Estimating Mineral Requirements of Wild Herbivores: Modelling Arctic Caribou (Rangifer tarandus granti) in Summer
Abstract
:Simple Summary
Abstract
1. Introduction
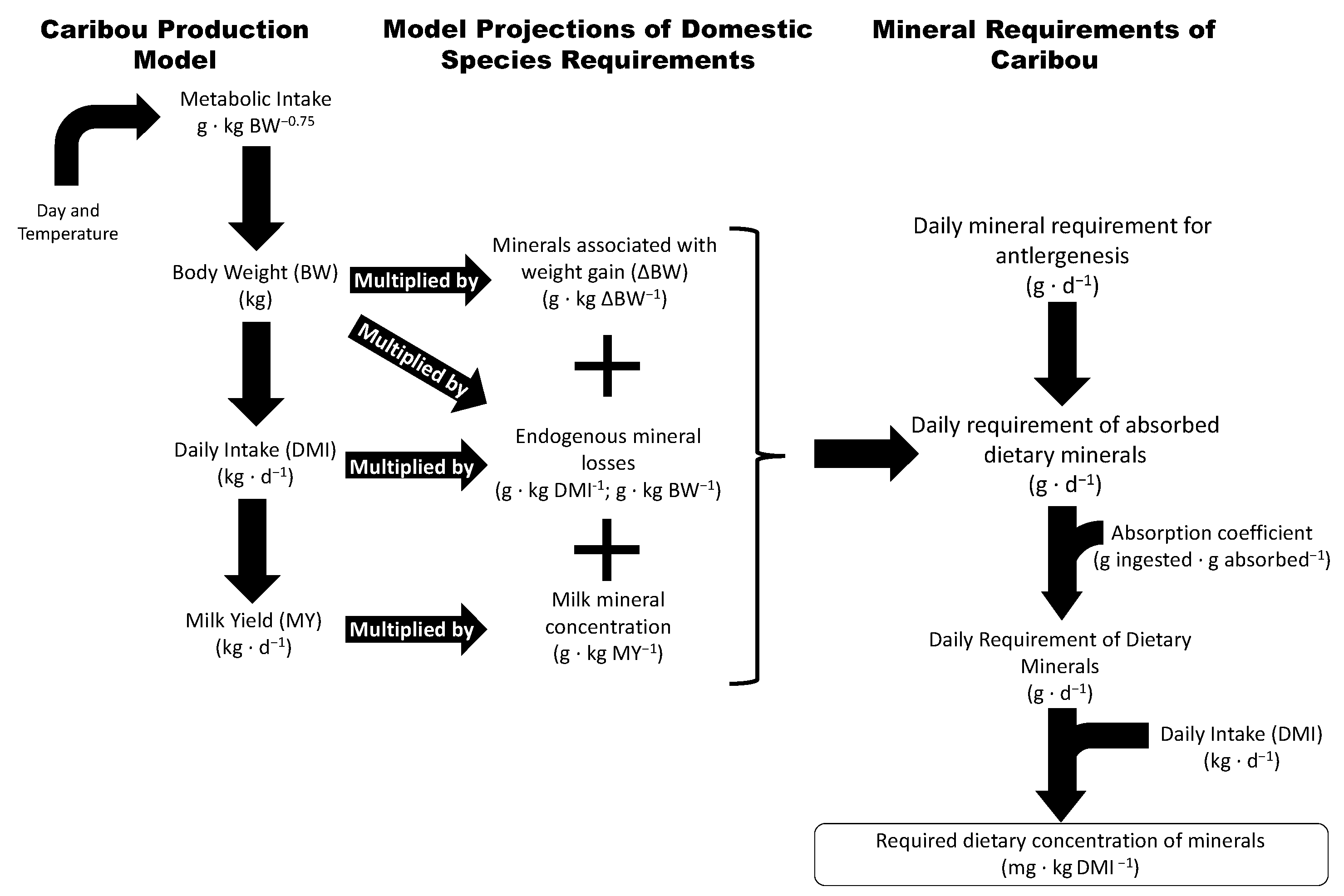
2. Materials and Methods
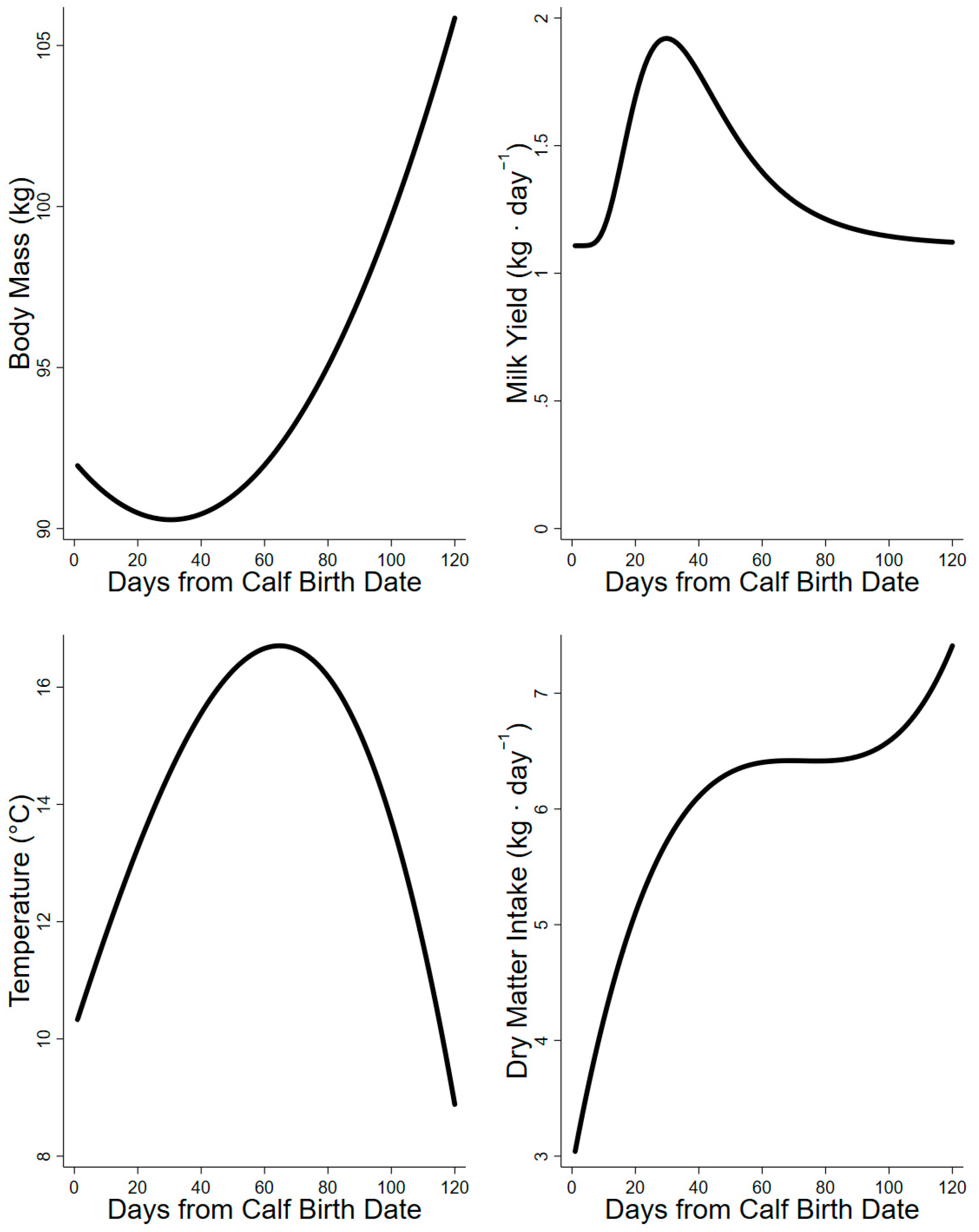
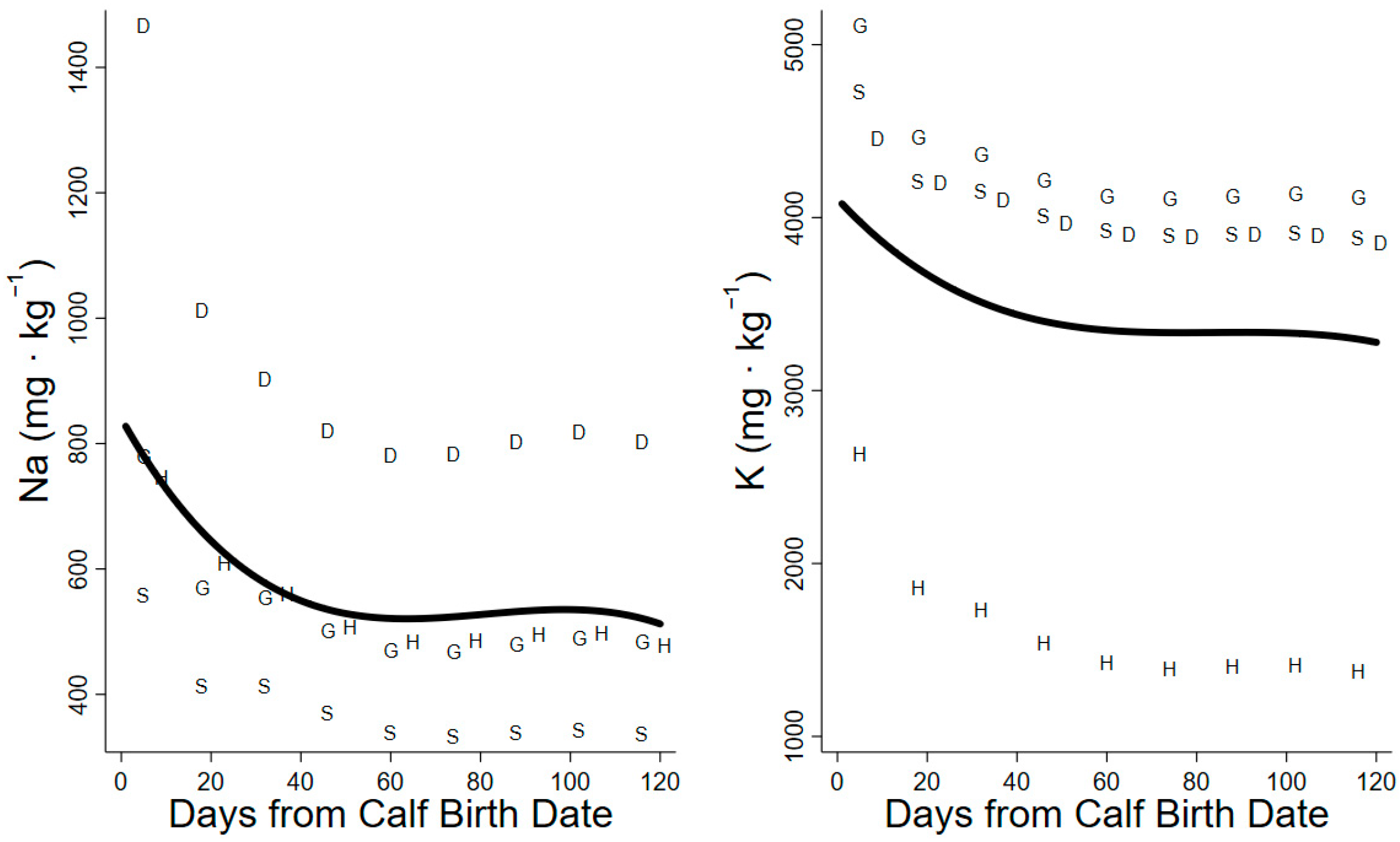
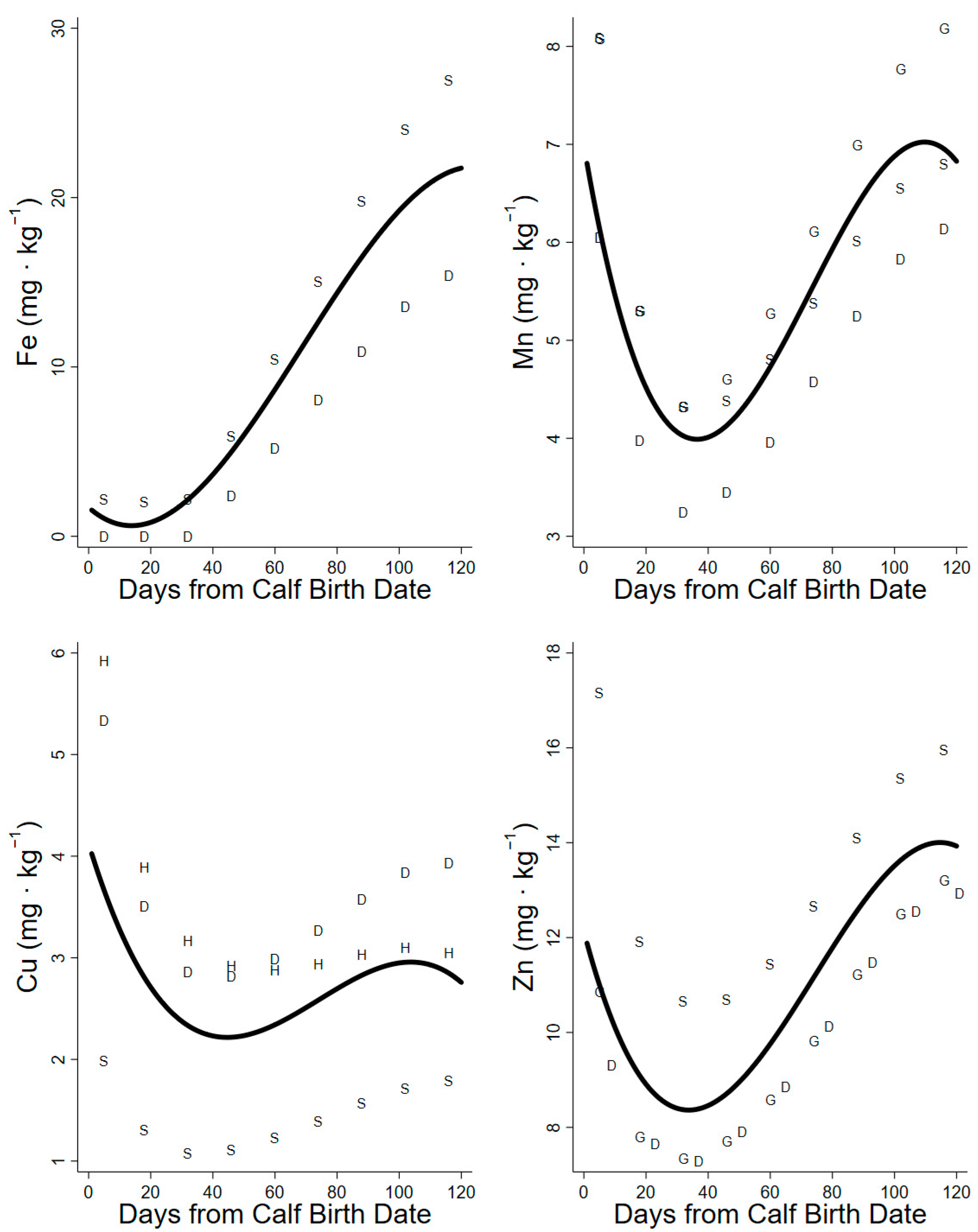
3. Results
3.1. Calcium, Phosphorus, and Magnesium
3.2. Sodium and Potassium
3.3. Iron, Manganese, Copper, and Zinc
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suttle, N.F. Mineral Nutrition of Livestock, 4th ed.; CABI: Wallingford, UK, 2010; p. 587. [Google Scholar]
- Kaspari, M. The invisible hand of the periodic table: How micronutrients shape ecology. Annu. Rev. Ecol. Evol. Syst. 2021, 52, 199–219. [Google Scholar] [CrossRef]
- McDowell, L.R. Minerals in Animal and Human Nutrition; Elsevier Science: Amsterdam, The Netherland, 2003. [Google Scholar]
- Barboza, P.S.; Parker, K.L.; Hume, I.D. Integrative Wildlife Nutrition; Springer: Heidelberg, Germany, 2009; p. 342, 119 illustrations. [Google Scholar]
- Spears, J.W. Minerals in forages. In Forage Quality, Evaluation, and Utilization; Fahey, G.C.J., Ed.; American Society of Agronomy: Madison, WI, USA, 1994; pp. 281–317. [Google Scholar]
- Ceacero, F.; Landete-Castillejos, T.; Olguin, A.; Miranda, M.; Garcia, A.; Martinez, A.; Cassinello, J.; Miguel, V.; Gallego, L. Avoiding toxic levels of essential minerals: A forgotten factor in deer diet preferences. PLoS ONE 2015, 10, e0115814. [Google Scholar] [CrossRef]
- Oster, K.W.; Barboza, P.S.; Gustine, D.D.; Joly, K.; Shively, R.D. Mineral constraints on Arctic caribou (Rangifer tarandus): A spatial and phenological perspective. Ecosphere 2018, 9, e02160. [Google Scholar] [CrossRef]
- Mincher, B.J.; Mionczynski, J.; Hnilicka, P.A. Soil redox chemistry limitation of selenium concentration in Carex species sedges. Soil Sci. 2007, 172, 733–739. [Google Scholar] [CrossRef]
- Wallis De Vries, M. Habitat quality and the performance of large herbivores. In Grazing and Conservation Management; Wallis De Vries, M., Bakker, J.P., Van Wieren, S., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 275–320. [Google Scholar]
- Worker, S.B.; Kielland, K.; Barboza, P.S. Effects of geophagy on food intake, body mass, and nutrient dynamics of snowshoe hares (Lepus americanus). Can. J. Zool. 2015, 93, 323–329. [Google Scholar] [CrossRef]
- Slabach, B.L.; Corey, T.B.; Aprille, J.R.; Starks, P.T.; Dane, B. Geophagic behavior in the mountain goat (Oreamnos americanus): Support for meeting metabolic demands. Can. J. Zool. 2015, 93, 599–604. [Google Scholar] [CrossRef]
- Jones, R.L.; Hanson, H.C. Mineral licks, geophagy, and biochemistry of North American ungulates; Iowa State University Press: Ames, IA, USA, 1985; p. 301. [Google Scholar]
- Ayotte, J.B.; Parker, K.L.; Arocena, J.M.; Gillingham, M.P. Chemical composition of lick soils: Functions of soil ingestion by four ungulate species. J. Mammal. 2006, 87, 878–888. [Google Scholar] [CrossRef]
- Kauffman, M.J.; Aikens, E.O.; Esmaeili, S.; Kaczensky, P.; Middleton, A.; Monteith, K.L.; Morrison, T.A.; Mueller, T.; Sawyer, H.; Goheen, J.R. Causes, consequences, and conservation of ungulate migration. Annu. Rev. Ecol. Evol. Syst. 2021, 52, 453–478. [Google Scholar] [CrossRef]
- McNaughton, S.J. Mineral nutrition and spatial concentrations of African ungulates. Nature 1988, 334, 343–345. [Google Scholar] [CrossRef]
- Staaland, H.; White, R.G. Regional variation in mineral contents of plants and its significance for migration by Arctic reindeer and caribou. Alces 2001, 37, 497–510. [Google Scholar]
- Robbins, C.T. Wildlife Feeding and Nutrition, 2nd ed.; Academic Press: San Diego, CA, USA, 1993. [Google Scholar]
- Hackmann, T.J. A system for predicting energy and protein requirements of wild ruminants. Zoo Biol. 2011, 30, 165–188. [Google Scholar] [CrossRef] [PubMed]
- Gordon, I.J.; Prins, H.H.T. (Eds.) The Ecology of Browsing and Grazing; Springer: Heidelberg, Germany, 2008. [Google Scholar]
- McNew, L.B.; Dahlgren, D.K.; Beck, J.L. (Eds.) Rangeland Wildlife Ecology and Conservation; Springer Nature: Cham, Switzerland, 2023; p. 1023. [Google Scholar]
- Briske, D.D. (Ed.) Rangeland Systems. Processes, Management and Challenges; Springer: Cham, Switzerland, 2017; p. 661. [Google Scholar]
- Joly, K.; Gurarie, E.; Sorum, M.S.; Kaczensky, P.; Cameron, M.D.; Jakes, A.F.; Borg, B.L.; Nandintsetseg, D.; Hopcraft, J.G.C.; Buuveibaatar, B.; et al. Longest terrestrial migrations and movements around the world. Sci. Rep. 2019, 9, 15333. [Google Scholar] [CrossRef] [PubMed]
- Laforge, M.P.; Bonar, M.; Vander Wal, E. Tracking snowmelt to jump the green wave: Phenological drivers of migration in a northern ungulate. Ecology 2020, 102, e03268. [Google Scholar] [CrossRef] [PubMed]
- Gerhart, K.L.; White, R.G.; Cameron, R.D.; Russell, D.E. Body composition and nutrient reserves of Arctic caribou. Can. J. Zool. 1996, 74, 136–146. [Google Scholar] [CrossRef]
- Adams, L.G.; Dale, B.W. Reproductive performance of female Alaskan caribou. J. Wildl. Manag. 1998, 62, 1184–1195. [Google Scholar] [CrossRef]
- Barboza, P.S.; VanSomeren, L.L.; Gustine, D.D.; Bret-Harte, M.S. The nitrogen window for Arctic herbivores: Plant phenology and protein gain of migratory caribou (Rangifer tarandus). Ecosphere 2018, 9, e02073. [Google Scholar] [CrossRef]
- Barboza, P.S.; Shively, R.D.; Thompson, D.P. Robust responses of female caribou to changes in food supply. Ecol. Evol. Physiol. 2024; in press. [Google Scholar]
- Richert, J.C.; Leffler, A.J.; Spalinger, D.E.; Welker, J.M. Snowier winters extend autumn availability of high-quality forage for caribou in Arctic Alaska. Ecosphere 2021, 12, e03617. [Google Scholar] [CrossRef]
- Dryden, G.M. Quantitative nutrition of deer: Energy, protein and water. Anim. Prod. Sci. 2011, 51, 292–302. [Google Scholar] [CrossRef]
- Coughenour, M.B. Savanna-Landscape and Regional Ecosystem Model. Model Description; National Resource Ecology Laboratory, Colorado State University: Fort Collins, CO, USA, 1993; p. 62. [Google Scholar]
- Parker, K.L.; White, R.G.; Gillingham, M.P.; Holleman, D.F. Comparison of energy metabolism in relation to daily activity and milk consumption by caribou and muskox neonates. Can. J. Zool. 1990, 68, 106–114. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids and New World Camelids; National Academy Press: Washington, DC, USA, 2007. [Google Scholar]
- National Research Council. Nutrient Requirements of Horses, 6th ed.; National Academy Press: Washington, DC, USA, 2007. [Google Scholar]
- National Research Council. Nutrient Requirements of Dairy Cattle, 7th ed.; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- National Research Council. Nutrient Requirements of Beef Cattle, 7th ed.; National Academy Press: Washington, DC, USA, 1996. [Google Scholar]
- Luick, J.R.; White, R.G.; Gau, A.M.; Jenness, R. Compositional changes in the milk secreted by grazing reindeer. I. Gross composition and ash. J. Dairy Sci. 1974, 57, 1325–1333. [Google Scholar] [CrossRef]
- Thomas, D.; Barry, S. Antler mass of barren-ground caribou relative to body condition and pregnancy rate. Arctic 2005, 58, 241–246. [Google Scholar] [CrossRef]
- Windholz, M.; Budvari, S.; Stroumtsos, L.Y.; Fertig, M.N. The Merck Index. An Excyclopedia of Chemicals and Drugs; Merck and Co.: Rahway, NJ, USA, 1976. [Google Scholar]
- Johnson, H.E.; Golden, T.S.; Adams, L.G.; Gustine, D.D.; Lenart, E.A.; Barboza, P.S. Dynamic selection for forage quality and quantity in response to phenology and insects in an Arctic ungulate. Ecol. Evol. 2021, 11, 11664–11688. [Google Scholar] [CrossRef]
- Barboza, P.S.; Parker, K.L. Allocating protein to reproduction in Arctic reindeer and caribou. Physiol. Biochem. Zool. 2008, 81, 835–855. [Google Scholar] [CrossRef]
- Miller, J.H.; Druckenmiller, P.; Bahn, V. Antlers on the Arctic Refuge: Capturing multi-generational patterns of calving ground use from bones on the landscape. Proc. R. Soc. B-Biol. Sci. 2013, 280, 1–9. [Google Scholar] [CrossRef]
- Thompson, D.P.; Barboza, P.S. Seasonal energy and protein requirements for Siberian reindeer (Rangifer tarandus). J. Mammal. 2017, 98, 1558–1567. [Google Scholar] [CrossRef]
- Trondrud, L.M.; Pigeon, G.; Krol, E.; Albon, S.; Ropstad, E.; Kumpula, J.; Evans, A.L.; Speakman, J.R.; Loe, L.E. A summer heat wave reduced activity, heart rate, and autumn body mass in a cold-adapted ungulate. Physiol. Biochem. Zool. 2023, 96, 282–293. [Google Scholar] [CrossRef]
- Rombach, E.P.; Barboza, P.S.; Blake, J.E. Utilization of copper during lactation and neonatal development in muskoxen. Can. J. Zool. 2002, 80, 1460–1469. [Google Scholar] [CrossRef]
- Swor, R.M. The Effect of Marginal Copper Diet on Muskox Calf Growth and Immune Function; University of Alaska: Fairbanks, AK, USA, 2002. [Google Scholar]
- Whitten, K.R.; Cameron, R.D. Nutrient dynamics of caribou forage on Alaska’s Arctic slope. In Proceedings of the 2nd International Reindeer/Caribou Symposium, 17–21 September 1979; Reimers, E., Gaare, E., Skjenneberg, S., Eds.; Direktoratet for Vilt og Ferskvannsfisk: Trondheim Norway, 1980; pp. 159–166. [Google Scholar]
- Jorgenson, J.C.; Udevitz, M.S. The distribution and seasonal quality of habitat available for key wildlife species of the Arctic Coastal Plain. In Terestrial Research: 1002 Area—Arctic National Wildlife Refuge, Interim. Report, 1988–1990; McCabe, T.R., Griffith, D.B., Walsh, N.E., Young, D.D., Eds.; US Fish and Wildlife Service: Anchorage, AK, USA, 1992; pp. 87–118. [Google Scholar]
- Jones, M.O.; Allred, B.W.; Naugle, D.E.; Maestas, J.D.; Donnelly, P.; Metz, M.J.; Karl, J.; Smith, R.; Bestelmeyer, B.; Boyd, C.S.; et al. Innovation in rangeland monitoring: Annual, 30 m, plant functional type percent cover maps for U.S. rangelands, 1984–2017. Ecosphere 2018, 9, e02430. [Google Scholar] [CrossRef]
- du Toit, J.T.; Cross, P.C.; Valeix, M. Managing the livestock–wildlife interface on rangelands. In Rangeland Systems; Briske, D.D., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 395–425. [Google Scholar]
- Rioux, È.; Pelletier, F.; Mosbacher, J.B.; Lesmerises, F.; St-Louis, R.; Kutz, S.; St-Laurent, M.-H. Links between individual performance, trace elements and stable isotopes in an endangered caribou population. Glob. Ecol. Conserv. 2022, 38, e02234. [Google Scholar] [CrossRef]
- Aguilar, X.F.; Leclerc, L.M.; Kugluktuk Angoniatit, A.; Ekaluktutiak, H.; Trappers, O.; Olokhaktomiut, H.; Trappers, C.; Mavrot, F.; Roberto-Charron, A.; Tomaselli, M.; et al. An integrative and multi-indicator approach for wildlife health applied to an endangered caribou herd. Sci. Rep. 2023, 13, 16524. [Google Scholar] [CrossRef]
- van Der Wal, R.; Brooker, R.W. Mosses mediate grazer impacts on grass abundance in Arctic ecosystems. Funct. Ecol. 2004, 18, 77–86. [Google Scholar] [CrossRef]
- van der Wal, R. Do herbivores cause habitat degradation or vegetation state transition? Evidence from the tundra. Oikos 2006, 114, 177–186. [Google Scholar]
- Miller, J.H.; Crowley, B.E.; Bataille, C.P.; Wald, E.J.; Kelly, A.; Gaetano, M.; Bahn, V.; Druckenmiller, P. Historical landscape use of migratory caribou: New insights from old antlers. Front. Ecol. Evol. 2021, 8, 590837. [Google Scholar] [CrossRef]
- Mann, D.H.; Groves, P.; Gaglioti, B.V.; Shapiro, B.A. Climate-driven ecological stability as a globally shared cause of late quaternary megafaunal extinctions: The plaids and stripes hypothesis. Biol. Rev. Camb. Philos. Soc. 2019, 94, 328–352. [Google Scholar] [CrossRef]
- Zamin, T.J.; Côté, S.D.; Tremblay, J.-P.; Grogan, P. Experimental warming alters migratory caribou forage quality. Ecol. Appl. 2017, 27, 2061–2073. [Google Scholar] [CrossRef]
- Rantanen, M.; Karpechko, A.Y.; Lipponen, A.; Nordling, K.; Hyvärinen, O.; Ruosteenoja, K.; Vihma, T.; Laaksonen, A. The Arctic has warmed nearly four times faster than the globe since 1979. Commun. Earth Environ. 2022, 3, 168. [Google Scholar] [CrossRef]
- Speed, J.D.M.; Skjelbred, I.Å.; Barrio, I.C.; Martin, M.D.; Berteaux, D.; Bueno, C.G.; Christie, K.S.; Forbes, B.C.; Forbey, J.; Fortin, D.; et al. Trophic interactions and abiotic factors drive functional and phylogenetic structure of vertebrate herbivore communities across the Arctic tundra biome. Ecography 2019, 42, 1152–1163. [Google Scholar] [CrossRef]

| Mineral | Taxa | AC (g·g−1) | Maintenance (g·d−1) | Body Mass Change (g·d−1) | Lactation (g·d−1) |
|---|---|---|---|---|---|
| Ca | Sheep | 0.5 | (0.623·DMI−1) + 0.228 | LWG·6.25·MW0.28·BW−0.28 | 3.2·MY−1 |
| Goats | 0.45 | (0.623·DMI−1) + 0.228 | 11·LWG−1 | 3.2·MY−1 | |
| Beef cattle | 0.5 | 0.0154·BW−1 | a 71·LMG−1 | 3.2·MY−1 | |
| Dairy cattle | 0.3 | 0.0154·BW−1 | 9.83·(MW0.22)·(BW−0.22))·LWG | 3.2·MY−1 | |
| Horses | 0.5 | 0.043·BW−1 | 16·LWG−1 | 3.2·MY−1 | |
| P | Sheep | 0.6 | 1.6·(0.603·DMI − 0.06) | LWG·(1.2 + 3.188·MW0.28·BW−0.28) | 2.7·MY−1 |
| Goats | 0.65 | 0.081 + (0.88·DMI−1) | 5.8·LWG−1 | 2.7·MY−1 | |
| Beef cattle | 0.64 | 0.016·BW−1 | a 39·LMG−1 | 2.7·MY−1 | |
| Dairy cattle | 0.68 | (1.2·DMI−1) + (0.002·BW−1) | 1.2 + (4.635·MW0.22)·(BW−0.22)·LWG | 2.7·MY−1 | |
| Horses | 0.35 | 0.028·BW−1 | 8.0·LWG−1 | 2.7·MY−1 | |
| Mg | Sheep | 0.17 | 0.003·BW−1 | 0.41·LWG−1 | 0.193·MY−1 |
| Goats | 0.2 | 0.0035·BW−1 | 0.4·LWG−1 | 0.193·MY−1 | |
| Beef cattle | 0.2 | 0.003·BW−1 | 0.45·LWG−1 | 0.193·MY−1 | |
| Dairy cattle | 0.16 | 0.003·BW−1 | 0.45·LWG−1 | 0.193·MY−1 | |
| Horses | 0.5 | 0.0015·BW−1 | 1.25·LWG−1 | 0.193·MY−1 | |
| Na | Sheep | 0.91 | 0.0108·BW−1 | 1.1·LWG−1 | 0.6·MY−1 |
| Goats | 0.8 | 0.015·BW−1 | 1.6·LWG−1 | 0.6·MY−1 | |
| Dairy cattle | 0.9 | 0.038·BW−1 | 1.4·LWG−1 | 0.6·MY−1 | |
| Horses | 0.9 | 0.02·BW−1 | 1.0·LWG−1 | 0.6·MY−1 | |
| K | Sheep | 0.9 | (2.6 g·DMI−1) + (0.038 g·BW−1) | 1.8·LWG−1 | 1.56·MY−1 |
| Goats | 0.9 | (2.6 g·DMI−1) + (0.05 g·BW−1) | 2.4·LWG−1 | 1.56·MY−1 | |
| Dairy cattle | 0.9 | (2.6·DMI−1) + (0.038 g·BW−1) | 1.6·LWG−1 | 1.56·MY−1 | |
| Horses | 0.75 | 0.048·BW−1 | 1.5·LWG−1 | 1.56·MY−1 |
| Mineral | Taxa | AC (g·g−1) | Maintenance (mg·d−1) | Body Mass Change (mg·d−1) | Lactation (mg·d−1) |
|---|---|---|---|---|---|
| Fe | Sheep | 0.1 | 0.014·BW−1 | 55·LWG−1 | 0.007·MY−1 |
| Dairy cattle | 0.1 | 0·BW−1 | 34·LWG−1 | 0.007·MY−1 | |
| Mn | Sheep | 0.0075 | 0.002·BW−1 | 0.47·LWG−1 | 0.0008·MY−1 |
| Goats | 0.0075 | 0.002·BW−1 | 0.7·LWG−1 | 0.0008·MY−1 | |
| Dairy cattle | 0.01 | 0.002·BW−1 | 0.7 mg·LWG−1 | 0.0008·MY−1 | |
| Cu | Sheep | 0.06 | 0.004·BW−1 | 1.06·LWG−1 | 0.0008·MY−1 |
| Dairy cattle | 0.04 | 0.0071·BW−1 | 1.15·LWG−1 | 0.0008·MY−1 | |
| Horses | 0.35 | 0.069·BW−1 | 1·LWG−1 | 0.0008·MY−1 | |
| Zn | Sheep | 0.15 | 0.076·BW−1 | 24·LWG−1 | 1.13·MY−1 |
| Goats | 0.15 | 0.045·BW−1 | 25·LWG−1 | 1.13·MY−1 | |
| Dairy cattle | 0.15 | 0.045·BW−1 | 24·LWG−1 | 1.13·MY−1 |
| Mineral | Requirement Phenology Regression | Adjusted r2 |
|---|---|---|
| Ca | Y = −25.32x + 0.2375x2 − 0.00056x3 + 4719.26 | 0.20 |
| P | Y = −11.92x + 0.0399x2 + 0.00019x3 + 3732.65 | 0.11 |
| Mg | Y = −11.31x + 0.1407x2 − 0.00055x3 + 829.89 | 0.64 |
| Na | Y = −12.8x + 0.1656x2 − 0.00068x3 + 840.15 | 0.14 |
| K | Y = −28.08x + 0.3377x2 − 0.00134x3 + 4108.27 | 0.03 |
| Fe | Y = −0.16x + 0.0065x2 − 0.00003x3 + 1.7 | 0.81 |
| Mn | Y = −0.19x + 0.0034x2 − 0.00002x3 + 6.99 | 0.71 |
| Cu | Y = −0.1x + 0.0016x2 − 0.00001x3 + 4.12 | 0.14 |
| Zn | Y = −0.25x + 0.0047x2 − 0.00002x3 + 12.13 | 0.61 |
| Mineral | Incorporated Models | Peak Dietary Requirement (mg·kg DMI−1) | Day of Peak Requirement | Early Lactation Requirement (mg·kg DMI−1 ± SD) | Late Lactation Requirement (mg·kg DMI−1 ± SD) |
|---|---|---|---|---|---|
| Ca | S,G,D,H | 4694 | 120 | 4406 ± 484 | 3999 ± 397 |
| P | S,G,D,H | 3721 | 1 | 3567 ± 696 | 3202 ± 499 |
| Mg | S,G,C,D,H | 819 | 1 | 719 ± 42 | 570 ± 40 |
| Na | S,G,D,H | 828 | 1 | 691 ± 267 | 535 ± 194 |
| K | S,G,D,H | 4653 | 1 | 3775 ± 1213 | 3364 ± 1285 |
| Fe | S,D | 21.74 | 120 | 1.03 ± 1.46 | 12.43 ± 5.26 |
| Mn | S,G,D | 7.02 | 110 | 5.10 ± 0.81 | 5.56 ± 0.79 |
| Cu | S,D,H | 4.02 | 1 | 3.04 ± 1.46 | 2.60 ± 1.02 |
| Zn | S,G,D | 14 | 115 | 9.67 ± 2.48 | 11.22 ± 1.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oster, K.W.; Gustine, D.D.; Smeins, F.E.; Barboza, P.S. Estimating Mineral Requirements of Wild Herbivores: Modelling Arctic Caribou (Rangifer tarandus granti) in Summer. Animals 2024, 14, 868. https://doi.org/10.3390/ani14060868
Oster KW, Gustine DD, Smeins FE, Barboza PS. Estimating Mineral Requirements of Wild Herbivores: Modelling Arctic Caribou (Rangifer tarandus granti) in Summer. Animals. 2024; 14(6):868. https://doi.org/10.3390/ani14060868
Chicago/Turabian StyleOster, Keith W., David D. Gustine, Fred E. Smeins, and Perry S. Barboza. 2024. "Estimating Mineral Requirements of Wild Herbivores: Modelling Arctic Caribou (Rangifer tarandus granti) in Summer" Animals 14, no. 6: 868. https://doi.org/10.3390/ani14060868
APA StyleOster, K. W., Gustine, D. D., Smeins, F. E., & Barboza, P. S. (2024). Estimating Mineral Requirements of Wild Herbivores: Modelling Arctic Caribou (Rangifer tarandus granti) in Summer. Animals, 14(6), 868. https://doi.org/10.3390/ani14060868






