Detection of Booroola Polymorphism of Bone Morphogenetic Protein Receptor 1b and Embrapa Polymorphism of Growth Differentiation Factor 9 in Sheep in Thailand
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sample Collection
2.2. DNA Extraction
2.3. Primers Designing
2.4. Polymerase Chain Reaction (PCR) Condition
2.5. Restriction Fragment Length Polymorphism (RFLP) and Gel Electrophoresis
2.6. Direct DNA Sequencing of PCR Products
2.7. Data Collection and Statistical Analysis
3. Results
3.1. PCR-RFLP Results of FecB and FecGE
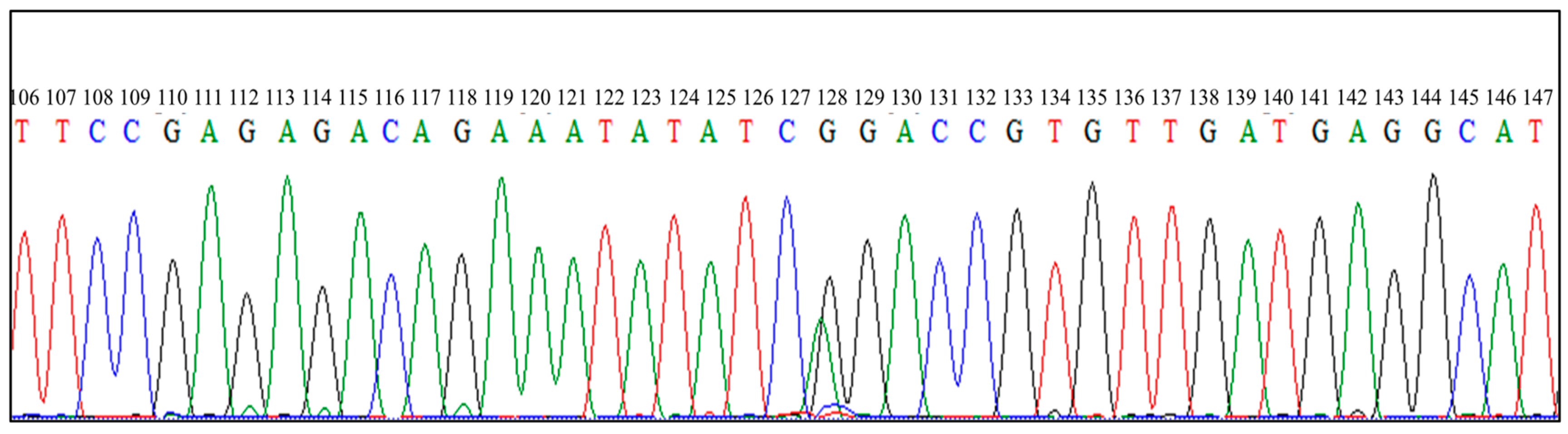
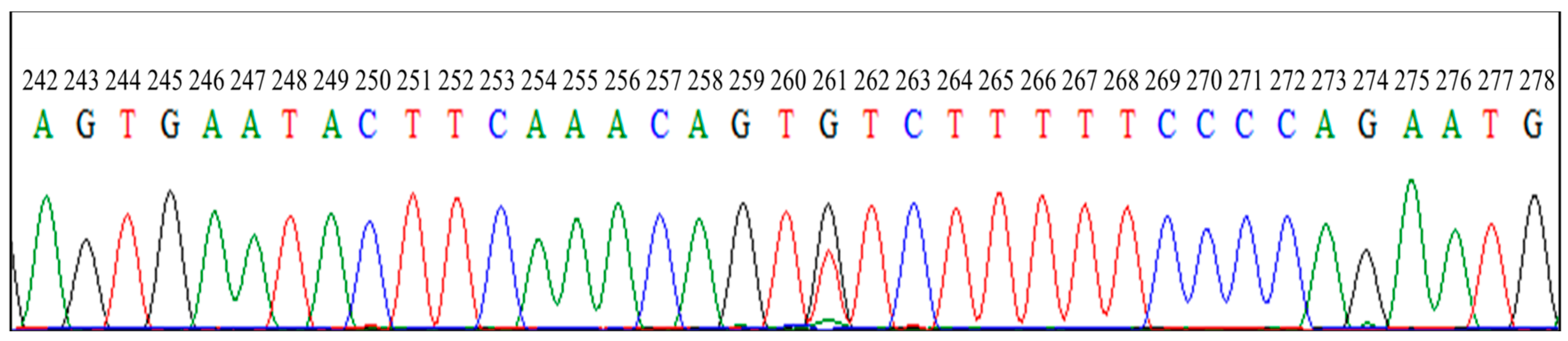
3.2. Direct Sequencing of PCR Products
3.3. History of Birth Types of Ewe and Association between Fec Genotypes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drouilhet, L.; Lecerf, F.; Bodin, L.; Fabre, S. Fine mapping of the FecL locus influencing prolificacy in Lacaune sheep. Anim. Genet. 2009, 40, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Mulsant, P.; Lecerf, F.; Fabre, S.; Schibler, L.; Monget, P.; Lanneluc, I.; Pisselet, C.; Riquet, J.; Monniaus, D.; Callebaut, I.; et al. Mutation in bone morphogenetic protein receptor-IB is associated with increased ovulation rate in Booroola Mérino ewes. Proc. Natl. Acad. Sci. USA 2001, 98, 5104–5109. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.; Wu, X.Y.; Juengel, J.L.; Ross, I.K.; Lumsden, J.M.; Lord, E.A.; Dodds, K.G.; Walling, G.A.; McEwan, J.C.; O’Connell, A.R.; et al. Highly prolific Booroola sheep have a mutation in the intracellular kinase domain of bone morphogenetic protein IB recepter (ALK-6) that is expressed in both oocytes and granulosa cells. Biol. Reprod. 2001, 64, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.H.; Montgomery, G.W.; Allison, A.J.; Kelly, R.W.; Bray, A.R. Segregation of a major gene influencing fecundity in progeny of Booroola sheep. N. Z. J. Agric. Res. 1982, 25, 525–529. [Google Scholar] [CrossRef]
- Fabre, S.; Pierre, A.; Pisselet, C.; Mulsant, P.; Lecerf, F.; Pohl, J.; Monget, P.; Monniaux, D. The Booroola mutation in sheep is associated with an alteration of the bone morphogenetic protein receptor-IB functionality. J. Endocrinol. 2003, 177, 435–444. [Google Scholar] [CrossRef][Green Version]
- Montgomery, G.W.; McNatty, K.P.; Davis, G.H. Physiology and molecular genetics of mutations that increase ovulation rate in sheep. Endocr. Rev. 1992, 13, 309–328. [Google Scholar] [CrossRef] [PubMed]
- McNatty, K.P.; Henderson, K.M. Gonadotrophins, fecundity genes and ovarian follicular function. J. Steroid Biochem. 1987, 27, 365–373. [Google Scholar] [CrossRef]
- Potki, P.; Mirhoseini, S.Z.; Afraz, F.; Vahidi, S.M.F. A profile of single nucleotide polymorphisms in fecundity genes among Iranian sheep breeds by using polymerase chain reaction fragment length polymorphism (PCR-RFLP) method. Iran. J. Applied Anim. Sci. 2020, 10, 265–285. [Google Scholar]
- Fogarty, N. A review of the effects of the Booroola gene (FecB) on sheep production. Small Rumin. Res. 2009, 85, 75–84. [Google Scholar] [CrossRef]
- Hanrahan, J.P.; Gregan, S.M.; Mulsant, P.; Mullen, M.; Davis, G.H.; Powell, R.; Galloway, S.M. Mutations in the genes for oocyte-derived growth factors GDF9 and BMP15 are associated with both increased ovulation rate and sterility in Cambridge and Belclare sheep (Ovis aries). Biol. Reprod. 2004, 70, 900–909. [Google Scholar] [CrossRef]
- Nicol, L.; Bishop, S.C.; Pong-Wong, R.; Bendixen, C.; Holm, L.E.; Rhind, S.M.; McNeilly, A.S. Homozygosity for a single base-pair mutation in the oocyte-specific GDF9 gene results in sterility in Thoka sheep. Reproduction 2009, 138, 921–933. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.D.M.; Castro, E.A.; Souza, C.J.H.; Paiva, S.R.; Sartori, R.; Franco, M.M.; Azevedo, H.C.; Silva, T.A.S.N.; Vieira, A.M.C.; Neves, J.P.; et al. A new polymorphism in the Growth and Differentiation Factor 9 (GDF9) gene is associated with increased ovulation rate and prolificacy in homozygous sheep. Anim. Genet. 2011, 42, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.J.H.; McNeilly, A.S.; Benavides, M.V.; Melo, E.O.; Moraes, J.C.F. Mutation in the protease cleavage site of GDF9 increases ovulation rate and litter size in heterozygous ewes and causes infertility in homozygous ewes. Anim. Genet. 2014, 45, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Mullen, M.P.; Hanrahan, J.P. Direct evidence on the contribution of a missense mutation in GDF9 to variation in ovulation rate of Finnsheep. PLoS ONE 2014, 9, e95251. [Google Scholar] [CrossRef] [PubMed]
- Våge, D.I.; Husdal, M.; Kent, M.P.; Klemetsdal, G.; Boman, I.A. A missense mutation in growth differentiation factor 9 (GDF9) is strongly associated with litter size in sheep. BMC Genet. 2013, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ruiz, E.; Gallegos-Sánchez, J.; Cortez-Romero, C.; Segura-León; Salinas-Ruíz, J.; Salazar-Ortiz, J. FecGE mutation in pelibuey sheep. Anim. Genet. 2020, 51, 346–347. [Google Scholar] [CrossRef] [PubMed]
- DLD. National Animal Statistics. 2022. Available online: http://ict.dld.go.th/webnew/index.php/th/service-ict/report/247-report-thailand-livestock (accessed on 25 April 2022).
- Abdoli, R.; Zamani, P.; Deljou, A.; Rezvan, H. Association of BMPR-1B and GDF9 genes polymorphisms and secondary protein structure changes with reproduction traits in Mehraban ewes. Gene 2013, 524, 296–303. [Google Scholar] [CrossRef]
- Davis, G.H.; Galloway, S.M.; Ross, I.K.; Gregan, S.M.; Ward, J.; Nimbkar, B.V.; Ghalsasi, P.M.; Nimbkar, C.; Grey, G.D.; Subandriyo; et al. DNA tests in prolific sheep from eight countries provide new evidence on origin of the Booroola (FecB) mutation. Biol. Reprod. 2002, 66, 1869–1874. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids. Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Allaire, J.J. RStudio: Integrated Development Environment for R; RStudio; PBC: Boston, MA, USA, 2022. [Google Scholar]
- DLD. Sheep Farming, 1st ed.; Guide Book, DLD, Ministry of Agriculture and Cooperatives; Banlengthong, S., Ed.; The Agricultural Cooperative Federation of Thailand., Limited, Publishing and Public Relations Group, Division of Livestock Development and Technology Transfer, DLD: Bangkok, Thailand, 2005; p. 36. [Google Scholar]
- Zuo, B.; Qian, H.; Wang, Z.; Wang, X.; Nisa, N.; Bayier, A.; Ying, S.; Hu, X.; Gong, C.; Guo, Z.; et al. A study on BMPR-IB genes of Bayanbulak sheep. Asian-Australas. J. Anim. Sci. 2013, 26, 36–42. [Google Scholar] [CrossRef]
- Zhang, C.S.; Geng, L.Y.; Du, L.X.; Liu, X.Z.; Fu, Z.X.; Feng, M.S.; Gong, Y.F. Polymorphic study of FecXG, FecGH, and FecB mutations in four domestic sheep breeds in the lower Yellow River Valley of China. J. Anim. Vet. Adv. 2011, 10, 2198–2201. [Google Scholar] [CrossRef]
- Nanekarani, S.; Goodarzi, M.; Khederzadeh, S. Detection of polymorphism in booroola gene and growth differentiation factor 9 in Lori sheep breed. Trop. J. Pharm. Res. 2016, 15, 1605–16011. [Google Scholar] [CrossRef]
- Jia, C.; Li, N.; Zhao, X.; Zhu, X.; Jia, Z. Association of single nucleotide polymorphisms in exon 6 region of BMPRIB gene with litter size traits in sheep. Asian-Australas. J. Anim. Sci. 2005, 18, 1375–1378. [Google Scholar] [CrossRef]
- Moradband, F.; Rahimi, G.; Gholizadeh, M. Assocation of polymorphisms in fecundity genes of GDF9, BMP15 and BMP15-1B with litter size in Iranian Baluchi sheep. Asian-Australas. J. Anim. Sci. 2011, 24, 1179–1183. [Google Scholar] [CrossRef]
- Liandris, E.; Kominakis, A.; Andreadou, M.; Kapeoldassi, K.; Chadio, S.; Tsiligianni, T.; Gazouli, M.; Ikonomopoulos, J.; Kominakis, I. Association between single nucleotide polymorphisms of GD9 and BMP15 genes and litter size in two dairy sheep breeds of Greece. Small Rumin. Res. 2012, 107, 16–21. [Google Scholar] [CrossRef]


| Gene | Fec Alleles (b) | Primer Name | Primer Sequences (5′-3′) | Product Size |
|---|---|---|---|---|
| BMPR1B | FecB | FecB-F2 * | CCAGAGGACAATAGCAAAGCAAA | 190 bp |
| FecB-R15 * | CAAGATGTTTTCATGCCTCATCAACACGGTC | |||
| GDF9 | FecGE | GE-outF4 | CAGCCTGTTTAACATGACTC | 457 bp |
| GE-outR4 | GTTCTGCACCATGGTGT |
| Fec Alleles (a) | No. of Animals | Genotypic Frequency (b) (%) | Allelic Frequency (%) | HWE + (c) | |||
|---|---|---|---|---|---|---|---|
| FecB (A > G) | 454 | +/+ | +/B | B/B | B | + | p < 0.01 |
| 446 (98.24) | 7 (1.54) | 1 (0.22) | 9 (0.99) | 899 (99.01) | |||
| FecGE (T > G) | 454 | +/+ | +/E | E/E | E | + | p = 0.97 |
| 443 (97.58) | 11 (2.42) | - (-) | 11 (1.21) | 897 (98.79) | |||
| Birth Types | Combined FecB and FecGE Haplotypes; n (%) | Total (%) | ||||
|---|---|---|---|---|---|---|
| +/+, +/+ | +/B, +/+ | +/+, +/E | +/B, +/E | B/B, +/E | ||
| History of lambing multiple births | 9 (60.00) | 3 (20.00) | 1 (6.67) | 2 (13.33) | - (-) | 15 (3.49) |
| Lambing only single birth | 380 (98.96) | 1 (0.26) | 3 (0.78) | - (-) | - (-) | 384 (89.30) |
| Nulliparous | 29 (93.55) | 1 (3.22) | - (-) | - (-) | 1 (3.22) | 31 (7.21) |
| Fec Allele (a) | Genotype (b) | History of Lambing Multiple Births | Lambing Only Single Birth | p-Value | Odds Ratio | 95% CI (c) |
|---|---|---|---|---|---|---|
| Non-carrier | +/+ | 9 | 380 | |||
| FecB | +/B | 3 | 1 | <0.01 | 117.5 | 8.6, 6295.3 |
| FecGE | +/E | 1 | 3 | 0.098 | 13.7 | 0.2, 192.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sae-Foo, P.; Triwutanon, S.; Rukkwamsuk, T. Detection of Booroola Polymorphism of Bone Morphogenetic Protein Receptor 1b and Embrapa Polymorphism of Growth Differentiation Factor 9 in Sheep in Thailand. Animals 2024, 14, 809. https://doi.org/10.3390/ani14050809
Sae-Foo P, Triwutanon S, Rukkwamsuk T. Detection of Booroola Polymorphism of Bone Morphogenetic Protein Receptor 1b and Embrapa Polymorphism of Growth Differentiation Factor 9 in Sheep in Thailand. Animals. 2024; 14(5):809. https://doi.org/10.3390/ani14050809
Chicago/Turabian StyleSae-Foo, Poothana, Supawit Triwutanon, and Theera Rukkwamsuk. 2024. "Detection of Booroola Polymorphism of Bone Morphogenetic Protein Receptor 1b and Embrapa Polymorphism of Growth Differentiation Factor 9 in Sheep in Thailand" Animals 14, no. 5: 809. https://doi.org/10.3390/ani14050809
APA StyleSae-Foo, P., Triwutanon, S., & Rukkwamsuk, T. (2024). Detection of Booroola Polymorphism of Bone Morphogenetic Protein Receptor 1b and Embrapa Polymorphism of Growth Differentiation Factor 9 in Sheep in Thailand. Animals, 14(5), 809. https://doi.org/10.3390/ani14050809






