Evaluation of the Difference in the Content of Essential and Non-Essential Elements in Wild Boar and Swine Tissues Sampled in the Same Area of Northern Italy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
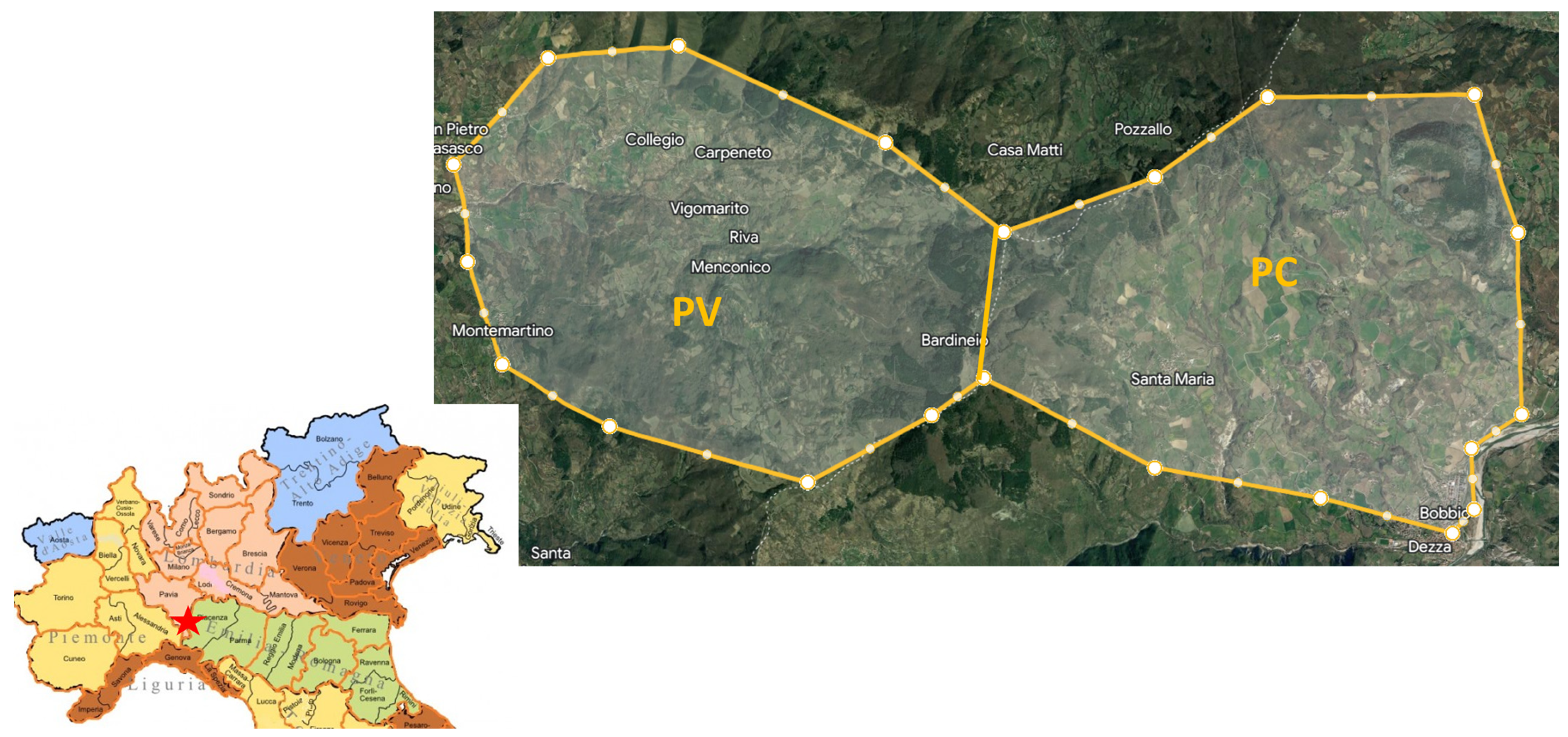
2.1. Sample Collection
2.2. Essential and Non-Essential Elements Analysis with ICP-MS
2.2.1. Preparation of Standard Solutions and Method Validation
2.2.2. Sample Treatment
2.3. Statistical Analysis
3. Results
3.1. Detected Concentrations of Essential and Non-Essential Elements in Muscle and Liver of Wild Boar and Swine
3.2. Comparison between Concentrations of Essential and Non-Essential Elements in Wild Boar Tissues According to the Two Different Areas
3.3. Comparison between Concentrations of Essential and Non-Essential Elements in Wild Boar Tissues According to Different Age Classes
3.4. Comparison between Mean Concentrations of Essential and Non-Essential Elements in Wild Boar Tissues According to Sex
3.5. Comparison between Concentrations of Essential and Non-Essential Elements in Tissues of the Two Species
4. Discussion
4.1. Tissue Concentration of Essential and Non-Essential Elements in Wild Boar and Swine
4.2. Comparison between Concentrations of Essential and Non-Essential Elements in Tissues of Wild Boar According to the Two Different Areas
4.3. Comparison between Concentrations of Essential and Non-Essential Elements in Tissues of Wild Boar According to the Two Different Age Classes
4.4. Comparison between Concentrations of Essential and Non-Essential Elements in Tissues of Wild Boar According to Sex
4.5. Comparison between Concentrations of Essential and Non-Essential Elements in Tissues of Wild Boar and Swine
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malmsten, A.; Dalin, A.-M.; Pettersson, J.; Persson, S. Concentrations of cadmium, lead, arsenic, and some essential metals in wild boar from Sweden. Eur. J. Wildl. Res. 2021, 67, 18. [Google Scholar] [CrossRef]
- Kamunda, C.; Mathuthu, M.; Madhuku, M. Health Risk Assessment of Heavy Metals in Soils from Witwatersrand Gold Mining Basin, South Africa. Int. J. Environ. Res. Public Health 2016, 13, 663. [Google Scholar] [CrossRef]
- Pieczyńska, J.; Grajeta, H. The role of selenium in human conception and pregnancy. J. Trace Elem. Med. Biol. 2015, 29, 31–38. [Google Scholar] [CrossRef]
- Chan, W.Y.; Rennert, O.M. The role of copper in iron metabolism. Ann. Clin. Lab. Sci. 1980, 10, 338–344. [Google Scholar]
- Zimmermam, J.J.; D’Allaire, S.; Taylor, D.J. Diseases of Swine; Institute for Clinical Science, Inc.: London, UK, 2013. [Google Scholar]
- Hill, G.M.; Ku, P.K.; Miller, E.R.; Ullrey, D.E.; Losty, T.A.; O’Dell, B.L. A Copper Deficiency in Neonatal Pigs Induced by a High Zinc Maternal Diet. J. Nutr. 1983, 113, 867–872. [Google Scholar] [CrossRef]
- Novotny, J.A.; Peterson, C.A. Molybdenum. Adv. Nutr. 2018, 9, 272–273. [Google Scholar] [CrossRef] [PubMed]
- Pantopoulos, K.; Porwal, S.K.; Tartakoff, A.; Devireddy, L. Mechanisms of Mammalian Iron Homeostasis. Biochemistry 2012, 51, 5705–5724. [Google Scholar] [CrossRef]
- Wallace, D.F. The Regulation of Iron Absorption and Homeostasis. Clin. Biochem. Rev. 2016, 37, 51–62. [Google Scholar] [PubMed]
- IARC. List of classifications-Agents classified by the IARC Monographs. Available online: https://monographs.iarc.who.int/agents-classified-by-the-iarc/ (accessed on 27 October 2023).
- Li, L.; Yang, X. The Essential Element Manganese, Oxidative Stress, and Metabolic Diseases: Links and Interactions. Oxidative Med. Cell. Longev. 2018, 2018, 7580707. [Google Scholar] [CrossRef]
- Edmunds, C.E.; Cornelison, A.S.; Farmer, C.; Rapp, C.; Ryman, V.E.; Schweer, W.P.; Wilson, M.E.; Dove, C.R. The Effect of Increasing Dietary Manganese from an Organic Source on the Reproductive Performance of Sows. Agriculture 2022, 12, 2168. [Google Scholar] [CrossRef]
- Avila, D.S.; Gubert, P.; Roos, D.H.; Puntel, R.; Aschner, M. Manganese. In Encyclopedia of Food and Health; Academic Press: Cambridge, MA, USA, 2016; pp. 637–640. [Google Scholar]
- Yang, Y.; Khan, Z.; Ahmad, K.; Arshad, N.; Rehman, S.U.; Ullah, M.F.; Wajid, K.; Mahpara, S.; Bashir, H.; Nadeem, M.; et al. Does the Chromium Element in Forages and Fodders Grown in Contaminated Pasture Lands Cause Toxicity in Livestock: Assessing the Potential Risk. Rev. De Chim. 2020, 71, 397–405. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef]
- Squadrone, S.; Brizio, P.; Mancini, C.; Abete, M.C.; Brusco, A. Altered homeostasis of trace elements in the blood of SCA2 patients. J. Trace Elem. Med. Biol. 2018, 47, 111–114. [Google Scholar] [CrossRef]
- Zavaliy, L.B.; Petrikov, S.S.; Simonova, A.Y.; Potskhveriya, M.M.; Zaker, F.; Ostapenko, Y.N.; Ilyashenko, K.K.; Dikaya, T.I.; Shakhova, O.B.; Evseev, A.K.; et al. Diagnosis and treatment of persons with acute thallium poisoning. Toxicol. Rep. 2021, 8, 277–281. [Google Scholar] [CrossRef]
- Rehder, D. The role of vanadium in biology. Metallomics 2015, 7, 730–742. [Google Scholar] [CrossRef]
- Lam, P.K.S. Use of biomarkers in environmental monitoring. Ocean Coast. Manag. 2009, 52, 348–354. [Google Scholar] [CrossRef]
- Holt, E.A.; Miller, S.W. Bioindicators: Using Organisms to Measure Environmental Impacts. Available online: https://www.nature.com/scitable/knowledge/library/bioindicators-using-organisms-to-measure-environmental-impacts-16821310/ (accessed on 13 October 2023).
- Kalisińska, E. Endothermic Animals as Biomonitors of Terrestrial Environments. In Mammals and Birds as Bioindicators of Trace Element Contaminations in Terrestrial Environments, 1st ed.; Kalisińska, E., Ed.; Springer Cham: Cham, Switzerland, 2019; p. 708. [Google Scholar]
- Puschenreiter, M. Trace Elements as Contaminants and Nutrients: Consequences in Ecosystems and Human Health.Edited by M. N. V. Prasad. ChemSusChem 2009, 2, 677. [Google Scholar] [CrossRef]
- Agradi, S.; Munga, A.; Barbato, O.; Palme, R.; Dokuzeylül, B.; Ercan, A.M.; Or, M.E.; Brecchia, G.; Curone, G.; Draghi, S. Goat Hair as a Bioindicator of Environmental Contaminants and Adrenal Activation During Vertical Transhumance. Front. Vet. Sci. 2023, 10, 1274081. [Google Scholar] [CrossRef]
- Draghi, S.; Pavlovic, R.; Pellegrini, A.; Fidani, M.; Riva, F.; Brecchia, G.; Agradi, S.; Arioli, F.; Vigo, D.; Di Cesare, F.; et al. First Investigation of the Physiological Distribution of Legacy and Emerging Perfluoroalkyl Substances in Raw Bovine Milk According to the Component Fraction. Foods 2023, 12, 2449. [Google Scholar] [CrossRef]
- Russo, D.; Salinas-Ramos, V.B.; Cistrone, L.; Smeraldo, S.; Bosso, L.; Ancillotto, L. Do We Need to Use Bats as Bioindicators? Biology 2021, 10, 693. [Google Scholar] [CrossRef]
- Lee, S.-M.; Lee, E.-J. Diet of the wild boar (Sus scrofa): Implications for management in forest-agricultural and urban environments in South Korea. PeerJ 2019, 7, e7835. [Google Scholar] [CrossRef]
- Schley, L.; Roper, T.J. Diet of wild boar Sus scrofa in Western Europe, with particular reference to consumption of agricultural crops. Mammal Rev. 2003, 33, 43–56. [Google Scholar] [CrossRef]
- Barrios-Garcia, M.N.; Ballari, S.A. Impact of wild boar (Sus scrofa) in its introduced and native range: A review. Biol. Invasions 2012, 14, 2283–2300. [Google Scholar] [CrossRef]
- Kowalczyk, J.; Numata, J.; Zimmermann, B.; Klinger, R.; Habedank, F.; Just, P.; Schafft, H.; Lahrssen-Wiederholt, M. Suitability of Wild Boar (Sus scrofa) as a Bioindicator for Environmental Pollution with Perfluorooctanoic Acid (PFOA) and Perfluorooctanesulfonic Acid (PFOS). Arch. Environ. Contam. Toxicol. 2018, 75, 594–606. [Google Scholar] [CrossRef]
- Channon, H.A.; Dybing, N.A.; Marshall, D.; Gentle, M.N. Feral Pigs; Elsevier Inc.: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Jota Baptista, C.; Seixas, F.; Gonzalo-Orden, J.M.; Patinha, C.; Pato, P.; Ferreira Da Silva, E.; Merino-Goyenechea, L.J.; Oliveira, P.A. Heavy metals and metalloids in wild boars (Sus scrofa)—Silent but serious public health hazard. Vet. Res. Commun. 2023. [Google Scholar] [CrossRef]
- European Union. COMMISSION REGULATION (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006. Available online: https://eur-lex.europa.eu/eli/reg/2023/915/oj (accessed on 17 October 2023).
- Bilandžić, N.; Sedak, M.; Čalopek, B.; Đokić, M.; Varenina, I.; Solomun Kolanović, B.; Božić Luburić, Đ.; Varga, I.; Roncarati, A. Evaluation of Element Concentrations in Beef and Pork Meat Cuts Available to the Population in the Croatian Capital. Foods 2020, 9, 1861. [Google Scholar] [CrossRef]
- Lénárt, Z.; Bartha, A.; Abonyi-Tóth, Z.; Lehel, J. Monitoring of metal content in the tissues of wild boar (Sus scrofa) and its food safety aspect. Environ. Sci. Pollut. Res. 2022, 30, 15899–15910. [Google Scholar] [CrossRef]
- Piskorová, L.; Vasilkova, Z.; Krupicer, I. Heavy metal residues in tissues of wild boar (Sus scrofa) and red fox (Vulpes vulpes) in the Central Zemplin region of the Slovak Republic. Czech J. Anim. Sci. 2003, 48, 134–138. [Google Scholar]
- Draghi, S.; Agradi, S.; Riva, F.; Tarhan, D.; Bilgiç, B.; Dokuzeylül, B.; Ercan, A.M.; Or, M.E.; Brecchia, G.; Vigo, D.; et al. Roe Deer (Capreolus capreolus) Hair as a Bioindicator for the Environmental Presence of Toxic and Trace Elements. Toxics 2023, 11, 49. [Google Scholar] [CrossRef]
- Pilarczyk, B.; Tomza-Marciniak, A.; Pilarczyk, R.; Udała, J.; Kruzhel, B.; Ligocki, M. Content of essential and non-essential elements in wild animals from western Ukraine and the health risks associated with meat and liver consumption. Chemosphere 2020, 244, 125506. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, F.; Domínguez, R.; Maggiolino, A.; Pateiro, M.; Carballo, J.; De Palo, P.; Barba, F.J.; Lorenzo, J.M. Meat Quality of Commercial Chickens Reared in Different Production Systems: Industrial, Range and Organic. Ann. Anim. Sci. 2020, 20, 263–285. [Google Scholar] [CrossRef]
- Nkosi, D.V.; Bekker, J.L.; Hoffman, L.C. Toxic Metals in Wild Ungulates and Domestic Meat Animals Slaughtered for Food Purposes: A Systemic Review. Foods 2021, 10, 2853. [Google Scholar] [CrossRef] [PubMed]
- Falandysz, J.; Szymczyk–Kobrzyńska, K.; Brzostowski, A.; Zalewski, K.; Zasadowski, A. Concentrations of heavy metals in the tissues of red deer (Cervus elaphus) from the region of Warmia and Mazury, Poland. Food Addit. Contam. 2005, 22, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Giżejewska, A.; Szkoda, J.; Nawrocka, A.; Żmudzki, J.; Giżejewski, Z. Can red deer antlers be used as an indicator of environmental and edible tissues’ trace element contamination? Environ. Sci. Pollut. Res. 2017, 24, 11630–11638. [Google Scholar] [CrossRef]
- Mtemi, W.M.; Liu, S.; Liu, K.; Wei, L.; Wang, X.; Jiang, A.; Goodale, E. Concentrations and biomagnification of multiple metals/metalloids are higher in rice than in sugarcane agroecosystems of southern China. Ecol. Indic. 2023, 150, 110266. [Google Scholar] [CrossRef]
- ISPRA. Geological and Geotematics Map. Available online: https://www.isprambiente.gov.it/en/databases/data-base-collection/soil-and-territory/geological-and-geotematics-map (accessed on 11 November 2023).
- Silver, W.L.; Neff, J.; McGroddy, M.; Veldkamp, E.; Keller, M.; Cosme, R. Effects of Soil Texture on Belowground Carbon and Nutrient Storage in a Lowland Amazonian Forest Ecosystem. Ecosystems. 2000, 3, 193–209. [Google Scholar] [CrossRef]
- Battini, F.; Valli, R.; Corradi, C. Coltivazioni Erbacee e Arboree; Edagricole, Ed.; Rizzoli: Segrate, MI, Italy, 2005; p. 528. [Google Scholar]
- Regione Campagna. Guida Alla Concimazione: Metodi, Procedure e Strumenti per un Servizio di Consulenza. Available online: http://www.agricoltura.regione.campania.it/concimazione/pdf/GUIDA2012.pdf (accessed on 12 November 2023).
- McDowell, L.R. Minerals in Animal Nutrition; Cunha, T.J., Ed.; Academic Press: Cambridge, MA, USA, 1992. [Google Scholar]
- Gupta, R.C. Veterinary Toxicology Basic and Clinical Principles; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Sydor, A.M.; Zamble, D.B. Nickel Metallomics: General Themes Guiding Nickel Homeostasis. In Metallomics and the Cell, 1st ed.; Banci, L., Ed.; Metals Ion in Life Sciences; Springer: Dordrecht, The Netherlands; Cham, Switzerland, 2012; Volume 12, p. 609. [Google Scholar]
- Hunt, N.J.; Kang, S.W.S.; Lockwood, G.P.; Le Couteur, D.G.; Cogger, V.C. Hallmarks of Aging in the Liver. Comput. Struct. Biotechnol. J. 2019, 17, 1151–1161. [Google Scholar] [CrossRef]
- García-Muñoz, J.; Cacciola, N.A.; Plazzi, F.; Prado Míguez-Santiyán, M.; Rodríguez, F.S.; López-Beceiro, A.; Fidalgo, L.E.; Martínez-Morcillo, S.; Pérez-López, M. Metal and metalloid concentrations in wild mammals from SW Europe: European hedgehog (Erinaceus europaeus) and badger (Meles meles). Environ. Sci. Pollut. Res. 2023, 30, 118855–118870. [Google Scholar] [CrossRef] [PubMed]
- Underwood, E.J.; Suttle, N.F. Copper. In The Mineral Nutrition of Livestock; CABI, Ed.; CABI Books, 2022; Available online: https://en.wikipedia.org/wiki/CAB_International (accessed on 11 November 2023).
- Espinosa, C.D.; Stein, H.H. Digestibility and metabolism of copper in diets for pigs and influence of dietary copper on growth performance, intestinal health, and overall immune status: A review. J. Anim. Sci. Biotechnol. 2021, 12, 13. [Google Scholar] [CrossRef] [PubMed]
- Pajarillo, E.A.B.; Lee, E.; Kang, D.K. Trace metals and animal health: Interplay of the gut microbiota with iron, manganese, zinc, and copper. Anim. Nutr. 2021, 7, 750–761. [Google Scholar] [CrossRef]
- Vizuete, J.; Hernández-Moreno, D.; López-Beceiro, A.; Fidalgo, L.E.; Soler, F.; Pérez-López, M.; Míguez-Santiyán, M.P. Heavy metals and metalloid levels in the tissues of yellow-legged gulls (Larus michahellis) from Spain: Sex, age, and geographical location differences. Environ. Sci. Pollut. Res. 2022, 29, 54292–54308. [Google Scholar] [CrossRef]
- Burger, J.; Fossi, C.; McClellan-Green, P.; Orlando, E.F. Methodologies, bioindicators, and biomarkers for assessing gender-related differences in wildlife exposed to environmental chemicals. Env. Res. 2007, 104, 135–152. [Google Scholar] [CrossRef]
- Kasprzyk, A.; Kilar, J.; Chwil, S.; Rudaś, M. Content of Selected Macro- and Microelements in the Liver of Free-Living Wild Boars (Sus scrofa L.) from Agricultural Areas and Health Risks Associated with Consumption of Liver. Animals 2020, 10, 1519. [Google Scholar] [CrossRef]
- Cygan-Szczegielniak, D.; Stasiak, K. Effects of age and sex on the content of heavy metals in the hair, liver and the longissimus lumborum muscle of roe deer Capreolus capreolus L. Environ. Sci. Pollut. Res. Int. 2022, 29, 10782–10790. [Google Scholar] [CrossRef]
- Suzuki, K.T.; Tamagawa, H.; Takahashi, K.; Shimojo, N. Pregnancy-induced mobilization of copper and zinc bound to renal metallothionein in cadmium-loaded rats. Toxicology 1990, 60, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Neila, C.; Hernández-Moreno, D.; Fidalgo, L.E.; López-Beceiro, A.; Soler, F.; Pérez-López, M. Does gender influence the levels of heavy metals in liver of wild boar? Ecotoxicol. Env. Saf. 2017, 140, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Perez-Lopez, M.; Soler Rodriguez, F.; Hernandez-Moreno, D.; Rigueira, L.; Fidalgo, L.E.; Lopez Beceiro, A. Bioaccumulation of cadmium, lead and zinc in liver and kidney of red fox (Vulpes vulpes) from NW Spain: Influence of gender and age. Toxicol. Environ. Chem. 2015, 98, 109–117. [Google Scholar] [CrossRef]
- Muhetaer, M.; Yang, M.; Xia, R.; Lai, Y.; Wu, J. Gender difference in arsenic biotransformation is an important metabolic basis for arsenic toxicity. BMC Pharmacol. Toxicol. 2022, 23, 15. [Google Scholar] [CrossRef] [PubMed]
- Torres-Sánchez, L.; López-Carrillo, L.; Rosado, J.L.; Rodriguez, V.M.; Vera-Aguilar, E.; Kordas, K.; García-Vargas, G.G.; Cebrian, M.E. Sex differences in the reduction of arsenic methylation capacity as a function of urinary total and inorganic arsenic in Mexican children. Env. Res. 2016, 151, 38–43. [Google Scholar] [CrossRef]
- Albiach-Serrano, A.; Bräuer, J.; Cacchione, T.; Zickert, N.; Amici, F. The effect of domestication and ontogeny in swine cognition (Sus scrofa scrofa and S. s. domestica). Appl. Anim. Behav. Sci. 2012, 141, 25–35. [Google Scholar] [CrossRef]
- Bullers, K. Merck Manuals. J. Med. Libr. Assoc. JMLA 2016, 104, 369–371. [Google Scholar] [CrossRef]
- Jánoska, F.; Farkas, A.; Marosan, M.; Fodor, J.-T. Wild boar (Sus scrofa) home range and habitat use in two Romanian habitats. Acta Silv. Lignaria Hung. 2018, 14, 51. [Google Scholar] [CrossRef]
- Zackular, J.P.; Moore, J.L.; Jordan, A.T.; Juttukonda, L.J.; Noto, M.J.; Nicholson, M.R.; Crews, J.D.; Semler, M.W.; Zhang, Y.; Ware, L.B.; et al. Dietary zinc alters the microbiota and decreases resistance to Clostridium difficile infection. Nat. Med. 2016, 22, 1330–1334. [Google Scholar] [CrossRef] [PubMed]
- Agradi, S.; Cremonesi, P.; Menchetti, L.; Balzaretti, C.; Severgnini, M.; Riva, F.; Castiglioni, B.; Draghi, S.; Di Giancamillo, A.; Castrica, M.; et al. Bovine Colostrum Supplementation Modulates the Intestinal Microbial Community in Rabbits. Animals 2023, 13, 976. [Google Scholar] [CrossRef]
- Hegde, N.V.; Kariyawasam, S.; DebRoy, C. Comparison of antimicrobial resistant genes in chicken gut microbiome grown on organic and conventional diet. Vet. Anim. Sci. 2016, 1–2, 9–14. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jami, E.; White, B.A.; Mizrahi, I. Potential Role of the Bovine Rumen Microbiome in Modulating Milk Composition and Feed Efficiency. PloS ONE 2014, 9, e85423. [Google Scholar] [CrossRef] [PubMed]
- Das, N.K.; Schwartz, A.J.; Barthel, G.; Inohara, N.; Liu, Q.; Sankar, A.; Hill, D.R.; Ma, X.; Lamberg, O.; Schnizlein, M.K.; et al. Microbial Metabolite Signaling Is Required for Systemic Iron Homeostasis. Cell Metab. 2020, 31, 115–130.e6. [Google Scholar] [CrossRef] [PubMed]
- Shannon, M.C.; Hill, G.M. Trace Mineral Supplementation for the Intestinal Health of Young Monogastric Animals. Front. Vet. Sci. 2019, 6, 73. [Google Scholar] [CrossRef]
- Villagómez-Estrada, S.; Pérez, J.F.; Darwich, L.; Vidal, A.; van Kuijk, S.; Melo-Durán, D.; Solà-Oriol, D. Effects of copper and zinc sources and inclusion levels of copper on weanling pig performance and intestinal microbiota. J. Anim. Sci. 2020, 98, skaa117. [Google Scholar] [CrossRef]
- Kociova, S.; Dolezelikova, K.; Horky, P.; Skalickova, S.; Baholet, D.; Bozdechova, L.; Vaclavkova, E.; Belkova, J.; Nevrkla, P.; Skladanka, J.; et al. Zinc phosphate-based nanoparticles as alternatives to zinc oxide in diet of weaned piglets. J. Anim. Sci. Biotechnol. 2020, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Labrie, J.; Tremblay, Y.D.N.; Haine, D.; Mourez, M.; Jacques, M. Zinc as an agent for the prevention of biofilm formation by pathogenic bacteria. J. Appl. Microbiol. 2013, 115, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Huynh, U.; Zastrow, M.L. Metallobiology of Lactobacillaceae in the gut microbiome. J. Inorg. Biochem. 2023, 238, 112023. [Google Scholar] [CrossRef] [PubMed]
- Gokulan, K.; Arnold, M.G.; Jensen, J.; Vanlandingham, M.; Twaddle, N.C.; Doerge, D.R.; Cerniglia, C.E.; Khare, S. Exposure to Arsenite in CD-1 Mice during Juvenile and Adult Stages: Effects on Intestinal Microbiota and Gut-Associated Immune Status. mBio 2018, 9, e01418-18. [Google Scholar] [CrossRef]
- Zmora, N.; Bashiardes, S.; Levy, M.; Elinav, E. The Role of the Immune System in Metabolic Health and Disease. Cell Metab. 2017, 25, 506–521. [Google Scholar] [CrossRef]
- Bist, P.; Choudhary, S. Impact of Heavy Metal Toxicity on the Gut Microbiota and Its Relationship with Metabolites and Future Probiotics Strategy: A Review. Biol. Trace Elem. Res. 2022, 200, 5328–5350. [Google Scholar] [CrossRef]
- Ley, R.E.; Hamady, M.; Lozupone, C.; Turnbaugh, P.J.; Ramey, R.R.; Bircher, J.S.; Schlegel, M.L.; Tucker, T.A.; Schrenzel, M.D.; Knight, R.; et al. Evolution of Mammals and Their Gut Microbes. Science 2008, 320, 1647–1651. [Google Scholar] [CrossRef]
- Wei, L.; Zhou, W.; Zhu, Z. Comparison of Changes in Gut Microbiota in Wild Boars and Domestic Pigs Using 16S rRNA Gene and Metagenomics Sequencing Technologies. Animals 2022, 12, 2270. [Google Scholar] [CrossRef] [PubMed]
| Element | Wild Boar (n = 40) | |||||
|---|---|---|---|---|---|---|
| Liver (n = 40) | Muscle (n = 40) | |||||
| Mean ± SD | Median (P25; P75) | Min–Max | Mean ± SD | Median (P25; P75) | Min–Max | |
| Co | 27.05 ± 4.42 | 25.33 (23.67; 30.15) | 21.43–36.63 | 7.37 ± 5.53 | 5.19 (3.91; 5.86) | 3.07–20.54 |
| Cu | 3424.16 ± 569.92 | 3495.93 (2906.65; 3797.72) | 2393.55–4606.05 | 1740.77 ± 497.28 | 1572.22 (1429.93; 1786.96) | 1155.87–2909.51 |
| Fe | 218,701.16 ± 78,400.76 | 206,287.79 (160,623.34; 299,503.53) | 109,920.34–356,189.4 | 30,066.85 ± 8479.75 | 28,726.03 (22,563.59; 34,355.85) | 20,134.93–47,678.89 |
| Mn | 2374.59 ± 835.9 | 2301.61 (1582.97; 3088.43) | 1396.41–3917.74 | 607.92 ± 332.26 | 562.58 (305.99; 931.27) | 157.88–1126.62 |
| Mo | 724.90 ± 192.66 | 626.64 (559.68; 909.07) | 481.11–1039.25 | 9.95 ± 3.76 | 11.04 (8.39; 12.41) | 0–14.18 |
| Ni | 27.61 ± 8.16 | 26.25 (21.35; 35.23) | 15.59–41.06 | 70.89 ± 35.27 | 71.78 (57.05; 94.83) | 17.98–140.74 |
| Se | 239.06 ± 47.47 | 245.12 (208.26; 276.96) | 148.57–309.41 | 101.73 ± 62.24 | 84.80 (66.12; 99.84) | 34–235.83 |
| Zn | 31,236.67 ± 3644.436 | 30,197.55 (28,716.08; 32,883.00) | 25,470.24–40,956.24 | 30,725.82 ± 16,568.12 | 21,048.99 (18,542.67; 47,944.67) | 15,558.35–65,909.36 |
| Al | 4055.12 ± 585.28 | 3997.19 (3650.7; 4275.25) | 3155.220–5814.65 | 5343.46 ± 2089.05 | 4160.85 (3411.58; 7398.73) | 2943.25–8990.14 |
| As | 14.49 ± 9.250 | 10.71 (7.81; 14.87) | 7.06–34.65 | 8.76 ± 1.48 | 8.44 (7.56; 9.74) | 6.75–11.84 |
| Ba | 73.00 ± 20.69 | 68.69 (53.4; 94.38) | 49.83–105.29 | 136.41 ± 92.82 | 108.53 (63.73; 148.91) | 53.88–358.98 |
| Cd | 138.67 ± 109.89 | 53.87 (48.35; 243.55) | 38.52–313.41 | 12.6 ± 4.57 | 10.43 (8.87; 15.80) | 7.16–21.59 |
| Cr | 53.39 ± 24.19 | 44.23 (39.83; 48.61) | 35.91–122.78 | 137.29 ± 96.16 | 99.02 (64.94; 138) | 45.25–344.67 |
| Pb | 53.75 ± 11.06 | 54.09 (42.79; 61.06) | 38.94–71.96 | 49.56 ± 8.09 | 49.60 (43.13; 55.72) | 35.76–65.91 |
| Tl | 5.46 ± 3.99 | 5.33 (1.94; 9.80) | 0.690–11.55 | 0.06 ± 0.17 | 0 (0; 0) | 0–0.61 |
| V | 46.14 ± 9.06 | 44.45 (38.96; 51.55) | 33.56–68.9 | 72.7 ± 9.15 | 72.18 (65.58; 79.67) | 54.96–97.24 |
| Element | Swine (n = 40) | |||||
|---|---|---|---|---|---|---|
| Liver (n = 40) | Muscle (n = 40) | |||||
| Mean ± SD | Median (P25; P75) | Min–Max | Mean ± SD | Median (P25; P75) | Min–Max | |
| Co | 28.25 ± 8.16 | 27.91 (23.882; 34.16) | 13.79–44.65 | 3.3 ± 0.99 | 3.26 (2.402; 3.98) | 2.07–5.82 |
| Cu | 16,700.72 ± 8969.23 | 16820 (9115.433; 18,882.5) | 7279.59–34,576.3 | 2192.88 ± 267.98 | 2234.89 (1958.62; 2421.32) | 1696.51–2661.15 |
| Fe | 336,705.3 ± 95,226.41 | 318,963 (282,535.73; 390318) | 186,823.61–572,838.78 | 30,023.03 ± 5011.51 | 29,857.6 (26,520.222; 33,008.9) | 20,760.52–42414 |
| Mn | 2634.62 ± 977.11 | 2657.7 (1656.914; 3181.25) | 1315.67–4737.56 | 344.42 ± 72.71 | 353.97 (283.923; 402.32) | 188.29–445.62 |
| Mo | 2054.06 ± 307.38 | 2076.3 (1927.266; 2198.3) | 1421.12–2718.25 | 32.14 ± 5.93 | 32.7 (29.2; 35.49) | 18.64–42.71 |
| Ni | 58.99 ± 57.33 | 35.05 (13.145; 132.07) | 10.55–153.44 | 22.19 ± 23.45 | 10.5 (9.352; 12.75) | 8.17–71.17 |
| Se | 320.24 ± 25.86 | 318.9 (297.485; 341.33) | 273.18–371.9 | 103.71 ± 13.69 | 102.44 (92.343; 112.35) | 84.83–130.56 |
| Zn | 67,454.11 ± 18,018.53 | 67,345.9 (51,419.637; 80,832.1) | 36,310.74–100,655.95 | 46,536.27 ± 5740.44 | 47,079.3 (42,291.388; 50,876.6) | 36,327.3–59,087.38 |
| Al | 12,029.17 ± 9551.85 | 13,290.7 (2683.48; 18,216.7) | 2176.35–28,549.38 | 3191.31 ± 839.44 | 2842.53 (2602.878; 3995.05) | 2126.67–4965.56 |
| As | 6.28 ± 1.73 | 6.31 (5.083; 7.13) | 3.30–10.24 | 6.41 ± 1.47 | 5.77 (5.13; 7.82) | 4.4–8.85 |
| Ba | 73.94 ± 42.03 | 75.77 (38.763; 84.54) | 32.65–162.66 | 49.47 ± 17.19 | 44.83 (35.442; 65.02) | 19.92–80.39 |
| Cd | 48.34 ± 11.25 | 45.45 (42.146; 51.07) | 33.074–75.59 | 2.49 ± 1.79 | 1.98 (0.682; 3.24) | 0.56–6.1 |
| Cr | 116.37 ± 127.26 | 47.8 (6.71; 281.41) | 5.52–329.07 | 25.02 ± 15.67 | 19.9 (14.027; 39.67) | 8.73–54.67 |
| Pb | 41.17 ± 12.60 | 44.03 (35.387; 50.52) | 17.18–63.36 | 41.06 ± 39.81 | 10.59 (7.09; 38.22) | 4.97–115.45 |
| Tl | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| V | 68.4 ± 21.47 | 66.94 (51.26; 72.46) | 35.93–113.46 | 59.44 ± 7.17 | 60.58 (53.547; 64.4) | 46.39–74.19 |
| Element | Liver (n = 40) | Muscle (n = 40) | ||||
|---|---|---|---|---|---|---|
| PV (n = 20) | PC (n = 20) | p-Value | PV (n = 20) | PC (n = 20) | p-Value | |
| Median | Median | Median | Median | |||
| Co | 26.58 | 24.25 | 5.53 | 4.09 | p = 0.02 | |
| Cu | 3516.74 | 3435.96 | 1574.78 | 1535.15 | ||
| Fe | 207,533.6 | 189,854.52 | 29,112.98 | 25,517.2 | ||
| Mn | 2441.56 | 2232.57 | 624.47 | 340.34 | ||
| Mo | 769.26 | 575.11 | 10.59 | 11.85 | ||
| Ni | 26.15 | 26.99 | 78.82 | 59.82 | p = 0.03 | |
| Se | 249.75 | 240.49 | 89.57 | 73.7 | p = 0.05 | |
| Zn | 29,814.06 | 31,057.21 | 23,017.68 | 18,878.96 | p = 0.004 | |
| Al | 4054.09 | 3996.69 | 4359.30 | 3961.37 | ||
| As | 13.76 | 8.77 | 8.41 | 8.47 | ||
| Ba | 78.56 | 55.65 | 117.89 | 68.64 | ||
| Cd | 206.31 | 52 | 10.04 | 13.26 | ||
| Cr | 43.42 | 43.84 | 99.35 | 91.1 | ||
| Pb | 48.77 | 56.75 | 49.21 | 50.42 | ||
| Tl | 2.29 | 5.63 | 0.00 | 0 | ||
| V | 47.04 | 41.56 | 72.31 | 71.82 | ||
| Element | Liver (n = 40) | Muscle (n = 40) | ||||
|---|---|---|---|---|---|---|
| <2 y.o. (n = 20) | >2 y.o. (n = 20) | p-Value | <2 y.o. (n = 20) | >2 y.o. (n = 20) | p-Value | |
| Median | Median | Median | Median | |||
| Co | 25.34 | 25.32 | 4.24 | 5.21 | ||
| Cu | 3511.46 | 3485.72 | 1599.14 | 1470.63 | p = 0.03 | |
| Fe | 189,923.4 | 211,863.3 | 23,477.91 | 30,163.97 | ||
| Mn | 2433.28 | 1853.24 | 340.34 | 725.86 | p = 0.02 | |
| Mo | 607.01 | 659.91 | 11.73 | 10.08 | ||
| Ni | 25.01 | 30.2 | 75.9 | 68.48 | ||
| Se | 251.88 | 230.21 | 89.57 | 71.06 | p = 0.02 | |
| Zn | 29,585.43 | 31,264.43 | 20,037.37 | 21,271.08 | ||
| Al | 3976.28 | 4094.93 | 3780.52 | 6379.95 | ||
| As | 8.64 | 12.9 | 8.35 | 8.59 | ||
| Ba | 59.59 | 77.79 | 70.07 | 124.32 | ||
| Cd | 51.76 | 55 | 13.52 | 9.29 | p = 0.01 | |
| Cr | 42.31 | 44.71 | 99.38 | 65.09 | p = 0.03 | |
| Pb | 54.93 | 47.32 | 48.39 | 53.17 | ||
| Tl | 5.49 | 4.96 | 0 | 0 | ||
| V | 41.34 | 47.43 | 72.88 | 71.71 | ||
| Element | Liver (n = 40) | Muscle (n = 40) | ||||
|---|---|---|---|---|---|---|
| Female (n = 20) | Male (n = 20) | p-Value | Female (n = 20) | Male (n = 20) | p-Value | |
| Median | Median | Median | Median | |||
| Co | 24.95 | 27.14 | 4.29 | 5.38 | ||
| Cu | 3496.14 | 3493.19 | 1545.97 | 1599.36 | ||
| Fe | 194,796.2 | 208,452.6 | 26,209.71 | 29,981.99 | ||
| Mn | 2368.74 | 2076.13 | 356.83 | 651.45 | ||
| Mo | 577.57 | 714.59 | 11.62 | 10.51 | ||
| Ni | 26.25 | 27.81 | 61.72 | 78.93 | ||
| Se | 245.12 | 245.37 | 84.8 | 85.77 | ||
| Zn | 29,885.11 | 31,688.08 | 19,800.6 | 22,250.11 | ||
| Al | 3962.66 | 4057.99 | 3686.66 | 5362.61 | ||
| As | 8.43 | 13.56 | 8.47 | 8.44 | p = 0.015 | |
| Ba | 58.34 | 81.69 | 66.45 | 122.19 | ||
| Cd | 51.88 | 130.16 | 13.39 | 9.9 | ||
| Cr | 44.23 | 42.79 | 99.36 | 96.38 | ||
| Pb | 56.12 | 46.59 | 47.75 | 50.2 | ||
| Tl | 5.43 | 3.9 | 0 | 0 | ||
| V | 41.8 | 47.89 | 74.19 | 71.37 | ||
| Element | Liver (n = 80) | Muscle (n = 80) | ||||
|---|---|---|---|---|---|---|
| Swine (n = 40) | Wild Boar (n = 40) | p-Value | Swine (n = 40) | Wild Boar (n = 40) | p-Value | |
| Median | Median | Median | Median | |||
| Co | 27.91 | 25.33 | 0.287 | 3.26 | 5.19 | <0.001 |
| Cu | 16,819.97 | 3495.93 | <0.001 | 2234.89 | 1572.22 | <0.001 |
| Fe | 318,962.8 | 206,287.8 | <0.001 | 29,857.6 | 28,726.03 | 0.423 |
| Mn | 2657.7 | 2301.61 | 0.284 | 353.97 | 562.58 | 0.009 |
| Mo | 2076.3 | 626.64 | <0.001 | 32.7 | 11.04 | <0.001 |
| Ni | 35.05 | 26.25 | 0.854 | 10.5 | 71.78 | <0.001 |
| Se | 318.91 | 245.12 | <0.001 | 102.44 | 84.8 | 0.002 |
| Zn | 66,518.42 | 30,197.55 | <0.001 | 47,079.32 | 21,048.99 | <0.001 |
| Al | 13,290.72 | 3997.19 | 0.537 | 2842.53 | 4160.85 | <0.001 |
| As | 6.31 | 10.71 | <0.001 | 5.77 | 8.44 | <0.001 |
| Ba | 75.77 | 68.69 | 0.124 | 44.83 | 108.53 | <0.001 |
| Cd | 45.45 | 53.87 | <0.001 | 1.98 | 10.43 | <0.001 |
| Cr | 47.8 | 44.23 | 0.27 | 19.9 | 99.02 | <0.001 |
| Pb | 44.03 | 54.09 | <0.001 | 10.59 | 49.6 | 0.079 |
| Tl | N.D. | 5.33 | N.D. | 0 | ||
| V | 66.94 | 44.45 | <0.001 | 60.58 | 72.18 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Draghi, S.; Spinelli, M.; Fontanarosa, C.; Curone, G.; Amoresano, A.; Pignoli, E.; Cagnardi, P.; Vigo, D.; Arioli, F.; Materazzi, S.; et al. Evaluation of the Difference in the Content of Essential and Non-Essential Elements in Wild Boar and Swine Tissues Sampled in the Same Area of Northern Italy. Animals 2024, 14, 827. https://doi.org/10.3390/ani14060827
Draghi S, Spinelli M, Fontanarosa C, Curone G, Amoresano A, Pignoli E, Cagnardi P, Vigo D, Arioli F, Materazzi S, et al. Evaluation of the Difference in the Content of Essential and Non-Essential Elements in Wild Boar and Swine Tissues Sampled in the Same Area of Northern Italy. Animals. 2024; 14(6):827. https://doi.org/10.3390/ani14060827
Chicago/Turabian StyleDraghi, Susanna, Michele Spinelli, Carolina Fontanarosa, Giulio Curone, Angela Amoresano, Elisabetta Pignoli, Petra Cagnardi, Daniele Vigo, Francesco Arioli, Stefano Materazzi, and et al. 2024. "Evaluation of the Difference in the Content of Essential and Non-Essential Elements in Wild Boar and Swine Tissues Sampled in the Same Area of Northern Italy" Animals 14, no. 6: 827. https://doi.org/10.3390/ani14060827
APA StyleDraghi, S., Spinelli, M., Fontanarosa, C., Curone, G., Amoresano, A., Pignoli, E., Cagnardi, P., Vigo, D., Arioli, F., Materazzi, S., Risoluti, R., & Di Cesare, F. (2024). Evaluation of the Difference in the Content of Essential and Non-Essential Elements in Wild Boar and Swine Tissues Sampled in the Same Area of Northern Italy. Animals, 14(6), 827. https://doi.org/10.3390/ani14060827











