Bovine Peripheral Blood-Derived Mesenchymal Stem Cells (PB-MSCs) and Spermatogonial Stem Cells (SSCs) Display Contrasting Expression Patterns of Pluripotency and Germ Cell Markers under the Effect of Sertoli Cell Conditioned Medium
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Experimental Design
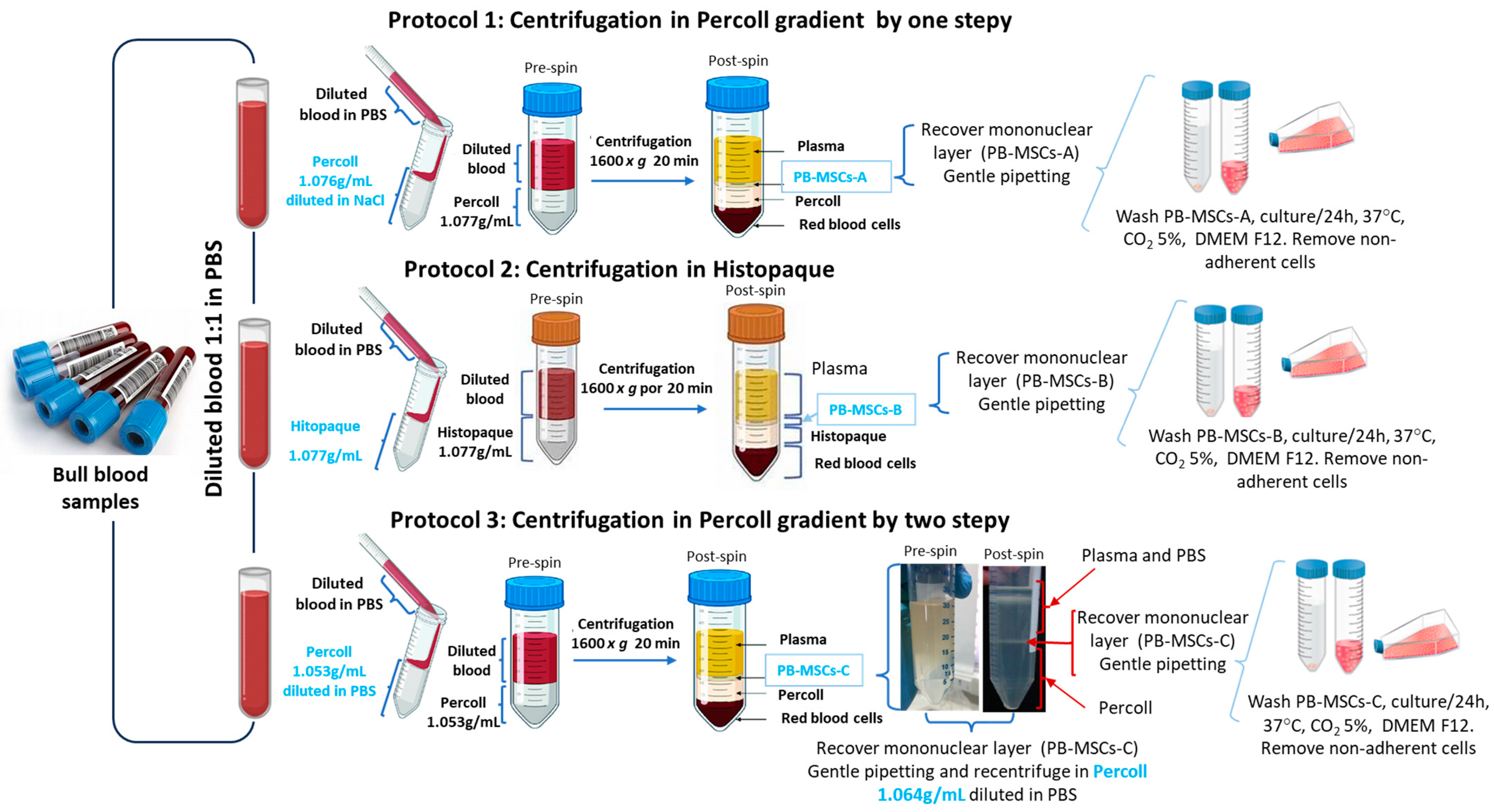
2.3. Isolation, Purification, and Characterization of Bull PB-MSCs
2.4. Isolation, Purification, and Characterization of Bull SCs
2.5. Isolation, Purification, and Characterization of Bull SSCs
2.6. Culture of PB-MSCs and SSCs under SCs/CM Conditions
2.7. Q-PCR Analysis
2.8. Immunofluorescence Analysis
2.9. Statistical Analysis
3. Results
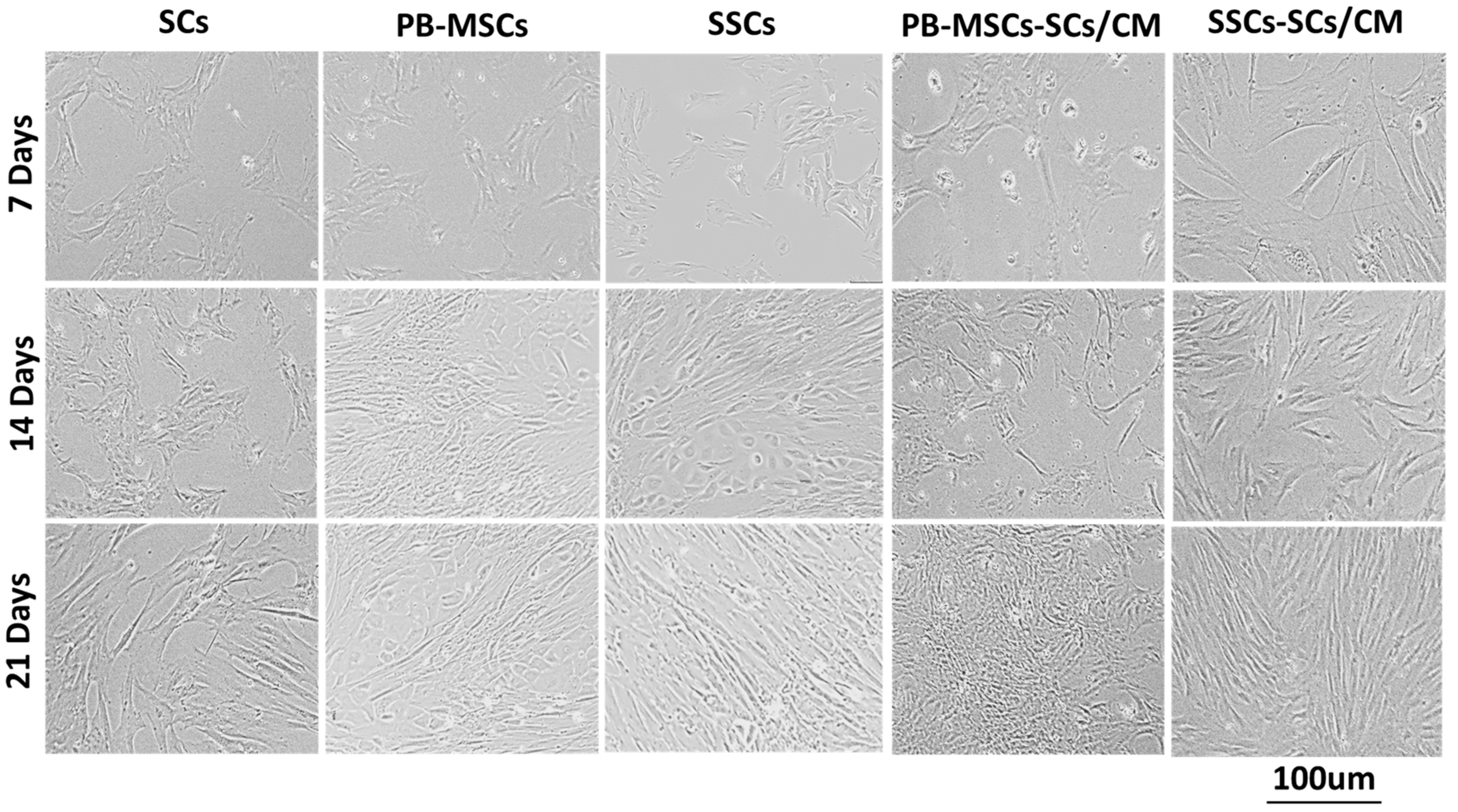
3.1. Morphology of PB-MSCs, SSCs, and SCs Derived from Bull Tissues Isolated by Different Cell Isolation Protocols
3.2. Analysis of Cell-Specific Marker Expression in PB-MSCs, SSCs, and SCs Isolated by Different Protocols
3.3. Effect of SCs/CM on In Vitro Differentiation of PB-MSCs-B and SSCs-A into GCs
3.3.1. Morphology of PB-MSCs, SCs, and SSCs Derived from Bull Tissues and Cultured with SCs/CM
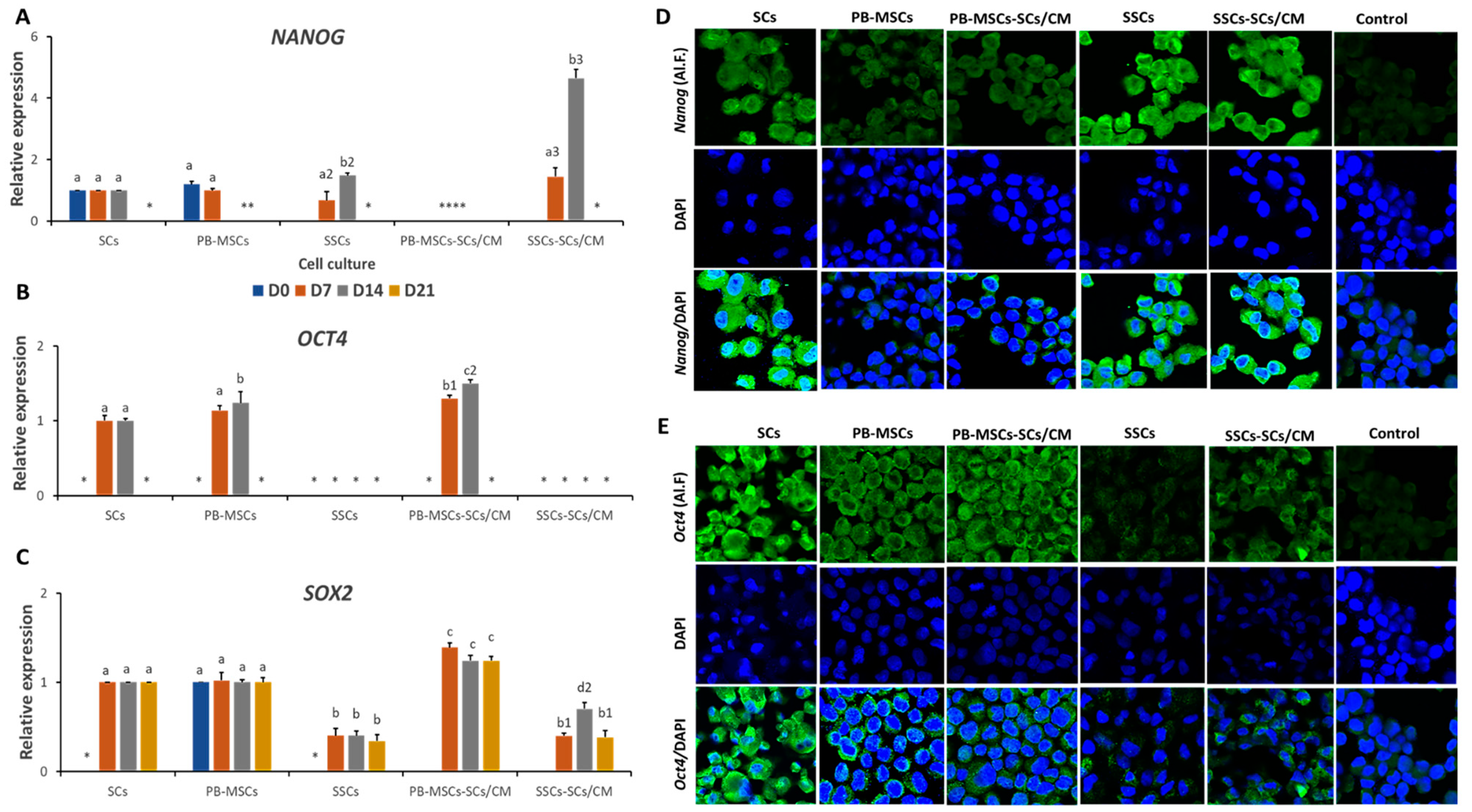
3.3.2. Expression of Pluripotency Markers in PB-MSCs or SSCs Cultured with SCs/CM for 21 Days
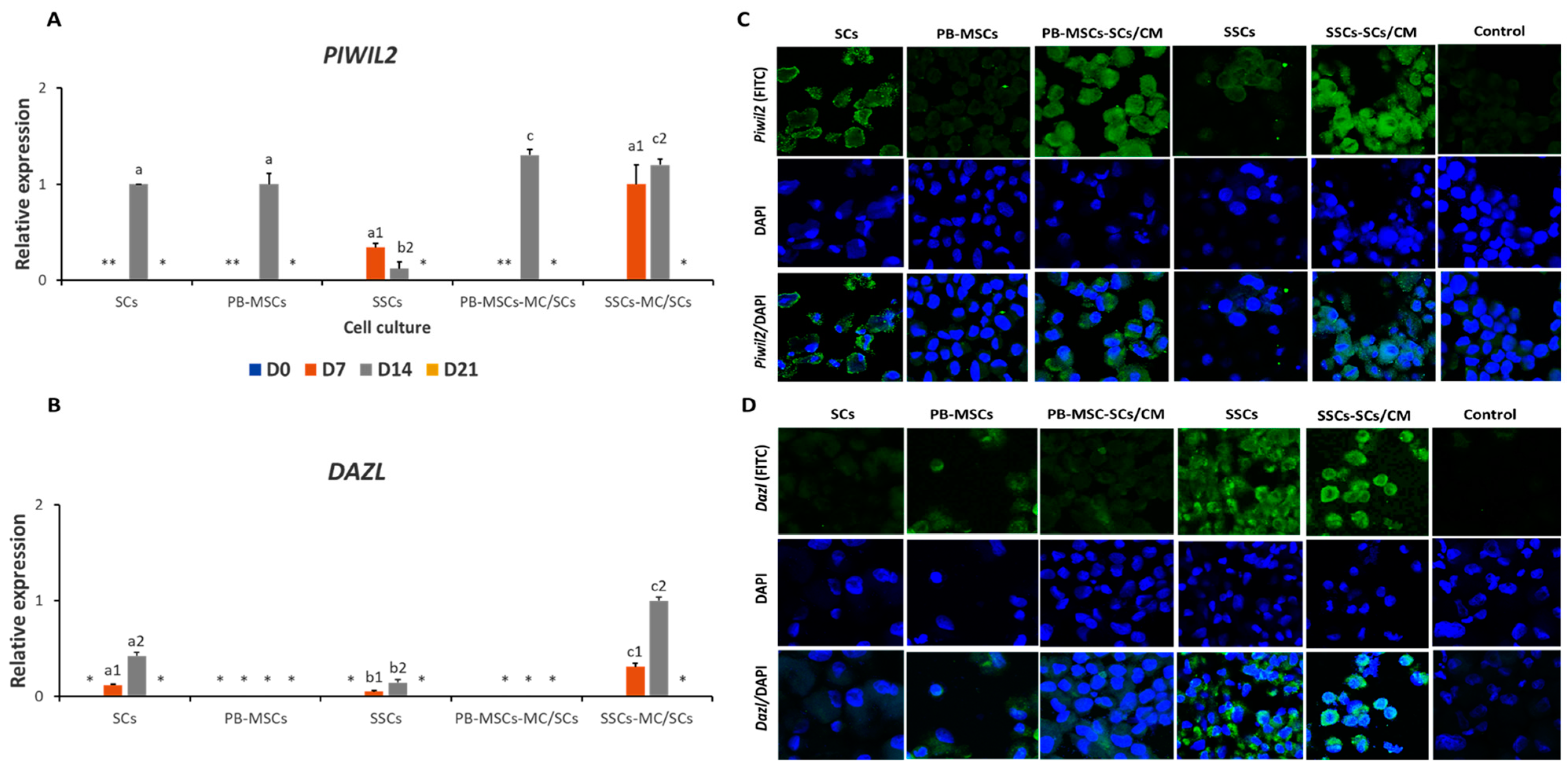
3.3.3. Expression of GCs Markers in PB-MSCs or SSCs Cultured with SCs/CM for 21 Days
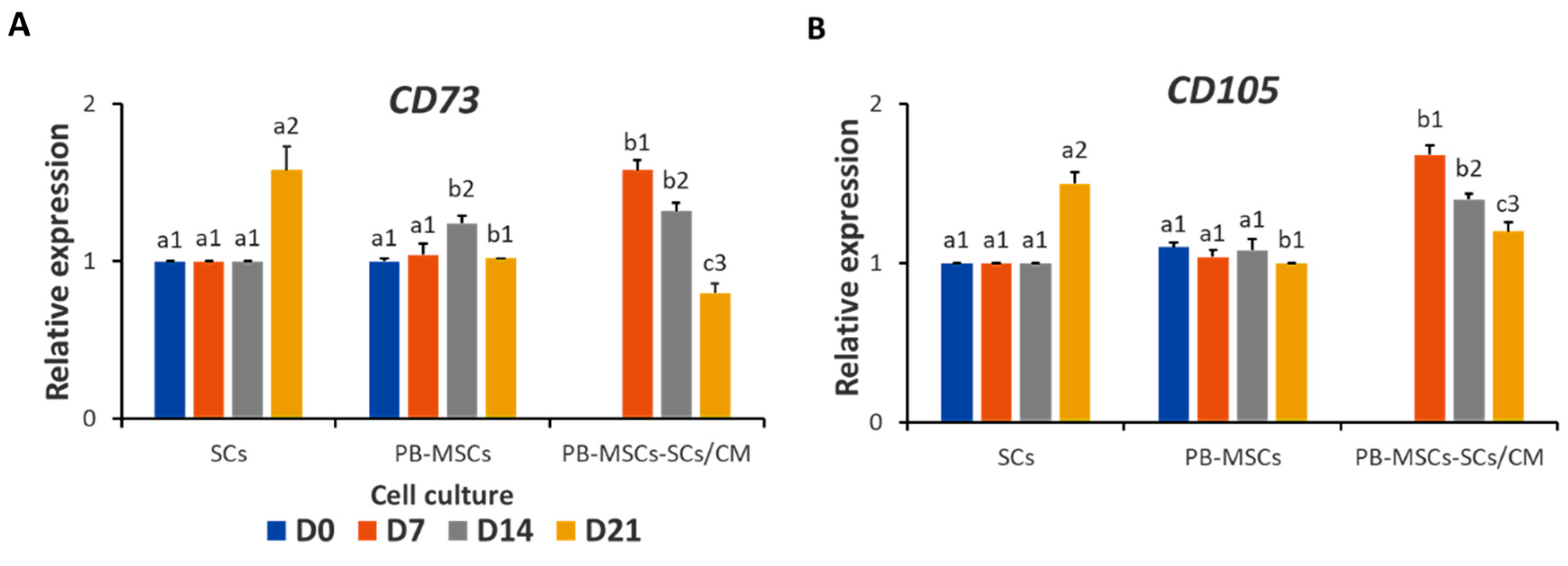
3.3.4. Expression of MSCs Markers in PB-MSCs or SSCs Cultured with SCs/CM for 21 Days
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dominici, M.L.B.K.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Oliveira, É.M.D. Diferenciação de Células-Tronco em Hepatócitos e Desenvolvimento de Modelo Pré-Clínico de Fibrose Hepática para Ensaios de Terapia Celular. Ph.D. Thesis, Universidade de São Paulo, Sao Paulo, Brazil, 2013. [Google Scholar]
- Kohyama, J.; Abe, H.; Shimazaki, T.; Koizumi, A.; Okano, H.; Hata, J.; Gojo, S. Brain from bone: Efficient “meta-differentiation” of marrow stroma-derived mature osteoblasts to neurons with Noggin or a demethylating agent. Differentiation 2001, 68, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Dueñas, F.; Becerra, V.; Cortes, Y.; Vidal, S.; Sáenz, L.; Palomino, J.; Peralta, O.A. Hepatogenic and neurogenic differentiation of bone marrow mesenchymal stem cells from abattoir-derived bovine fetuses. BMC Vet. Res. 2014, 10, 154. [Google Scholar] [CrossRef] [PubMed]
- Vater, C.; Kasten, P.; Stiehler, M. Culture media for the differentiation of mesenchymal stromal cells. Acta Biometer. 2011, 7, 463–477. [Google Scholar] [CrossRef]
- Li, S.; Huang, K.J.; Wu, J.C.; Hu, M.S.; Sanyal, M.; Hu, M.; Logaker, H.Y.; Lorenz, H.P. Peripheral blood-derived mesenchymal stem cells: Candidate cells responsible for healing critical-sized calvarial bone defects. Stem Cells Transl. Med. 2015, 4, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Calle, A.; Gutiérrez-Reinoso, M.Á.; Re, M.; Blanco, J.; De La Fuente, J.; Monguió-Tortajada, M.; Ramírez, M.Á. Bovine peripheral blood MSC chemotax towards inflammation and embryo implantation stimuli. J. Cell. Physiol. 2021, 236, 1054–1067. [Google Scholar] [CrossRef]
- Lin, W.; Xu, L.; Lin, S.; Shi, L.; Wang, B.; Pan, Q.; Li, G. Characterisation of multipotent stem cells from human peripheral blood using an improved protocol. J. Orthop. Translat. 2019, 19, 18–28. [Google Scholar] [CrossRef]
- He, Q.; Wan, C.; Li, G. Concise review: Multipotent mesenchymal stromal cells in blood. Stem Cells 2007, 25, 69–77. [Google Scholar] [CrossRef]
- Cortez, J.; Bahamonde, J.; De Los Reyes, M.; Palomino, J.; Torres, C.G.; Peralta, O.A. In vitro differentiation of bovine bone marrow-derived mesenchymal stem cells into male germ cells by exposure to exogenous bioactive factors. Reprod. Domest. Anim. 2018, 53, 700–709. [Google Scholar] [CrossRef]
- Cordero, P.; Guerrero-Moncayo, A.; De Los Reyes, M.; Varas-Godoy, M.; Cortez, J.; Torres, C.G.; Peralta, O.A. Overexpression of DAZL, STRA8, and BOULE genes and treatment with BMP4 or retinoic acid modulate the expression of MSC overexpressing germ cell genes. Front. Vet. Sci. 2021, 8, 667547. [Google Scholar] [CrossRef]
- Segunda, M.N.; Bahamonde, J.; Muñoz, I.; Sepulveda, S.; Cortez, J.; De Los Reyes, M.; Peralta, O.A. Sertoli cell-mediated differentiation of bovine fetal mesenchymal stem cells into germ cell lineage using an in vitro coculture system. Theriogenology 2019, 130, 8–18. [Google Scholar] [CrossRef]
- Segunda, M.N.; Díaz, C.; Torres, C.G.; Parraguez, V.H.; De los Reyes, M.; Peralta, O.A. Comparative Analysis of the Potential for Germ Cell (GC) Differentiation of Bovine Peripheral Blood Derived-Mesenchymal Stem Cells (PB-MSC) and Spermatogonial Stem Cells (SSC) in Co-Culture System with Sertoli Cells (SC). Animals 2023, 13, 318. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Peng, G.; Zheng, S.; Wu, X. Differentiation of umbilical cord mesenchymal stem cells into steroidogenic cells in comparison to bone marrow mesenchymal stem cells. Cell Prolif. 2012, 45, 101–110. [Google Scholar] [CrossRef]
- Ghaem Maghami, R.; Mirzapour, T.; Bayrami, A. Differentiation of mesenchymal stem cells to germ-like cells under induction of Sertoli cell-conditioned medium and retinoic acid. Andrologia 2018, 50, e12887. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Lin, L.; Tang, Q.; Li, W.; Huang, T.; Huo, X.; Ma, L. Sertoli cell-mediated differentiation of male germ cell-like cells from human umbilical cord Wharton’s jelly-derived mesenchymal stem cells in an in vitro coculture system. Eur. J. Med. Res. 2015, 20, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, X.; Peng, J.; He, D.; Lin, T.; Zhu, J.; Li, X.; Zhang, Y.; Wei, G. Potential spermatogenesis recovery with bone marrow mesenchymal stem cells in an azoospermic rat model. Int. J. Mol. Sci. 2014, 15, 13151–13165. [Google Scholar] [CrossRef]
- Jabari, A.; Gilani, M.A.S.; Koruji, M.; Gholami, K.; Mohsenzadeh, M.; Khadivi, F.; Movassagh, S.A. Three-dimensional coculture of human spermatogonial stem cells with Sertoli cells in soft agar culture system supplemented by growth factors and Laminin. Acta Histochem. 2020, 122, 151572. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.D.; Bicudo, S.D.; Toma, H.S. O papel das células de Sertoli na espermatogênese. Pubvet 2010, 855, 1–17. Available online: http://hdl.handle.net/11449/141236 (accessed on 5 May 2023).
- Lie, P.P.; Cheng, C.Y.; Mruk, D.D. Signaling pathways regulating the blood—Testis barrier. Int. J. Biochem. Cell Biol. 2013, 45, 621–625. [Google Scholar] [CrossRef]
- Huleihel, M.; Nourashrafeddin, S.; Plant, T.M. Application of three-dimensional culture systems to study mammalian spermatogenesis, with an emphasis on the rhesus monkey (Macaca mulatta). Asian J. Androl. 2015, 17, 972. [Google Scholar] [CrossRef]
- Diaz, T.; Marta, A. Impacto de la Nutrición Diferencial Durante la Preñez y Lactancia Sobre el Factor Neurotrófico Derivado de células Gliales (GDNF) en Testículos de Ratas Adultas. PhD Thesis, Universidad de la República, Facultad de Veterinaria, Montevideo, Uruguay, 2016. Available online: https://bibliotecadigital.fvet.edu.uy/handle/123456789/2087 (accessed on 27 September 2023).
- Monfared, M.H.; Minaee, B.; Rastegar, T.; Khrazineiad, E.; Barbarestani, M. Sertoli cell condition medium can induce germ like cells from bone marrow derived mesenchymal stem cells. Iran. J. Basic Med. Sci. 2016, 19, 1186. [Google Scholar] [CrossRef]
- Mohammadzadeh, E.; Mirzapour, T.; Nowroozi, M.R.; Nazarian, H.; Piryaei, A.; Alipour, F.; Ghaffari Novin, M. Differentiation of spermatogonial stem cells by soft agar three-dimensional culture system. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1772–1781. [Google Scholar] [CrossRef]
- Luo, Y.; Xie, L.; Mohsin, A.; Ahmed, W.; Xu, C.; Peng, Y.; Guo, M. Efficient generation of male germ-like cells derived during co-culturing of adipose-derived mesenchymal stem cells with Sertoli cells under retinoic acid and testosterone induction. Stem Cell Res. Ther. 2019, 10, 1–18. [Google Scholar] [CrossRef]
- Nicholls, P.K.; Schorle, H.; Naqvi, S.; Hu, Y.C.; Fan, Y.; Carmell, M.A.; Page, D.C. Mammalian germ cells are determined after PGC colonization of the nascent gonad. Proc. Natl. Acad. Sci. USA 2019, 116, 25677–25687. [Google Scholar] [CrossRef] [PubMed]
- Pawitan, J.A. Prospect of stem cell conditioned medium in regenerative medicine. BioMed Res. Int. 2014, 2024, 965849. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Tran, N.T.; Than, U.T.T.; Nguyen, M.Q.; Tran, A.M.; Do, P.T.X.; Hoang, N.T.M. Optimization of human umbilical cord blood-derived mesenchymal stem cell isolation and culture methods in serum-and xeno-free conditions. Stem Cell Res. Ther. 2022, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Harms, C.A.; Keller, J.; Kennedy-Stoskopf, S. Use of a two-step Percoll® gradient for separation of loggerhead sea turtle peripheral blood mononuclear cells. J. Wildl Dis. 2000, 36, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Ganan-Gomez, I.; Clise-Dwyer, K.; Colla, S. Isolation, culture, and immunophenotypic analysis of bone marrow HSPCs from patients with myelodysplastic syndromes. STAR Protoc. 2022, 3, 101764. [Google Scholar] [CrossRef]
- Hamidabadi, H.G.; Bojnordi, M.N. Coculture of mouse spermatogonial stem cells with sertoli cell as a feeder layer, stimulates the proliferation and spermatogonial stemness profile. Middle East Fertil. Soc. J. 2018, 23, 107–111. [Google Scholar] [CrossRef]
- Schefe, J.H.; Lehmann, K.E.; Buschmann, I.R.; Unger, T.; Funke-Kaiser, H. Quantitative real-time RT-PCR data analysis: Current concepts and the novel “gene expression’s C T difference” formula. J. Mol. Med. 2006, 84, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Meng, X.; Yu, X.; Wang, G.; Dong, Z.; Zhou, Z.; Wang, F. From 2D to 3D co-culture systems: A review of co-culture models to study the neural cells interaction. Int. J. Mol. Sci. 2022, 23, 13116. [Google Scholar] [CrossRef] [PubMed]
- Trivanović, D.; Kocić, J.; Mojsilović, S.; Krstić, A.; Ilić, V.; Djordjević, I.O.; Santibanez, J.F.; Jovcić, G.; Terzić, M.; Bugarski, D. Mesenchymal stem cells isolated from peripheral blood and umbilical cord Wharton’s jelly. Srp. Arh. Celok. Lek. 2013, 141, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Yamawaki-Ogata, A.; Kanemoto, I.; Usui, A.; Narita, Y. Isolation and characterisation of peripheral blood-derived feline mesenchymal stem cells. Vet J. 2016, 216, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Lyahyai, J.; Mediano, D.R.; Ranera, B.; Sanz, A.; Remacha, A.R.; Bolea, R.; Martín-Burriel, I. Isolation and characterization of ovine mesenchymal stem cells derived from peripheral blood. BMC Vet. Res. 2012, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, L.; Cui, X.; Lin, X.; Li, Y.; Wang, Y.; Gao, F. Wt1 directs the lineage specification of sertoli and granulosa cells by repressing Sf1 expression. Development 2017, 144, 44–53. [Google Scholar] [CrossRef]
- Wang, W.J.; Wu, S.P.; Liu, J.B.; Shi, Y.S.; Huang, X.; Zhang, Q.B.; Yao, K.T. MYC regulation of CHK1 and CHK2 promotes radio resistance in a stem cell-like population of nasopharyngeal carcinoma cells. Cancer Res. 2013, 73, 1219–1231. [Google Scholar] [CrossRef]
- Piprek, R.P.; Kloc, M.; Kubiak, J.Z. Early development of the gonads: Origin and differentiation of the somatic cells of the genital ridges. In Molecular Mechanisms of Cell Differentiation in Gonad Development; Springer: Cham, Switzerland, 2016; pp. 1–22. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, Y.; Qu, R.; He, Y.; Tian, X.; Zeng, W. Spermatogonial stem cells from domestic animals: Progress and prospects. Reproduction 2014, 147, R65–R74. [Google Scholar] [CrossRef]
- Fujihara, M.; Kim, S.M.; Minami, N.; Yamada, M.; Imai, H. Characterization and in vitro culture of male germ cells from developing bovine testis. J. Reprod. Dev. 2011, 57, 355–364. [Google Scholar] [CrossRef]
- Herrid, M.; Davey, R.J.; Hill, J.R. Characterization of germ cells from pre-pubertal bull calves in preparation for germ cell transplantation. Cell Tissue Res. 2007, 330, 321–329. [Google Scholar] [CrossRef]
- Luo, J.; Megee, S.; Dobrinski, I. Asymmetric distribution of UCH-L1 in spermatogonia is associated with maintenance and differentiation of spermatogonial stem cells. J. Cell. Physiol. 2009, 220, 460–468. [Google Scholar] [CrossRef]
- Ram, K.; Kannan, T.; Basha, S.; Geetha Ramesh, G.R.; William, B. In-vitro culture morphology of Spermatogonial stem cells (SSCs) in mice. Int. J. Livest. Res. 2017, 7, 52–59. [Google Scholar] [CrossRef]
- Bahadorani, M.; Hosseini, S.M.; Abedi, P.; Hajian, M.; Afrough, M.; Azhdari, T.Z.; Nasr-Esfahani, M.H. Comparative immunohistochemical analysis of VASA, PLZF and THY1 in goats and sheep suggests that these markers are also conserved in these species. J. Cytol. Histol. 2011, 2, 126. [Google Scholar] [CrossRef]
- Nabulindo, N.W.; Nguhiu-Mwangi, J.; Kipyegon, A.N.E.; Ogugo, M.; Muteti, C.; Christian, T.; Kemp, S. Culture of Kenyan goat (Capra hircus) undifferentiated spermatogonia in feeder-free conditions. Front Vet Sci. 2022, 9, 894075. [Google Scholar] [CrossRef] [PubMed]
- Costoya, J.A.; Hobbs, R.M.; Barna, M.; Cattoretti, G.; Manova, K.; Sukhwani, M.; Pandolfi, P.P. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat. Genet. 2004, 36, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ye, S.; Liang, D.; Wang, P.; Fu, J.; Ma, Q.; Wang, Y. In vitro modeling of human germ cell development using pluripotent stem cells. Stem Cell Rep. 2018, 10, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Ma, W.; Shen, Q.; Zhang, M.; Du, Z.; Wu, C.; Hua, J. Reconstitution of male germline cell specification from mouse embryonic stem cells using defined factors in vitro. Cell Death Differ. 2019, 26, 2115–2124. [Google Scholar] [CrossRef]
- Nazm, B.M.; Ghasemi, H.; Narimanpour, Z. An Efficient In Vitro Culture System to Amplify Spermatogonia Stem Cell Markers. Res. Mol. Med. 2020, 8, 10–20. [Google Scholar] [CrossRef]
- Zheng, Y.; Tian, X.; Zhang, Y.; Qin, J.; An, J.; Zeng, W. In vitro propagation of male germline stem cells from piglets. J. Assist. Reprod. Genet. 2013, 30, 945–952. [Google Scholar] [CrossRef]
- Aponte, P.M.; Soda, T.; Teerds, K.J.; Mizrak, S.C.; Van de Kant, H.J.; de Rooij, D.G. Propagation of bovine spermatogonial stem cells in vitro. Reproduction 2008, 136, 543–557. [Google Scholar] [CrossRef]
- Luo, J.; Megee, S.; Rathi, R.; Dobrinski, I. Protein gene product 9.5 is a spermatogonia-specific marker in the pig testis: Application to enrichment and culture of porcine spermatogonia. Mol. Reprod. Dev. 2006, 12, 1531–1540. [Google Scholar] [CrossRef]
- Silva, J.; Nichols, J.; Theunissen, T.W.; Guo, G.; Van Oosten, A.L.; Barrandon, O.; Smith, A. Nanog is the gateway to the pluripotent ground state. Cell 2009, 138, 722–737. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Arooj, M.; Rashid, F.A.; Gul, A. Role of epigenetic modifications in stem cell regulatory regions (Oct4, Sox2 and Nanog) and cancer. IOSR J. Pharm. Biol. Sci. 2013, 5, 76–81. [Google Scholar] [CrossRef]
- Nagano, M.; Ryu, B.Y.; Brinster, C.J.; Avarbock, M.R.; Brinster, R.L. Maintenance of mouse male germ line stem cells in vitro. Biol. Reprod. 2003, 68, 2207–2214. [Google Scholar] [CrossRef] [PubMed]
- Browne, S.; Jha, A.K.; Ameri, K.; Marcus, S.G.; Yeghiazarians, Y.; Healy, K.E. TGF-β1/CD105 signaling controls vascular network formation within growth factor sequestering hyaluronic acid hydrogels. PLoS ONE 2018, 13, e0194679. [Google Scholar] [CrossRef] [PubMed]
- Niwa, H.; Miyazaki, J.I.; Smith, A.G. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat. Genet. 2000, 24, 372–376. [Google Scholar] [CrossRef]
- Malaver-Ortega, L.F.; Sumer, H.; Liu, J.; Verma, P.J. Inhibition of JAK-STAT ERK/MAPK and glycogen synthase kinase-3 induces a change in gene expression profile of bovine induced pluripotent stem cells. Stem Cells Int. 2016, 2016, 5127984. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Li, P.Z.; Pang, J.; Wan, Y.J.; Zhang, G.M.; Fan, Y.X.; Wang, F. Induction of goat bone marrow mesenchymal stem cells into putative male germ cells using mRNA for STRA8, BOULE and DAZL. Cytotechnology 2019, 71, 563–572. [Google Scholar] [CrossRef]
- Lee, J.H.; Engel, W.; Nayernia, K. Stem cell protein Piwil2 modulates expression of murine spermatogonial stem cell expressed genes. Mol. Reprod. Dev. 2006, 73, 173–179. [Google Scholar] [CrossRef]
- Saiti, D.; Lacham-Kaplan, O. Mouse Germ Cell Development in-vivo and in-vitro. Biomarker Insights 2007, 2, 117727190700200024. [Google Scholar] [CrossRef]
- Yuan, L.; Liu, J.-G.; Zhao, J.; Brundell, E.; Daneholt, B.; Hoog, C. The murine SCP3 gene is required for synaptonemal complex assembly, chromosome synapsis, and male fertility. Mol. Cell 2000, 5, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.L.; Baltus, A.E.; Roepers-Gajadien, H.L.; Hassold, T.J.; de Rooij, D.G.; van Pelt, A.M.; Page, D.C. Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc. Natl. Acad. Sci. USA 2008, 105, 14976–14980. [Google Scholar] [CrossRef] [PubMed]

| Genes | Nucleotide sequence (5′-3′) | n.Access | |
|---|---|---|---|
| Forward | Reverse | ||
| Endogenous genes | |||
| β- ACTINA | CGCACCACTGGCATTGTCAT | TCCAAGGCGACGTAGCAGAG | NM_173979.3 |
| GAPDH | CCTTCATTGACCTTCACTACATGG TCTA | TGGAAGATGGTGATGGCCTTTCCATTG | NM_001034034.2 |
| Mesenchymal stem cell genes | |||
| CD73 | TGGTCCAGGCCTATGCTTTTG | GGGATGCTGCTGTTGAGAAGAA | NM_174129.3 |
| CD105 | CGGACAGTGACCGTGAAGTTG | TGTTGTGGTTGGCCTCGATTA | NM_00107639.1 |
| Hematopoietic cell genes | |||
| CD34 | CATGCCGTCTTAACCCATCT | CGGTCTACAGAGGTGGTGGT | NM_174009.1 |
| CD45 | CCACGGGTATTCAGCAAGTT | CCCAGATCATCCTCCAGAAA | NM_001206523 |
| Sertoli cell genes | |||
| WT1 | CGTGCGTACCATGTAGGGAA | CTCGTGCTTGAAGGAGTGGT | XM_015474834.2 |
| AR | CAGATGGCAGTCATTCAG | CTTGGTGAGCTGGTAGAAG | XM_001244127 |
| Spermatogonial stem cell genes | |||
| UCHL1 | AGAAGCAGCATCTCGGTTCC | CGTGGTTGAGGGTAAGTGCT | NM_001046172.2 |
| PLZF | GCCGTGATACCGAGAGCAAC | CTCTCCTCGCTGGAATGCTT | NM_001037476.2 |
| CD90 | ACTCATACCGCTCCCGAACCA | CATGTGTATGTCCCCTCGTCCTT | NM_001034765 |
| Germ cell genes | |||
| DAZL | TCCAAGTTCACCAGTTCAGG | CGT CTG TAT GCT TCT GTC CAC | NM_001081725.1 |
| STRA8 | TGTGCCCAGGTGTTCATCTC | GGGGACTGTCACCTCATTGG | XM_015463130 |
| PIWIL2 | TCGTATTGATGATGTGGATTGG | GGGAGCAGCAGGATTTCAC | XM_617223.3 |
| FRAGILIS | ATCTGCAGCGAGACCTCTGT | CCGATGGACATGATGATGAG | XM_002697323 |
| STELLA | TGCAAGTTGCCACTCAACTC | TCTTACCCCTCTCCGCCTAT | NM_00111110 |
| VASA | TGCTACTCCTGGAAGACTGA | CGGTCTGCTGAACATCTCTA | NM_001007819.1 |
| SCP3 | CTAGAATTGTTCAGAGCCAGAG | GTTCAAGTTCTTTCTTCAAAG | NM_001040588.2 |
| Pluripotency genes | |||
| OCT4 | GAAAGAGAAAGCGGACGAG | GTGAAAGGAGACCCAGCAG | NM_174580.2 |
| NANOG | TAAGCACAGGGGGCAAAAGT | ATGGCTAAAAGGGGTGGAGG | NM_001025344.1 |
| SOX2 | CCCGTGGTTACCTCTTCTTCC | CGCTCTGCTAGTGCTGGGAC | NM_001105463.2 |
| Marker | PB-MSCs-SCs/CM | SSCs- SCs/CM |
|---|---|---|
| CD73 | ↑ (Q-PCR) | - |
| CD105 | ↑ (Q-PCR) | - |
| DAZL | x (Q-PCR) | ↑ (Q-PCR) *(IF) |
| STRA8 | x (Q-PCR) | x (Q-PCR) |
| PIWIL2 | ↑ (Q-PCR) *(IF) | ↑ (Q-PCR) *(IF) |
| FRAGILIS | x (Q-PCR) | x (Q-PCR) |
| STELLA | x (Q-PCR) | x (Q-PCR) |
| OCT4 | ↑ (Q-PCR) *(IF) | x (Q-PCR) *(IF) |
| NANOG | x (Q-PCR) | ↑ (Q-PCR) *(IF) |
| SOX2 | ↑ (Q-PCR) | ↑ (Q-PCR) |
| SCP3 | x (Q-PCR) | x (Q-PCR) |
| VASA | x (Q-PCR) | x (Q-PCR) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segunda, M.N.; Díaz, C.; Torres, C.G.; Parraguez, V.H.; De los Reyes, M.; Peralta, O.A. Bovine Peripheral Blood-Derived Mesenchymal Stem Cells (PB-MSCs) and Spermatogonial Stem Cells (SSCs) Display Contrasting Expression Patterns of Pluripotency and Germ Cell Markers under the Effect of Sertoli Cell Conditioned Medium. Animals 2024, 14, 803. https://doi.org/10.3390/ani14050803
Segunda MN, Díaz C, Torres CG, Parraguez VH, De los Reyes M, Peralta OA. Bovine Peripheral Blood-Derived Mesenchymal Stem Cells (PB-MSCs) and Spermatogonial Stem Cells (SSCs) Display Contrasting Expression Patterns of Pluripotency and Germ Cell Markers under the Effect of Sertoli Cell Conditioned Medium. Animals. 2024; 14(5):803. https://doi.org/10.3390/ani14050803
Chicago/Turabian StyleSegunda, Moisés N., Carlos Díaz, Cristian G. Torres, Víctor H. Parraguez, Mónica De los Reyes, and Oscar A. Peralta. 2024. "Bovine Peripheral Blood-Derived Mesenchymal Stem Cells (PB-MSCs) and Spermatogonial Stem Cells (SSCs) Display Contrasting Expression Patterns of Pluripotency and Germ Cell Markers under the Effect of Sertoli Cell Conditioned Medium" Animals 14, no. 5: 803. https://doi.org/10.3390/ani14050803
APA StyleSegunda, M. N., Díaz, C., Torres, C. G., Parraguez, V. H., De los Reyes, M., & Peralta, O. A. (2024). Bovine Peripheral Blood-Derived Mesenchymal Stem Cells (PB-MSCs) and Spermatogonial Stem Cells (SSCs) Display Contrasting Expression Patterns of Pluripotency and Germ Cell Markers under the Effect of Sertoli Cell Conditioned Medium. Animals, 14(5), 803. https://doi.org/10.3390/ani14050803






