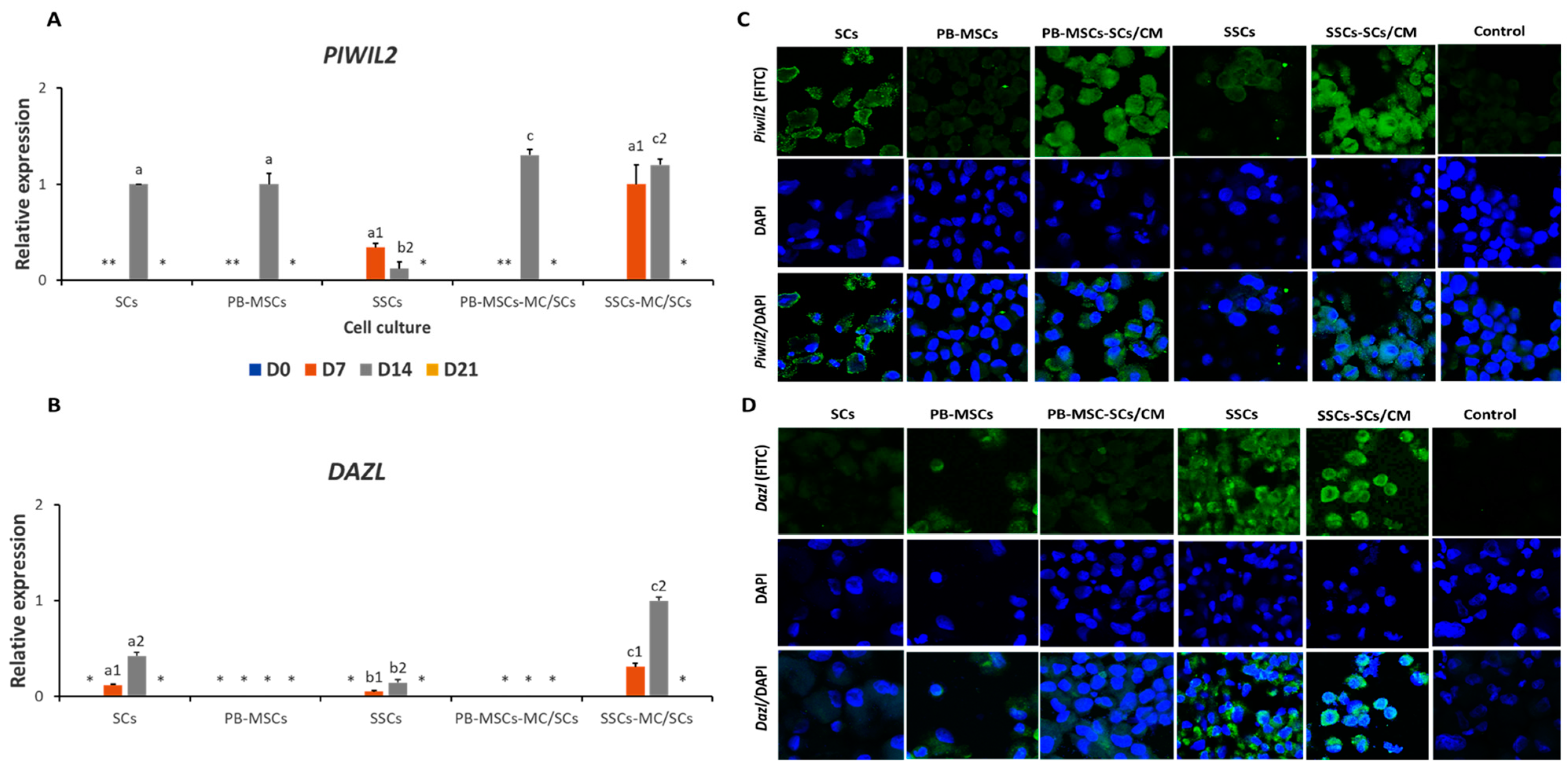
3.3.3. Expression of GCs Markers in PB-MSCs or SSCs Cultured with SCs/CM for 21 Days
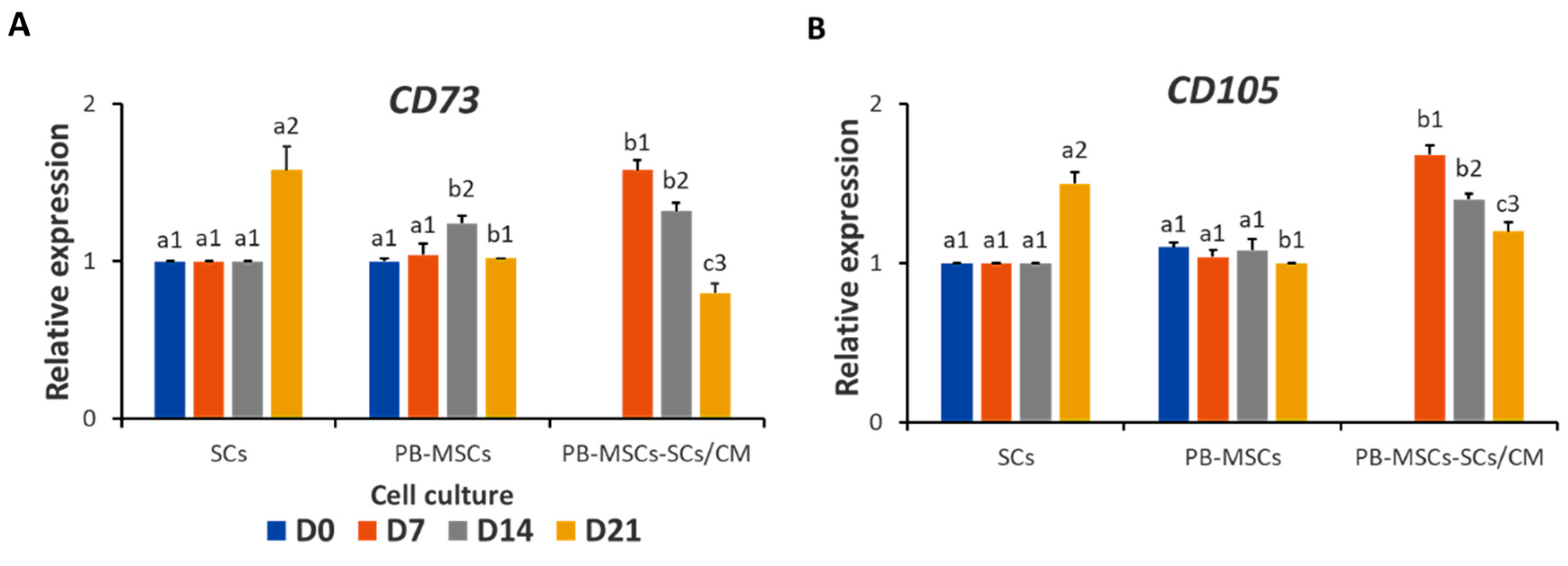
3.3.4. Expression of MSCs Markers in PB-MSCs or SSCs Cultured with SCs/CM for 21 Days
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dominici, M.L.B.K.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Oliveira, É.M.D. Diferenciação de Células-Tronco em Hepatócitos e Desenvolvimento de Modelo Pré-Clínico de Fibrose Hepática para Ensaios de Terapia Celular. Ph.D. Thesis, Universidade de São Paulo, Sao Paulo, Brazil, 2013. [Google Scholar]
- Kohyama, J.; Abe, H.; Shimazaki, T.; Koizumi, A.; Okano, H.; Hata, J.; Gojo, S. Brain from bone: Efficient “meta-differentiation” of marrow stroma-derived mature osteoblasts to neurons with Noggin or a demethylating agent. Differentiation 2001, 68, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Dueñas, F.; Becerra, V.; Cortes, Y.; Vidal, S.; Sáenz, L.; Palomino, J.; Peralta, O.A. Hepatogenic and neurogenic differentiation of bone marrow mesenchymal stem cells from abattoir-derived bovine fetuses. BMC Vet. Res. 2014, 10, 154. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vater, C.; Kasten, P.; Stiehler, M. Culture media for the differentiation of mesenchymal stromal cells. Acta Biometer. 2011, 7, 463–477. [Google Scholar] [CrossRef]
- Li, S.; Huang, K.J.; Wu, J.C.; Hu, M.S.; Sanyal, M.; Hu, M.; Logaker, H.Y.; Lorenz, H.P. Peripheral blood-derived mesenchymal stem cells: Candidate cells responsible for healing critical-sized calvarial bone defects. Stem Cells Transl. Med. 2015, 4, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Calle, A.; Gutiérrez-Reinoso, M.Á.; Re, M.; Blanco, J.; De La Fuente, J.; Monguió-Tortajada, M.; Ramírez, M.Á. Bovine peripheral blood MSC chemotax towards inflammation and embryo implantation stimuli. J. Cell. Physiol. 2021, 236, 1054–1067. [Google Scholar] [CrossRef]
- Lin, W.; Xu, L.; Lin, S.; Shi, L.; Wang, B.; Pan, Q.; Li, G. Characterisation of multipotent stem cells from human peripheral blood using an improved protocol. J. Orthop. Translat. 2019, 19, 18–28. [Google Scholar] [CrossRef]
- He, Q.; Wan, C.; Li, G. Concise review: Multipotent mesenchymal stromal cells in blood. Stem Cells 2007, 25, 69–77. [Google Scholar] [CrossRef]
- Cortez, J.; Bahamonde, J.; De Los Reyes, M.; Palomino, J.; Torres, C.G.; Peralta, O.A. In vitro differentiation of bovine bone marrow-derived mesenchymal stem cells into male germ cells by exposure to exogenous bioactive factors. Reprod. Domest. Anim. 2018, 53, 700–709. [Google Scholar] [CrossRef]
- Cordero, P.; Guerrero-Moncayo, A.; De Los Reyes, M.; Varas-Godoy, M.; Cortez, J.; Torres, C.G.; Peralta, O.A. Overexpression of DAZL, STRA8, and BOULE genes and treatment with BMP4 or retinoic acid modulate the expression of MSC overexpressing germ cell genes. Front. Vet. Sci. 2021, 8, 667547. [Google Scholar] [CrossRef]
- Segunda, M.N.; Bahamonde, J.; Muñoz, I.; Sepulveda, S.; Cortez, J.; De Los Reyes, M.; Peralta, O.A. Sertoli cell-mediated differentiation of bovine fetal mesenchymal stem cells into germ cell lineage using an in vitro coculture system. Theriogenology 2019, 130, 8–18. [Google Scholar] [CrossRef]
- Segunda, M.N.; Díaz, C.; Torres, C.G.; Parraguez, V.H.; De los Reyes, M.; Peralta, O.A. Comparative Analysis of the Potential for Germ Cell (GC) Differentiation of Bovine Peripheral Blood Derived-Mesenchymal Stem Cells (PB-MSC) and Spermatogonial Stem Cells (SSC) in Co-Culture System with Sertoli Cells (SC). Animals 2023, 13, 318. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Peng, G.; Zheng, S.; Wu, X. Differentiation of umbilical cord mesenchymal stem cells into steroidogenic cells in comparison to bone marrow mesenchymal stem cells. Cell Prolif. 2012, 45, 101–110. [Google Scholar] [CrossRef]
- Ghaem Maghami, R.; Mirzapour, T.; Bayrami, A. Differentiation of mesenchymal stem cells to germ-like cells under induction of Sertoli cell-conditioned medium and retinoic acid. Andrologia 2018, 50, e12887. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Lin, L.; Tang, Q.; Li, W.; Huang, T.; Huo, X.; Ma, L. Sertoli cell-mediated differentiation of male germ cell-like cells from human umbilical cord Wharton’s jelly-derived mesenchymal stem cells in an in vitro coculture system. Eur. J. Med. Res. 2015, 20, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, X.; Peng, J.; He, D.; Lin, T.; Zhu, J.; Li, X.; Zhang, Y.; Wei, G. Potential spermatogenesis recovery with bone marrow mesenchymal stem cells in an azoospermic rat model. Int. J. Mol. Sci. 2014, 15, 13151–13165. [Google Scholar] [CrossRef]
- Jabari, A.; Gilani, M.A.S.; Koruji, M.; Gholami, K.; Mohsenzadeh, M.; Khadivi, F.; Movassagh, S.A. Three-dimensional coculture of human spermatogonial stem cells with Sertoli cells in soft agar culture system supplemented by growth factors and Laminin. Acta Histochem. 2020, 122, 151572. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.D.; Bicudo, S.D.; Toma, H.S. O papel das células de Sertoli na espermatogênese. Pubvet 2010, 855, 1–17. Available online: http://hdl.handle.net/11449/141236 (accessed on 5 May 2023).
- Lie, P.P.; Cheng, C.Y.; Mruk, D.D. Signaling pathways regulating the blood—Testis barrier. Int. J. Biochem. Cell Biol. 2013, 45, 621–625. [Google Scholar] [CrossRef]
- Huleihel, M.; Nourashrafeddin, S.; Plant, T.M. Application of three-dimensional culture systems to study mammalian spermatogenesis, with an emphasis on the rhesus monkey (Macaca mulatta). Asian J. Androl. 2015, 17, 972. [Google Scholar] [CrossRef]
- Diaz, T.; Marta, A. Impacto de la Nutrición Diferencial Durante la Preñez y Lactancia Sobre el Factor Neurotrófico Derivado de células Gliales (GDNF) en Testículos de Ratas Adultas. PhD Thesis, Universidad de la República, Facultad de Veterinaria, Montevideo, Uruguay, 2016. Available online: https://bibliotecadigital.fvet.edu.uy/handle/123456789/2087 (accessed on 27 September 2023).
- Monfared, M.H.; Minaee, B.; Rastegar, T.; Khrazineiad, E.; Barbarestani, M. Sertoli cell condition medium can induce germ like cells from bone marrow derived mesenchymal stem cells. Iran. J. Basic Med. Sci. 2016, 19, 1186. [Google Scholar] [CrossRef]
- Mohammadzadeh, E.; Mirzapour, T.; Nowroozi, M.R.; Nazarian, H.; Piryaei, A.; Alipour, F.; Ghaffari Novin, M. Differentiation of spermatogonial stem cells by soft agar three-dimensional culture system. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1772–1781. [Google Scholar] [CrossRef]
- Luo, Y.; Xie, L.; Mohsin, A.; Ahmed, W.; Xu, C.; Peng, Y.; Guo, M. Efficient generation of male germ-like cells derived during co-culturing of adipose-derived mesenchymal stem cells with Sertoli cells under retinoic acid and testosterone induction. Stem Cell Res. Ther. 2019, 10, 1–18. [Google Scholar] [CrossRef]
- Nicholls, P.K.; Schorle, H.; Naqvi, S.; Hu, Y.C.; Fan, Y.; Carmell, M.A.; Page, D.C. Mammalian germ cells are determined after PGC colonization of the nascent gonad. Proc. Natl. Acad. Sci. USA 2019, 116, 25677–25687. [Google Scholar] [CrossRef] [PubMed]
- Pawitan, J.A. Prospect of stem cell conditioned medium in regenerative medicine. BioMed Res. Int. 2014, 2024, 965849. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Tran, N.T.; Than, U.T.T.; Nguyen, M.Q.; Tran, A.M.; Do, P.T.X.; Hoang, N.T.M. Optimization of human umbilical cord blood-derived mesenchymal stem cell isolation and culture methods in serum-and xeno-free conditions. Stem Cell Res. Ther. 2022, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Harms, C.A.; Keller, J.; Kennedy-Stoskopf, S. Use of a two-step Percoll® gradient for separation of loggerhead sea turtle peripheral blood mononuclear cells. J. Wildl Dis. 2000, 36, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Ganan-Gomez, I.; Clise-Dwyer, K.; Colla, S. Isolation, culture, and immunophenotypic analysis of bone marrow HSPCs from patients with myelodysplastic syndromes. STAR Protoc. 2022, 3, 101764. [Google Scholar] [CrossRef]
- Hamidabadi, H.G.; Bojnordi, M.N. Coculture of mouse spermatogonial stem cells with sertoli cell as a feeder layer, stimulates the proliferation and spermatogonial stemness profile. Middle East Fertil. Soc. J. 2018, 23, 107–111. [Google Scholar] [CrossRef]
- Schefe, J.H.; Lehmann, K.E.; Buschmann, I.R.; Unger, T.; Funke-Kaiser, H. Quantitative real-time RT-PCR data analysis: Current concepts and the novel “gene expression’s C T difference” formula. J. Mol. Med. 2006, 84, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Meng, X.; Yu, X.; Wang, G.; Dong, Z.; Zhou, Z.; Wang, F. From 2D to 3D co-culture systems: A review of co-culture models to study the neural cells interaction. Int. J. Mol. Sci. 2022, 23, 13116. [Google Scholar] [CrossRef] [PubMed]
- Trivanović, D.; Kocić, J.; Mojsilović, S.; Krstić, A.; Ilić, V.; Djordjević, I.O.; Santibanez, J.F.; Jovcić, G.; Terzić, M.; Bugarski, D. Mesenchymal stem cells isolated from peripheral blood and umbilical cord Wharton’s jelly. Srp. Arh. Celok. Lek. 2013, 141, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Yamawaki-Ogata, A.; Kanemoto, I.; Usui, A.; Narita, Y. Isolation and characterisation of peripheral blood-derived feline mesenchymal stem cells. Vet J. 2016, 216, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Lyahyai, J.; Mediano, D.R.; Ranera, B.; Sanz, A.; Remacha, A.R.; Bolea, R.; Martín-Burriel, I. Isolation and characterization of ovine mesenchymal stem cells derived from peripheral blood. BMC Vet. Res. 2012, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, L.; Cui, X.; Lin, X.; Li, Y.; Wang, Y.; Gao, F. Wt1 directs the lineage specification of sertoli and granulosa cells by repressing Sf1 expression. Development 2017, 144, 44–53. [Google Scholar] [CrossRef]
- Wang, W.J.; Wu, S.P.; Liu, J.B.; Shi, Y.S.; Huang, X.; Zhang, Q.B.; Yao, K.T. MYC regulation of CHK1 and CHK2 promotes radio resistance in a stem cell-like population of nasopharyngeal carcinoma cells. Cancer Res. 2013, 73, 1219–1231. [Google Scholar] [CrossRef]
- Piprek, R.P.; Kloc, M.; Kubiak, J.Z. Early development of the gonads: Origin and differentiation of the somatic cells of the genital ridges. In Molecular Mechanisms of Cell Differentiation in Gonad Development; Springer: Cham, Switzerland, 2016; pp. 1–22. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, Y.; Qu, R.; He, Y.; Tian, X.; Zeng, W. Spermatogonial stem cells from domestic animals: Progress and prospects. Reproduction 2014, 147, R65–R74. [Google Scholar] [CrossRef]
- Fujihara, M.; Kim, S.M.; Minami, N.; Yamada, M.; Imai, H. Characterization and in vitro culture of male germ cells from developing bovine testis. J. Reprod. Dev. 2011, 57, 355–364. [Google Scholar] [CrossRef]
- Herrid, M.; Davey, R.J.; Hill, J.R. Characterization of germ cells from pre-pubertal bull calves in preparation for germ cell transplantation. Cell Tissue Res. 2007, 330, 321–329. [Google Scholar] [CrossRef]
- Luo, J.; Megee, S.; Dobrinski, I. Asymmetric distribution of UCH-L1 in spermatogonia is associated with maintenance and differentiation of spermatogonial stem cells. J. Cell. Physiol. 2009, 220, 460–468. [Google Scholar] [CrossRef]
- Ram, K.; Kannan, T.; Basha, S.; Geetha Ramesh, G.R.; William, B. In-vitro culture morphology of Spermatogonial stem cells (SSCs) in mice. Int. J. Livest. Res. 2017, 7, 52–59. [Google Scholar] [CrossRef]
- Bahadorani, M.; Hosseini, S.M.; Abedi, P.; Hajian, M.; Afrough, M.; Azhdari, T.Z.; Nasr-Esfahani, M.H. Comparative immunohistochemical analysis of VASA, PLZF and THY1 in goats and sheep suggests that these markers are also conserved in these species. J. Cytol. Histol. 2011, 2, 126. [Google Scholar] [CrossRef]
- Nabulindo, N.W.; Nguhiu-Mwangi, J.; Kipyegon, A.N.E.; Ogugo, M.; Muteti, C.; Christian, T.; Kemp, S. Culture of Kenyan goat (Capra hircus) undifferentiated spermatogonia in feeder-free conditions. Front Vet Sci. 2022, 9, 894075. [Google Scholar] [CrossRef] [PubMed]
- Costoya, J.A.; Hobbs, R.M.; Barna, M.; Cattoretti, G.; Manova, K.; Sukhwani, M.; Pandolfi, P.P. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat. Genet. 2004, 36, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ye, S.; Liang, D.; Wang, P.; Fu, J.; Ma, Q.; Wang, Y. In vitro modeling of human germ cell development using pluripotent stem cells. Stem Cell Rep. 2018, 10, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Ma, W.; Shen, Q.; Zhang, M.; Du, Z.; Wu, C.; Hua, J. Reconstitution of male germline cell specification from mouse embryonic stem cells using defined factors in vitro. Cell Death Differ. 2019, 26, 2115–2124. [Google Scholar] [CrossRef]
- Nazm, B.M.; Ghasemi, H.; Narimanpour, Z. An Efficient In Vitro Culture System to Amplify Spermatogonia Stem Cell Markers. Res. Mol. Med. 2020, 8, 10–20. [Google Scholar] [CrossRef]
- Zheng, Y.; Tian, X.; Zhang, Y.; Qin, J.; An, J.; Zeng, W. In vitro propagation of male germline stem cells from piglets. J. Assist. Reprod. Genet. 2013, 30, 945–952. [Google Scholar] [CrossRef]
- Aponte, P.M.; Soda, T.; Teerds, K.J.; Mizrak, S.C.; Van de Kant, H.J.; de Rooij, D.G. Propagation of bovine spermatogonial stem cells in vitro. Reproduction 2008, 136, 543–557. [Google Scholar] [CrossRef]
- Luo, J.; Megee, S.; Rathi, R.; Dobrinski, I. Protein gene product 9.5 is a spermatogonia-specific marker in the pig testis: Application to enrichment and culture of porcine spermatogonia. Mol. Reprod. Dev. 2006, 12, 1531–1540. [Google Scholar] [CrossRef]
- Silva, J.; Nichols, J.; Theunissen, T.W.; Guo, G.; Van Oosten, A.L.; Barrandon, O.; Smith, A. Nanog is the gateway to the pluripotent ground state. Cell 2009, 138, 722–737. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Arooj, M.; Rashid, F.A.; Gul, A. Role of epigenetic modifications in stem cell regulatory regions (Oct4, Sox2 and Nanog) and cancer. IOSR J. Pharm. Biol. Sci. 2013, 5, 76–81. [Google Scholar] [CrossRef]
- Nagano, M.; Ryu, B.Y.; Brinster, C.J.; Avarbock, M.R.; Brinster, R.L. Maintenance of mouse male germ line stem cells in vitro. Biol. Reprod. 2003, 68, 2207–2214. [Google Scholar] [CrossRef] [PubMed]
- Browne, S.; Jha, A.K.; Ameri, K.; Marcus, S.G.; Yeghiazarians, Y.; Healy, K.E. TGF-β1/CD105 signaling controls vascular network formation within growth factor sequestering hyaluronic acid hydrogels. PLoS ONE 2018, 13, e0194679. [Google Scholar] [CrossRef] [PubMed]
- Niwa, H.; Miyazaki, J.I.; Smith, A.G. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat. Genet. 2000, 24, 372–376. [Google Scholar] [CrossRef]
- Malaver-Ortega, L.F.; Sumer, H.; Liu, J.; Verma, P.J. Inhibition of JAK-STAT ERK/MAPK and glycogen synthase kinase-3 induces a change in gene expression profile of bovine induced pluripotent stem cells. Stem Cells Int. 2016, 2016, 5127984. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Li, P.Z.; Pang, J.; Wan, Y.J.; Zhang, G.M.; Fan, Y.X.; Wang, F. Induction of goat bone marrow mesenchymal stem cells into putative male germ cells using mRNA for STRA8, BOULE and DAZL. Cytotechnology 2019, 71, 563–572. [Google Scholar] [CrossRef]
- Lee, J.H.; Engel, W.; Nayernia, K. Stem cell protein Piwil2 modulates expression of murine spermatogonial stem cell expressed genes. Mol. Reprod. Dev. 2006, 73, 173–179. [Google Scholar] [CrossRef]
- Saiti, D.; Lacham-Kaplan, O. Mouse Germ Cell Development in-vivo and in-vitro. Biomarker Insights 2007, 2, 117727190700200024. [Google Scholar] [CrossRef]
- Yuan, L.; Liu, J.-G.; Zhao, J.; Brundell, E.; Daneholt, B.; Hoog, C. The murine SCP3 gene is required for synaptonemal complex assembly, chromosome synapsis, and male fertility. Mol. Cell 2000, 5, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.L.; Baltus, A.E.; Roepers-Gajadien, H.L.; Hassold, T.J.; de Rooij, D.G.; van Pelt, A.M.; Page, D.C. Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc. Natl. Acad. Sci. USA 2008, 105, 14976–14980. [Google Scholar] [CrossRef] [PubMed]

| Genes | Nucleotide sequence (5′-3′) | n.Access | |
|---|---|---|---|
| Forward | Reverse | ||
| Endogenous genes | |||
| β- ACTINA | CGCACCACTGGCATTGTCAT | TCCAAGGCGACGTAGCAGAG | NM_173979.3 |
| GAPDH | CCTTCATTGACCTTCACTACATGG TCTA | TGGAAGATGGTGATGGCCTTTCCATTG | NM_001034034.2 |
| Mesenchymal stem cell genes | |||
| CD73 | TGGTCCAGGCCTATGCTTTTG | GGGATGCTGCTGTTGAGAAGAA | NM_174129.3 |
| CD105 | CGGACAGTGACCGTGAAGTTG | TGTTGTGGTTGGCCTCGATTA | NM_00107639.1 |
| Hematopoietic cell genes | |||
| CD34 | CATGCCGTCTTAACCCATCT | CGGTCTACAGAGGTGGTGGT | NM_174009.1 |
| CD45 | CCACGGGTATTCAGCAAGTT | CCCAGATCATCCTCCAGAAA | NM_001206523 |
| Sertoli cell genes | |||
| WT1 | CGTGCGTACCATGTAGGGAA | CTCGTGCTTGAAGGAGTGGT | XM_015474834.2 |
| AR | CAGATGGCAGTCATTCAG | CTTGGTGAGCTGGTAGAAG | XM_001244127 |
| Spermatogonial stem cell genes | |||
| UCHL1 | AGAAGCAGCATCTCGGTTCC | CGTGGTTGAGGGTAAGTGCT | NM_001046172.2 |
| PLZF | GCCGTGATACCGAGAGCAAC | CTCTCCTCGCTGGAATGCTT | NM_001037476.2 |
| CD90 | ACTCATACCGCTCCCGAACCA | CATGTGTATGTCCCCTCGTCCTT | NM_001034765 |
| Germ cell genes | |||
| DAZL | TCCAAGTTCACCAGTTCAGG | CGT CTG TAT GCT TCT GTC CAC | NM_001081725.1 |
| STRA8 | TGTGCCCAGGTGTTCATCTC | GGGGACTGTCACCTCATTGG | XM_015463130 |
| PIWIL2 | TCGTATTGATGATGTGGATTGG | GGGAGCAGCAGGATTTCAC | XM_617223.3 |
| FRAGILIS | ATCTGCAGCGAGACCTCTGT | CCGATGGACATGATGATGAG | XM_002697323 |
| STELLA | TGCAAGTTGCCACTCAACTC | TCTTACCCCTCTCCGCCTAT | NM_00111110 |
| VASA | TGCTACTCCTGGAAGACTGA | CGGTCTGCTGAACATCTCTA | NM_001007819.1 |
| SCP3 | CTAGAATTGTTCAGAGCCAGAG | GTTCAAGTTCTTTCTTCAAAG | NM_001040588.2 |
| Pluripotency genes | |||
| OCT4 | GAAAGAGAAAGCGGACGAG | GTGAAAGGAGACCCAGCAG | NM_174580.2 |
| NANOG | TAAGCACAGGGGGCAAAAGT | ATGGCTAAAAGGGGTGGAGG | NM_001025344.1 |
| SOX2 | CCCGTGGTTACCTCTTCTTCC | CGCTCTGCTAGTGCTGGGAC | NM_001105463.2 |
| Marker | PB-MSCs-SCs/CM | SSCs- SCs/CM |
|---|---|---|
| CD73 | ↑ (Q-PCR) | - |
| CD105 | ↑ (Q-PCR) | - |
| DAZL | x (Q-PCR) | ↑ (Q-PCR) *(IF) |
| STRA8 | x (Q-PCR) | x (Q-PCR) |
| PIWIL2 | ↑ (Q-PCR) *(IF) | ↑ (Q-PCR) *(IF) |
| FRAGILIS | x (Q-PCR) | x (Q-PCR) |
| STELLA | x (Q-PCR) | x (Q-PCR) |
| OCT4 | ↑ (Q-PCR) *(IF) | x (Q-PCR) *(IF) |
| NANOG | x (Q-PCR) | ↑ (Q-PCR) *(IF) |
| SOX2 | ↑ (Q-PCR) | ↑ (Q-PCR) |
| SCP3 | x (Q-PCR) | x (Q-PCR) |
| VASA | x (Q-PCR) | x (Q-PCR) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segunda, M.N.; Díaz, C.; Torres, C.G.; Parraguez, V.H.; De los Reyes, M.; Peralta, O.A. Bovine Peripheral Blood-Derived Mesenchymal Stem Cells (PB-MSCs) and Spermatogonial Stem Cells (SSCs) Display Contrasting Expression Patterns of Pluripotency and Germ Cell Markers under the Effect of Sertoli Cell Conditioned Medium. Animals 2024, 14, 803. https://doi.org/10.3390/ani14050803
Segunda MN, Díaz C, Torres CG, Parraguez VH, De los Reyes M, Peralta OA. Bovine Peripheral Blood-Derived Mesenchymal Stem Cells (PB-MSCs) and Spermatogonial Stem Cells (SSCs) Display Contrasting Expression Patterns of Pluripotency and Germ Cell Markers under the Effect of Sertoli Cell Conditioned Medium. Animals. 2024; 14(5):803. https://doi.org/10.3390/ani14050803
Chicago/Turabian StyleSegunda, Moisés N., Carlos Díaz, Cristian G. Torres, Víctor H. Parraguez, Mónica De los Reyes, and Oscar A. Peralta. 2024. "Bovine Peripheral Blood-Derived Mesenchymal Stem Cells (PB-MSCs) and Spermatogonial Stem Cells (SSCs) Display Contrasting Expression Patterns of Pluripotency and Germ Cell Markers under the Effect of Sertoli Cell Conditioned Medium" Animals 14, no. 5: 803. https://doi.org/10.3390/ani14050803
APA StyleSegunda, M. N., Díaz, C., Torres, C. G., Parraguez, V. H., De los Reyes, M., & Peralta, O. A. (2024). Bovine Peripheral Blood-Derived Mesenchymal Stem Cells (PB-MSCs) and Spermatogonial Stem Cells (SSCs) Display Contrasting Expression Patterns of Pluripotency and Germ Cell Markers under the Effect of Sertoli Cell Conditioned Medium. Animals, 14(5), 803. https://doi.org/10.3390/ani14050803






