The Use of Bi-Nasal Prongs for Delivery of Non-Invasive Ventilation to Foals
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
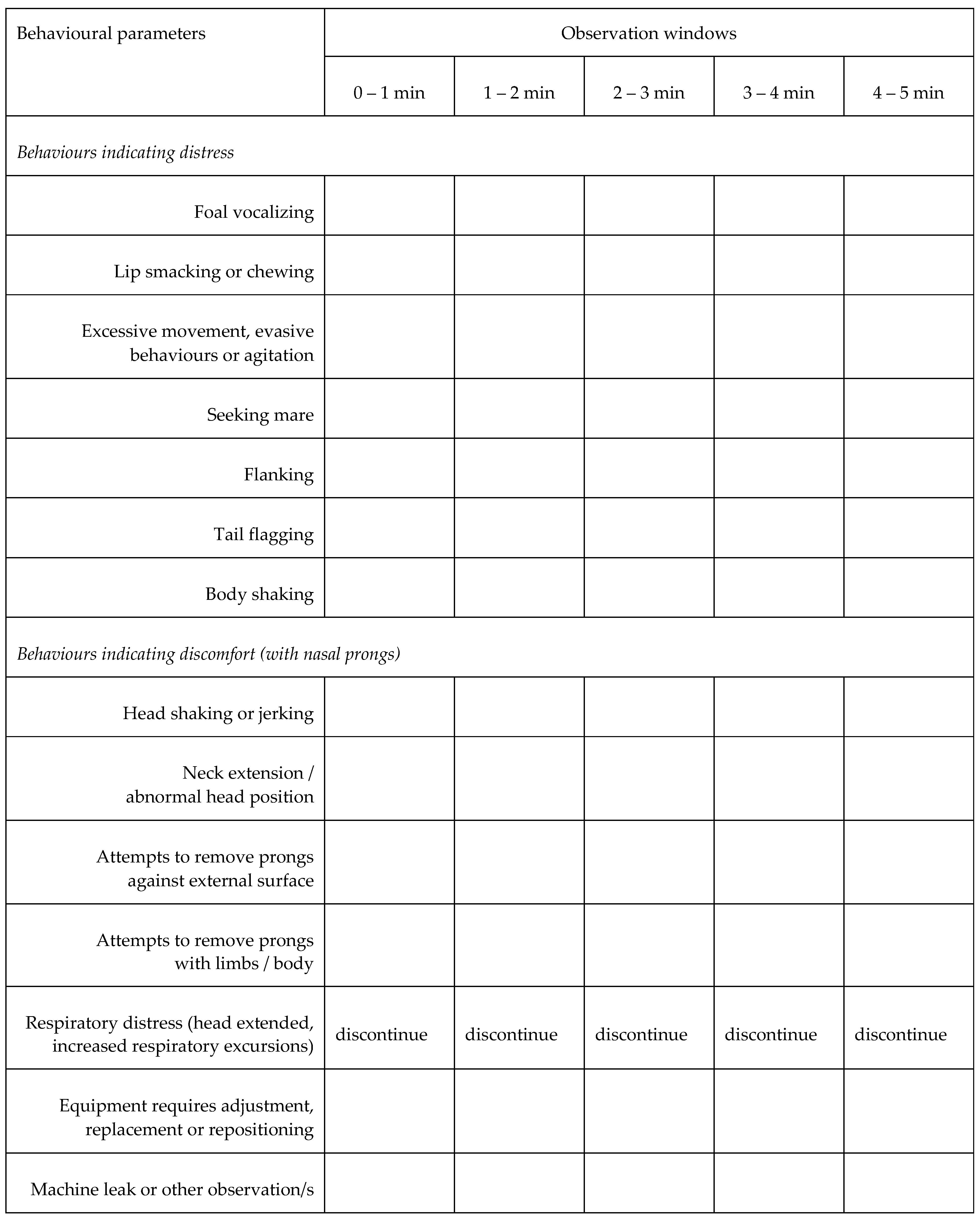
2.2. Experimental Design, and Physiological and Behavioural Responses
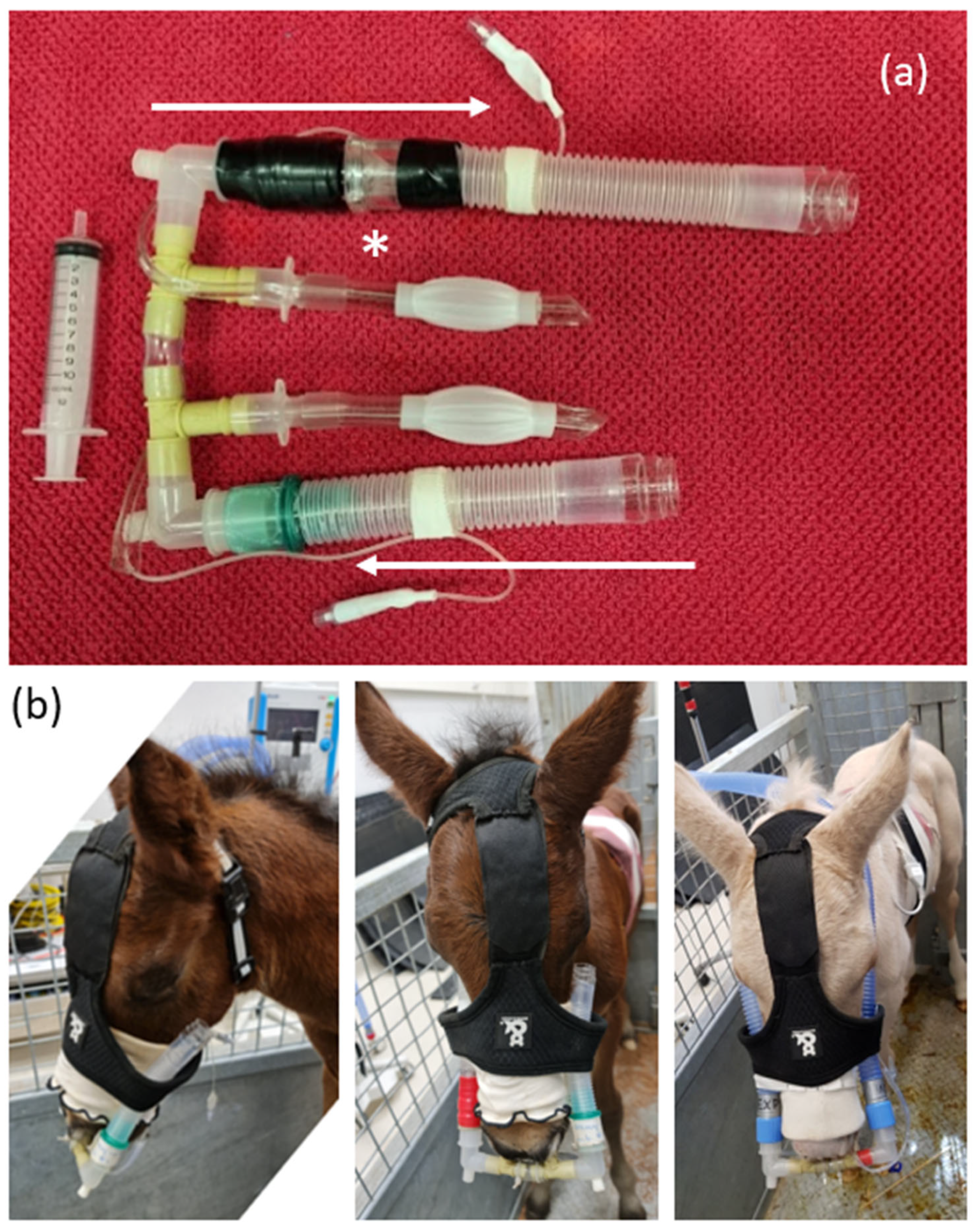
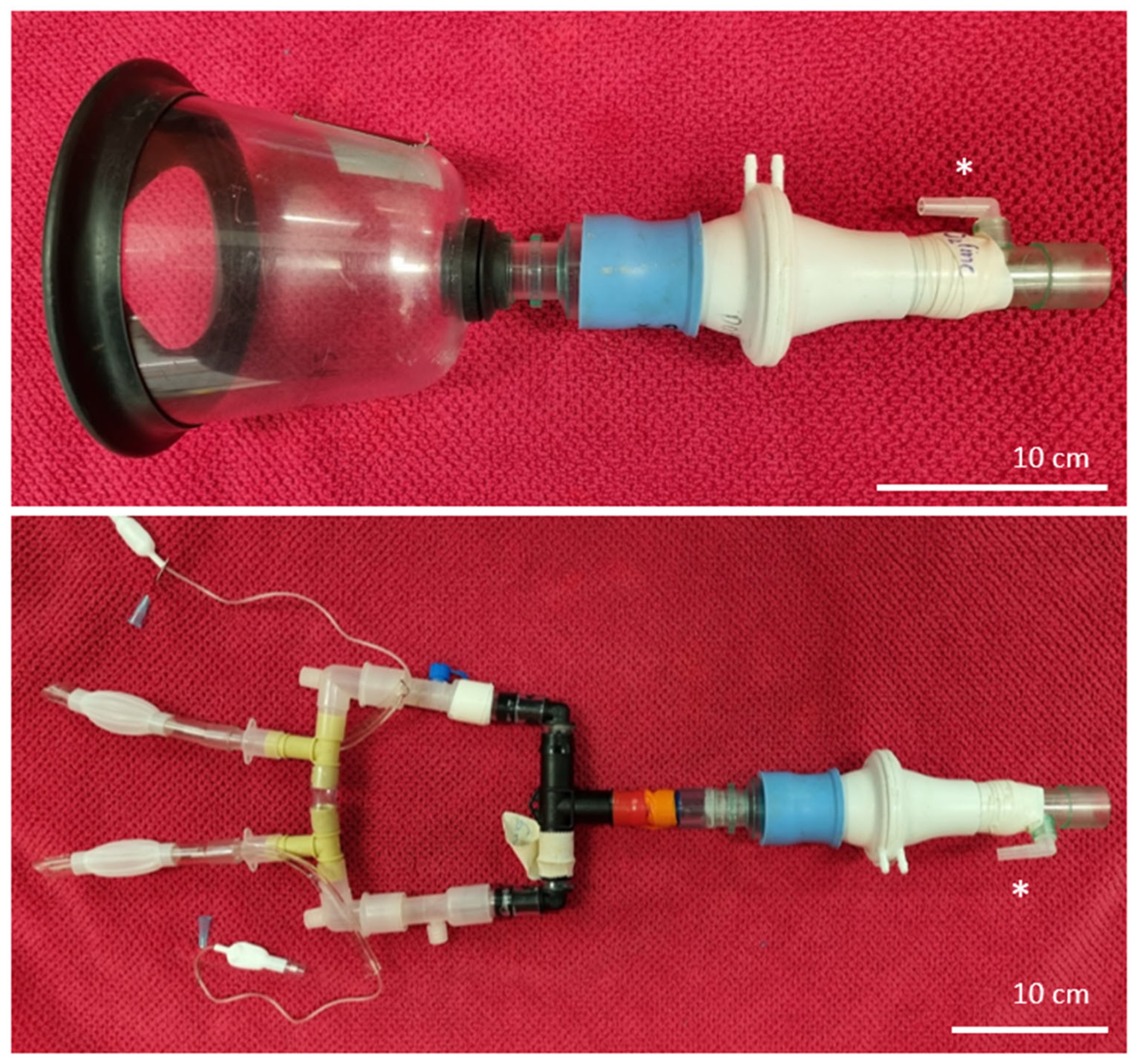
2.3. Instrumentation
2.4. Spirometry and Gas Analysis
2.5. Statistical Methods
3. Results
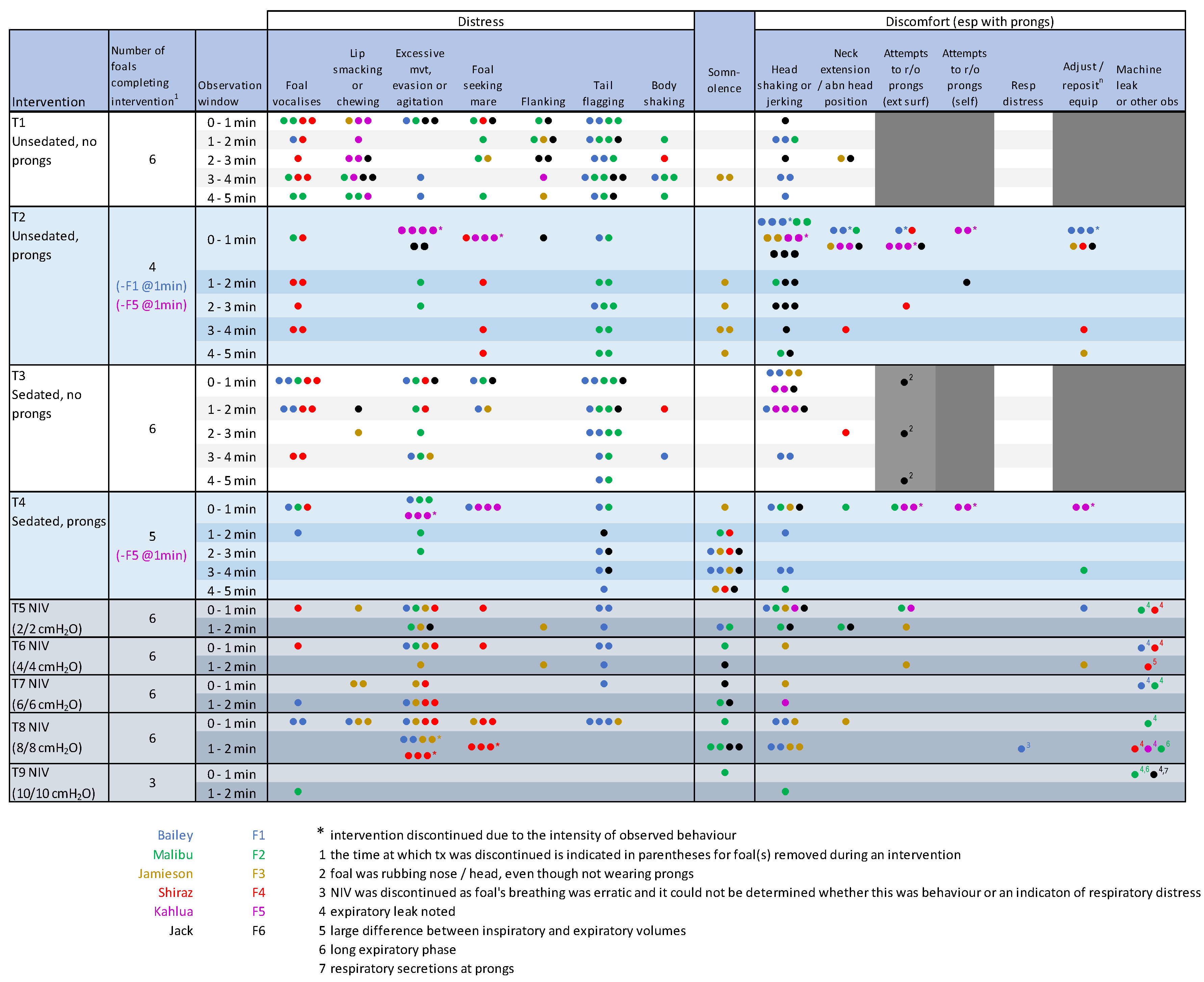
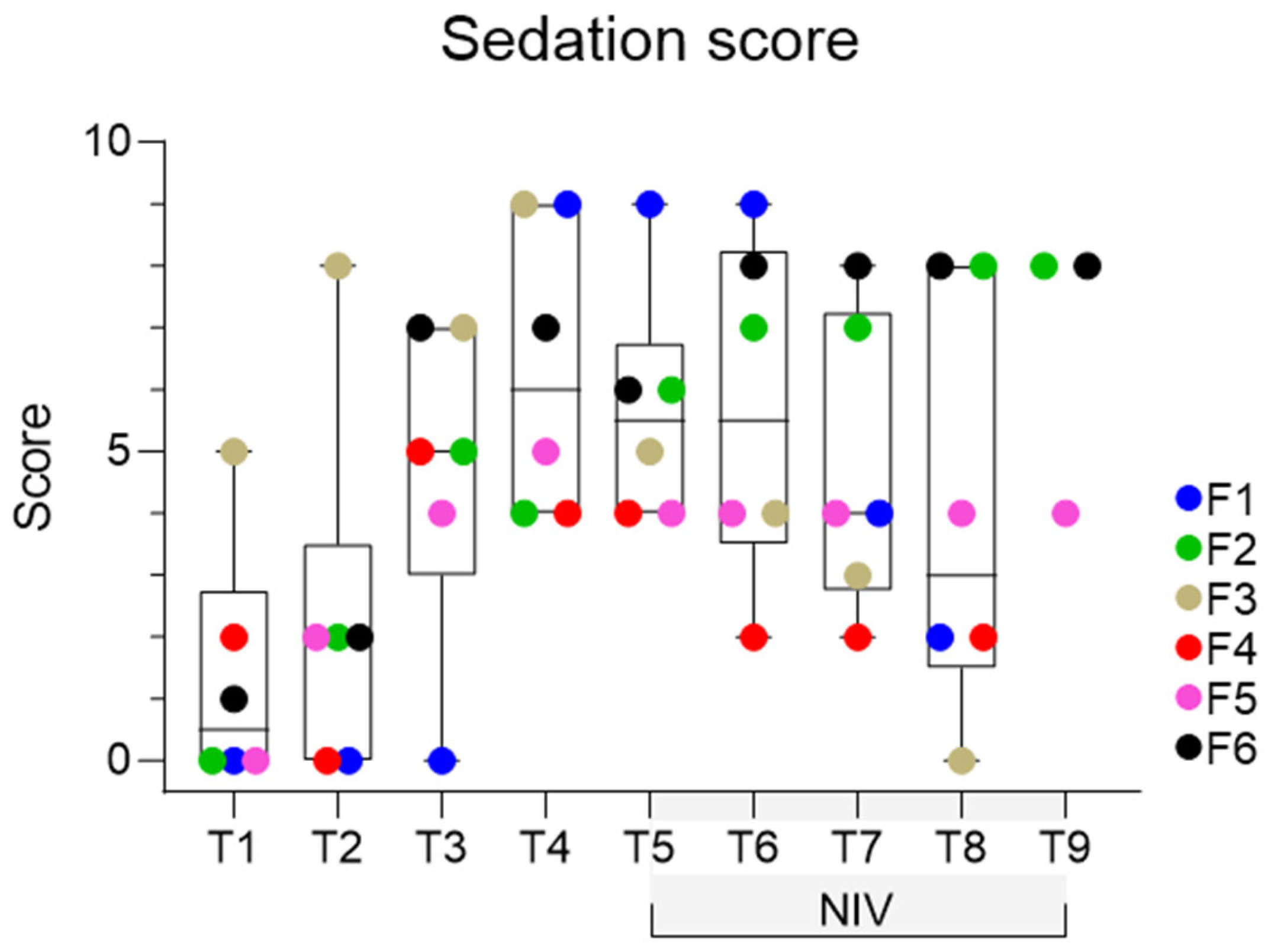
3.1. Behavioural Observations
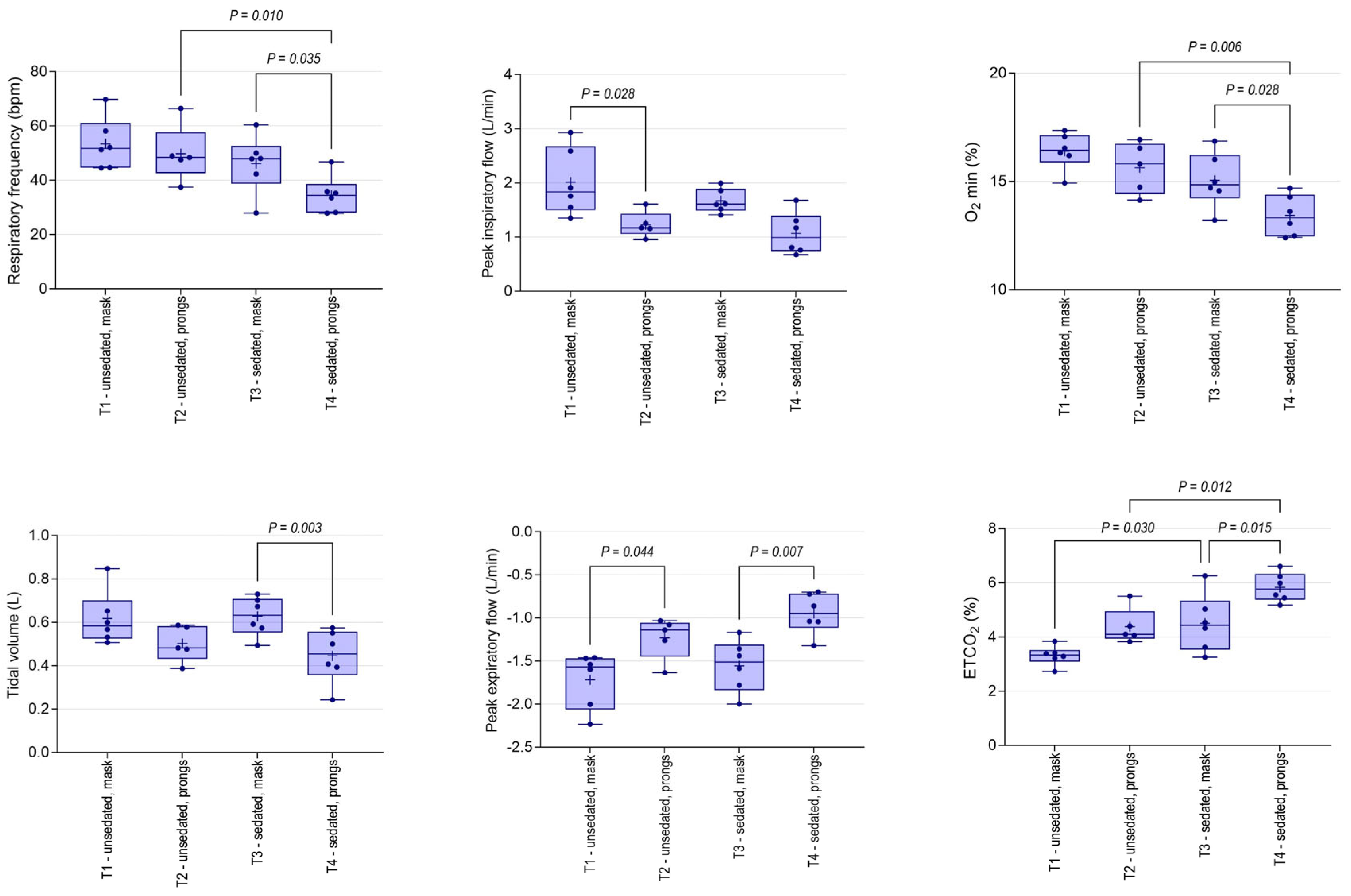
3.2. Physiological Changes
3.3. Spirometry Changes
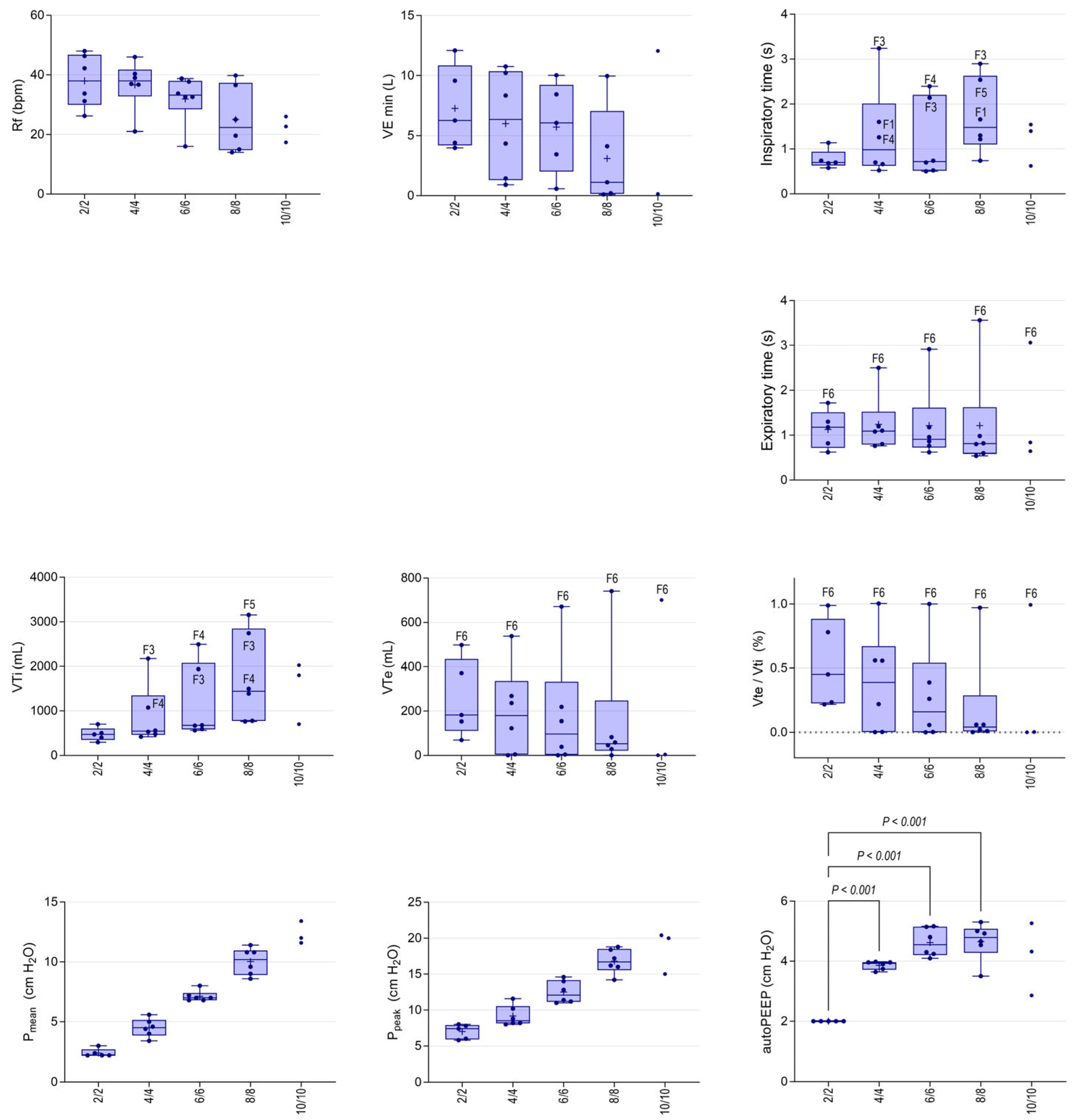
3.4. Non-Invasive Ventilation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mair, T.S.; Lane, J.G. The differential diagnosis of sudden onset respiratory distress. Equine Vet. Ed. 1996, 8, 131–136. [Google Scholar] [CrossRef]
- Wilkins, P.A.; Otto, C.M.; Baumgardner, J.E.; Dunkel, B.; Bedenice, D.; Paradis, M.R.; Staffieri, F.; Syring, R.S.; Slack, J.; Grasso, S.; et al. Acute lung injury and acute respiratory distress syndromes in veterinary medicine: Consensus definitions: The Dorothy Russell Havemeyer Working Group on ALI and ARDS in Veterinary Medicine. J. Vet. Emerg. Crit. Care 2007, 17, 333–339. [Google Scholar] [CrossRef]
- Kosch, P.C.; Koterba, A.M.; Coons, T.J.; Webb, A.I. Developments in management of the newborn foal in respiratory distress 1: Evaluation. Equine Vet. J. 1984, 16, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, V.V.; More, K.; Roehr, C.C.; Bandiya, P.; Nangia, S. Efficacy of non-invasive respiratory support modes for primary respiratory support in preterm neonates with Respiratory Distress Syndrome: Systematic review and network meta-analysis. Pediatr. Pulmonol. 2020, 55, 2940–2963. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Muniraman, H.; Biniwale, M.; Ramanathan, R.A. Review on non-invasive respiratory support for management of respiratory distress in extremely preterm infants. Front. Pediatr. 2020, 8, 270. [Google Scholar] [CrossRef] [PubMed]
- Raidal, S.L.; Catanchin, C.S.M.; Burgmeestre, L.; Quinn, C.T. Bi-level positive airway pressure for non-invasive respiratory support of foals. Front. Vet. Sci. 2021, 8, 741720. [Google Scholar] [CrossRef] [PubMed]
- Raidal, S.L.; McKean, R.; Ellul, P.A.; Nielsen, S.G.; Quinn, C.T. Effects of continuous positive airway pressure on respiratory function in sedated foals. J. Vet. Emerg. Crit. Care 2019, 29, 269–278. [Google Scholar] [CrossRef]
- Raidal, S.L.; Catanchin, C.S.M.; Sacks, M.; Carstens, A.; Quinn, C.T.; Mosing, M. Effects of 2 modes of positive pressure ventilation on respiratory mechanics and gas exchange in foals. J. Vet. Intern. Med. 2023, 37, 1233–1242. [Google Scholar] [CrossRef]
- Jasani, B.; Ismail, A.; Rao, S.; Patole, S. Effectiveness and safety of nasal mask versus binasal prongs for providing continuous positive airway pressure in preterm infants—A systematic review and meta-analysis. Pediatr. Pulmonol. 2018, 53, 987–992. [Google Scholar] [CrossRef]
- Pillow, J.J. Which continuous positive airway pressure system is better for the preterm infant with respiratory distress syndrome? Clin. Perinatol. 2012, 39, 483–496. [Google Scholar] [CrossRef]
- Sweet, D.G.; Carnielli, V.; Greisen, G.; Hallman, M.; Ozek, E.; Te Pas, A.; Plavka, R.; Roehr, C.C.; Saugstad, O.D.; Simeoni, U.; et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome—2019 Update. Neonatology 2019, 115, 432–450. [Google Scholar] [CrossRef]
- Floyd, E.; Danks, S.; Comyn, I.; Mackenzie, C.; Marr, C.M. Nasal high flow oxygen therapy in hospitalised neonatal foals. Equine Vet. J. 2022, 54, 946–951. [Google Scholar] [CrossRef]
- Erber, R.; Wulf, M.; Rose-Meierhöfer, S.; Becker-Birck, M.; Möstl, E.; Aurich, J.; Hoffmann, G.; Aurich, C. Behavioral and physiological responses of young horses to different weaning protocols: A pilot study. Stress 2012, 15, 184–194. [Google Scholar] [CrossRef]
- Delank, K.; Reese, S.; Erhard, M.; Wöhr, A.C. Behavioral and hormonal assessment of stress in foals (Equus caballus) throughout the weaning process. PLoS ONE 2023, 18, e0280078. [Google Scholar] [CrossRef]
- De Oliveira, A.R.; Gozalo-Marcilla, M.; Ringer, S.K.; Schauvliege, S.; Fonseca, M.W.; Trindade, P.H.E.; Puoli Filho, J.N.P.; Luna, S.P.L. Development, validation, and reliability of a sedation scale in horses (EquiSed). Front. Vet. Sci. 2021, 8, 611729. [Google Scholar] [CrossRef]
- Rabec, C.; Georges, M.; Kabeya, N.K.; Baudouin, N.; Massin, F.; Reybet-Degat, O.; Camus, P. Evaluating noninvasive ventilation using a monitoring system coupled to a ventilator: A bench-to-bedside study. Eur. Respir. J. 2009, 34, 902–913. [Google Scholar] [CrossRef] [PubMed]
- Racca, F.; Appendini, L.; Gregoretti, C.; Varese, I.; Berta, G.; Vittone, F.; Ferreyra, G.; Stra, E.; Ranieri, V.M. Helmet ventilation and carbon dioxide rebreathing: Effects of adding a leak at the helmet ports. Int. Care Med. 2008, 34, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Itagaki, T.; Chenelle, C.T.; Bennett, D.J.; Fisher, D.F.; Kacmarek, R.M. Effects of leak compensation on patient-ventilator synchrony during premature/neonatal invasive and noninvasive ventilation: A lung model study. Respir. Care 2017, 62, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Rabec, C.; Gonzalez-Bermejo, J.; Hardy, S.; Aouf, S.; Escourrou, P.; Roisman, G. Combined effects of leaks, respiratory system properties and upper airway patency on the performance of home ventilators: A bench study. BMC Pulm. Med. 2017, 17, 145. [Google Scholar] [CrossRef] [PubMed]
- Natalini, G.; Tuzzo, D.; Rosano, A.; Testa, M.; Grazioli, M.; Pennestrì, V.; Amodeo, G.; Berruto, F.; Fiorillo, M.; Peratoner, A.; et al. Effect of external PEEP in patients under controlled mechanical ventilation with an auto-PEEP of 5 cmH2O or higher. Ann. Intensive Care 2016, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- MacIntyre, N.R. Physiologic effects of noninvasive ventilation. Respir. Care 2019, 64, 617–628. [Google Scholar] [CrossRef]
- Peterson-Carmichael, S.; Seddon, P.C.; Cheifetz, I.M.; Frerichs, I.; Hall, G.L.; Hammer, J.; Hantos, Z.; van Kaam, A.H.; McEvoy, C.T.; Newth, C.J.; et al. An Official American Thoracic Society/European Respiratory Society Workshop Report: Evaluation of Respiratory Mechanics and Function in the Pediatric and Neonatal Intensive Care Units. Ann. Am. Thorac. Soc. 2016, 13, S1–S11. [Google Scholar] [CrossRef]
- Hammer, J.; Newth, C.J. Infant lung function testing in the intensive care unit. Intensive Care Med. 1995, 21, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Ergan, B.; Nasiłowski, J.; Winck, J.C. How should we monitor patients with acute respiratory failure treated with noninvasive ventilation? Eur. Respir. Rev. 2018, 27, 170101. [Google Scholar] [CrossRef] [PubMed]
- Georges, M.; Rabec, C.; Monin, E.; Aho, S.; Beltramo, G.; Janssens, J.P.; Bonniaud, P. Monitoring of noninvasive ventilation: Comparative analysis of different strategies. Respir. Res. 2020, 21, 324. [Google Scholar] [CrossRef] [PubMed]
- Carroll, C.L.; Napolitano, N.; Pons-Òdena, M.; Iyer, N.P.; Korang, S.K.; Essouri, S. Noninvasive respiratory support for pediatric acute respiratory distress syndrome: From the Second Pediatric Acute Lung Injury Consensus Conference. Pediatr. Crit. Care Med. 2023, 24 (Suppl. S2), S135–S147. [Google Scholar] [CrossRef]
- Brabant, O.A.; Byrne, D.P.; Sacks, M.; Moreno Martinez, F.; Raisis, A.L.; Araos, J.B.; Waldmann, A.D.; Schramel, J.P.; Ambrosio, A.; Hosgood, G.; et al. Thoracic electrical impedance tomography—The 2022 Veterinary Consensus Statement. Front. Vet. Sci. 2022, 9, 946911. [Google Scholar] [CrossRef]
- Sacks, M.; Raidal, S.; Catanchin, C.S.M.; Hosgood, G.; Mosing, M. Impact of sedation, body position change and continuous positive airway pressure on distribution of ventilation in healthy foals. Front. Vet. Sci. 2022, 9, 1075791. [Google Scholar] [CrossRef]
- Rauseo, M.; Spinelli, E.; Sella, N.; Slobod, D.; Spadaro, S.; Longhini, F.; Giarratano, A.; Gilda, C.; Mauri, T.; Navalesi, P. Expert opinion document: Electrical impedance tomography: Applications from the intensive care unit and beyond. J. Anesth. Analg. Crit. Care 2022, 2, 28. [Google Scholar] [CrossRef]
- Toth, B.; Aleman, M.; Brosnan, R.J.; Dickinson, P.J.; Conley, A.J.; Stanley, S.D.; Nogradi, N.; Williams, C.D.; Madigan, J.E. Evaluation of squeeze-induced somnolence in neonatal foals. Am. J. Vet. Res. 2012, 73, 1881–1889. [Google Scholar] [CrossRef]
- Fischer, C.; Bertelle, V.; Hohlfeld, J.; Forcada-Guex, M.; Stadelmann-Diaw, C.; Tolsa, J.F. Nasal trauma due to continuous positive airway pressure in neonates. Arch. Dis. Child. Fetal Neonatal Ed. 2010, 95, F447–F451. [Google Scholar] [CrossRef] [PubMed]
- Imbulana, D.I.; Manley, B.J.; Dawson, J.A.; Davis, P.G.; Owen, L.S. Nasal injury in preterm infants receiving non-invasive respiratory support: A systematic review. Arch Dis Child Fetal Neonatal Ed. 2018, 103, F29–F35. [Google Scholar] [CrossRef] [PubMed]
- Hess, D.R. Patient-ventilator interaction during noninvasive ventilation. Respir. Care 2011, 56, 153–165; discussion 165–167. [Google Scholar] [CrossRef] [PubMed]

| Time | Intervention | Duration (min) | Approximate Elapsed Time (min) |
|---|---|---|---|
| T1 | Unsedated, no PDI | 5 | 5 |
| Mask spirometry | 6 | ||
| Place nasal prongs, allow to settle | 8 | ||
| T2 | Unsedated, nasal prongs in place | 5 | 13 |
| Nasal prong spirometry | 14 | ||
| Remove nasal prongs | |||
| Sedate, allow to settle | 16 | ||
| T3 | Sedated, no PDI | 5 | 21 |
| Mask spirometry | 22 | ||
| Place nasal prongs, allow to settle | 24 | ||
| T4 | Sedated, nasal prongs in place | 5 | 29 |
| Nasal prong spirometry | 30 | ||
| Connect NIV circuit, allow to settle | 32 | ||
| T5 | NIV 2/2 (PS 1 2 cmH2O, PEEP 2 2 cmH2O) | 2 | 34 |
| T6 | NIV 4/4 (PS 4 cmH2O, PEEP 4 cmH2O) | 2 | 36 |
| T7 | NIV 6/6 (PS 6 cmH2O, PEEP 6 cmH2O) | 2 | 38 |
| T8 | NIV 8/8 (PS 8 cmH2O, PEEP 8 cmH2O) | 2 | 40 |
| T9 | NIV 10/10 (PS 10 cmH2O, PEEP 10 cmH2O) | 2 | 42 |
| Score | Head Height above Ground | Response to Tactile Stimuli | Response to Auditory Stimuli | Postural Stability |
|---|---|---|---|---|
| 0 | Head carried in a neutral, alert position | Intense movement of body/body part, steps away | Appropriate, rapid movement of head, neck and body, evasive action | No swaying, weight bearing on all limbs or appropriately resting one limb |
| 1 | Neck inclined above horizontal, but head not fully elevated | Moderate movement of body/body part, without evasive action | Rapid or slightly reduced movement of head, body or ears | Nil or minimal swaying, nil or minimal postural adaptation |
| 2 | Head lowered, neck horizontal or below | Slight movement of body/body part, minimal response to touch | Slow and limited movement of head, neck or ears | Moderate swaying, base-wide stance and/or limb/s malpositioned |
| 3 | Head lowered to <25% of full height | No response | No response | All above plus intense swaying/instability, risk of falling |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Diggelen, M.; Quinn, C.T.; Catanchin, C.S.M.; Lehmann, H.S.; Raidal, S.L. The Use of Bi-Nasal Prongs for Delivery of Non-Invasive Ventilation to Foals. Animals 2024, 14, 865. https://doi.org/10.3390/ani14060865
van Diggelen M, Quinn CT, Catanchin CSM, Lehmann HS, Raidal SL. The Use of Bi-Nasal Prongs for Delivery of Non-Invasive Ventilation to Foals. Animals. 2024; 14(6):865. https://doi.org/10.3390/ani14060865
Chicago/Turabian Stylevan Diggelen, Michael, Chris T. Quinn, Chee Sum M. Catanchin, Heidi S. Lehmann, and Sharanne L. Raidal. 2024. "The Use of Bi-Nasal Prongs for Delivery of Non-Invasive Ventilation to Foals" Animals 14, no. 6: 865. https://doi.org/10.3390/ani14060865
APA Stylevan Diggelen, M., Quinn, C. T., Catanchin, C. S. M., Lehmann, H. S., & Raidal, S. L. (2024). The Use of Bi-Nasal Prongs for Delivery of Non-Invasive Ventilation to Foals. Animals, 14(6), 865. https://doi.org/10.3390/ani14060865






