Circadian Corticosterone Profile in Laying Hens (Gallus gallus domesticus)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Procedure
2.2. Blood Sampling and Corticosterone Measurement
2.3. Behavioral Analysis
2.4. Statistical Analyses
3. Results
3.1. Behaviour and Sounds
3.2. CORT Concentrations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deviche, P.; Bittner, S.; Gao, S.; Valle, S. Roles and Mechanistic Bases of Glucocorticoid Regulation of Avian Reproduction. Integr. Comp. Biol. 2017, 57, 1184–1193. [Google Scholar] [CrossRef]
- Heiblum, R.; Arnon, E.; Gvaryahu, G.; Robinzon, B.; Snapir, N. Short-term stress increases testosterone secretion from testes in male domestic fowl. Gen. Comp. Endocrinol. 2000, 120, 55–66. [Google Scholar] [CrossRef]
- Deviche, P.J.; Hurley, L.L.; Fokidis, H.B.; Lerbour, B.; Silverin, B.; Silverin, B.; Sabo, J.; Sharp, P.J. Acute stress rapidly decreases plasma testosterone in a free-ranging male songbird: Potential site of action and mechanism. Gen. Comp. Endocrinol. 2010, 169, 82–90. [Google Scholar] [CrossRef]
- Deviche, P.; Gao, S.; Davies, S.; Sharp, P.J.; Dawson, A. Rapid stress-induced inhibition of plasma testosterone in free-ranging male rufous-winged sparrows, Peucaea carpalis: Characterization, time course, and recovery. Gen. Comp. Endocrinol. 2012, 177, 1–8. [Google Scholar] [CrossRef]
- Abolins-Abols, M.; Hanauer, R.E.; Rosvall, K.A.; Peterson, M.P.; Ketterson, E.D. The effect of chronic and acute stressors, and their interaction, on testes function: An experimental test during testicular recrudescence. J. Exp. Biol. 2018, 221, jeb180869. [Google Scholar] [CrossRef]
- Rich, E.L.; Romero, L.M. Exposure to chronic stress downregulates corticosterone responses to acute stressors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R1628–R1636. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wei, F.; Li, G. The evolution of the concept of stress and the framework of the stress system. Cell Stress 2021, 5, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Owen, D.J.; Lane, J.M. High levels of corticosterone in feather-plucking parrots (Psittacus erithacus). Vet. Rec. 2006, 158, 804–805. [Google Scholar] [CrossRef] [PubMed]
- El-Lethey, H.; Aerni, V.; Jungi, T.W.; Wechsler, B. Stress and feather pecking in laying hens in relation to housing conditions. Br. Poult. Sci. 2000, 41, 22–28. [Google Scholar] [CrossRef]
- Carere, C.; van Oers, K. Shy and bold great tits (Parus major): Body temperature and breath rate in response to handling stress. Physiol. Behav. 2004, 82, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, S.A.; Cromberg, V.U. Aggressive behavior in the genus Gallus sp. Rev. Bras. Cienc. Avic. 2006, 8, 1–14. [Google Scholar] [CrossRef][Green Version]
- Schmidt, K.L.; Soma, K.K. Cortisol and corticosterone in the songbird immune and nervous systems: Local vs. systemic levels during development. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R103–R110. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.L.J.; Zaytsoff, S.J.M.; Montina, T.; Inglis, G.D. Corticosterone-Mediated Physiological Stress Alters Liver, Kidney, and Breast Muscle Metabolomic Profiles in Chickens. Animals 2021, 11, 3056. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.L. Reproduction in the Female. In Sturkie’s Avian Physiology, 6th ed.; Scanes, C.G., Sturkie, P.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 635–665. ISBN 9780124071605. [Google Scholar]
- Beuving, G.; Vonder, G. Effect of stressing factors on corticosterone levels in the plasma of laying hens. Gen. Comp. Endocrinol. 1978, 35, 153–159. [Google Scholar] [CrossRef]
- Wingfield, J.C.; Smith, J.P.; Farner, D.S. Endocrine Responses of White-Crowned Sparrows to Environmental Stress. Condor 1982, 84, 399. [Google Scholar] [CrossRef]
- Dickens, M.J.; Balthazart, J.; Cornil, C.A. Brain aromatase and circulating corticosterone are rapidly regulated by combined acute stress and sexual interaction in a sex-specific manner. J. Neuroendocrinol. 2012, 24, 1322–1334. [Google Scholar] [CrossRef]
- Blas, J. Stress in Birds. In Sturkie’s Avian Physiology, 6th ed.; Scanes, C.G., Sturkie, P.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 769–810. ISBN 9780124071605. [Google Scholar]
- Wada, H.; Hahn, T.P.; Breuner, C.W. Development of stress reactivity in white-crowned sparrow nestlings: Total corticosterone response increases with age, while free corticosterone response remains low. Gen. Comp. Endocrinol. 2007, 150, 405–413. [Google Scholar] [CrossRef]
- Wada, H.; Salvante, K.G.; Wagner, E.; Williams, T.D.; Breuner, C.W. Ontogeny and individual variation in the adrenocortical response of zebra finch (Taeniopygia guttata) nestlings. Physiol. Biochem. Zool. 2009, 82, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Breuner, C.W.; Orchinik, M.; Hahn, T.P.; Meddle, S.L.; Moore, I.T.; Owen-Ashley, N.T.; Sperry, T.S.; Wingfield, J.C. Differential mechanisms for regulation of the stress response across latitudinal gradients. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, R594–R600. [Google Scholar] [CrossRef] [PubMed]
- Carere, C.; Eens, M. Unravelling animal personalities: How and why individuals consistently differ. Behaviour 2005, 142, 1149–1157. [Google Scholar] [CrossRef]
- Cockrem, J.F. Stress, corticosterone responses and avian personalities. J. Ornithol. 2007, 148, 169–178. [Google Scholar] [CrossRef]
- Beuving, G.; Vonder, G. Comparison of the adrenal sensitivity to ACTH of laying hens with immobilization and plasma baseline levels of corticosterone. Gen. Comp. Endocrinol. 1986, 62, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Ralph, C.R.; Hemsworth, P.H.; Leury, B.J.; Tilbrook, A.J. Relationship between plasma and tissue corticosterone in laying hens (Gallus gallus domesticus): Implications for stress physiology and animal welfare. Domest. Anim. Endocrinol. 2015, 50, 72–82. [Google Scholar] [CrossRef]
- Weimer, S.L.; Wideman, R.F.; Scanes, C.G.; Mauromoustakos, A.; Christensen, K.D.; Vizzier-Thaxton, Y. An evaluation of methods for measuring stress in broiler chickens. Poult. Sci. 2018, 97, 3381–3389. [Google Scholar] [CrossRef]
- Johnson, A.L.; van Tienhoven, A. Plasma concentrations of six steroids and LH during the ovulatory cycle of the hen, Gallus domesticus. Biol. Reprod. 1980, 23, 386–393. [Google Scholar] [CrossRef]
- Johnson, A.L. Comparison of three serial blood sampling techniques on plasma hormone concentrations in the laying hen. Poult. Sci. 1981, 60, 2322–2327. [Google Scholar] [CrossRef]
- de Jong, I.C.; van Voorst, A.; Erkens, J.H.; Ehlhardt, D.A.; Blokhuis, H.J. Determination of the circadian rhythm in plasma corticosterone and catecholamine concentrations in growing broiler breeders using intravenous cannulation. Physiol. Behav. 2001, 74, 299–304. [Google Scholar] [CrossRef]
- Fallahsharoudi, A.; de Kock, N.; Johnsson, M.; Ubhayasekera, S.J.; Kumari, A.; Bergquist, J.; Wright, D.; Jensen, P. Domestication effects on stress induced steroid secretion and adrenal gene expression in chickens. Sci. Rep. 2015, 5, 15345. [Google Scholar] [CrossRef]
- Carvalho, R.R.; Palme, R.; da Silva Vasconcellos, A. An integrated analysis of social stress in laying hens: The interaction between physiology, behaviour, and hierarchy. Behav. Process. 2018, 149, 43–51. [Google Scholar] [CrossRef]
- Törnqvist, E.; Annas, A.; Granath, B.; Jalkesten, E.; Cotgreave, I.; Öberg, M. Strategic focus on 3R principles reveals major reductions in the use of animals in pharmaceutical toxicity testing. PLoS ONE 2014, 9, e101638. [Google Scholar] [CrossRef]
- Aske, K.C.; Waugh, C.A. Expanding the 3R principles: More rigour and transparency in research using animals. EMBO Rep. 2017, 18, 1490–1492. [Google Scholar] [CrossRef]
- Directive 1999/74/EG. Available online: https://eur-lex.europa.eu/eli/dir/1999/74/oj (accessed on 23 January 2024).
- Directive 2010/63/EU. Available online: https://eur-lex.europa.eu/eli/dir/2010/63/oj (accessed on 23 January 2024).
- Korbel, R.; Reese, S.; Liebich, H.-G. Fixationstechniken und Anästhesieverfahren. In Anatomie der Vögel: Klinische Aspekte und Propädeutik. Zier-, Greif-, Zoo-, Wildvögel und Wirtschaftsgeflügel, 2. Aufl.; König, H.E., Korbel, R., Liebich, H.G., König, H.E., Liebich, H.-G., Korbel, R.T., Eds.; Schattauer: Stuttgart, Germany, 2012; pp. 293–304. ISBN 9783794564873. [Google Scholar]
- Webb, M.L.; Mashaly, M.M. Effect of adaptation to handling on the circulating corticosterone concentration of laying hens. Br. Poult. Sci. 1984, 25, 425–427. [Google Scholar] [CrossRef]
- Goymann, W.; Trappschuh, M.; Jensen, W.; Schwabl, I. Low ambient temperature increases food intake and dropping production, leading to incorrect estimates of hormone metabolite concentrations in European stonechats. Horm. Behav. 2006, 49, 644–653. [Google Scholar] [CrossRef]
- Collias, N.; Joos, M. The Vocal Repertoire of the Red Junglefowl: A Spectrographic Classification and the Code of Communication. Condor 1987, 89, 510–524. [Google Scholar] [CrossRef]
- Gyger, M.; Marler, P.; Pickert, R. Semantics of an Avian Alarm Call System: The Male Domestic Fowl, Gallus domesticus. Behaviour 1987, 102, 15–39. [Google Scholar] [CrossRef]
- Evans, C.S.; Marler, P. Food calling and audience effects in male chickens, Gallus gallus: Their relationships to food availability, courtship and social facilitation. Anim. Behav. 1994, 47, 1159–1170. [Google Scholar] [CrossRef]
- Zimmerman, P.; Koene, P. The effect of frustrative nonreward on vocalisations and behaviour in the laying hen, Gallus gallus domesticus. Behav. Process. 1998, 44, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.S.; Evans, L. Chicken food calls are functionally referential. Anim. Behav. 1999, 58, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, P.H.; Koene, P.; van Hooff, J.A. Thwarting of behaviour in different contexts and the gakel-call in the laying hen. Appl. Anim. Behav. Sci. 2000, 69, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.S.; Evans, L. Representational signalling in birds. Biol. Lett. 2007, 3, 8–11. [Google Scholar] [CrossRef]
- Kokolakis, A.; Smith, C.L.; Evans, C.S. Aerial alarm calling by male fowl (Gallus gallus) reveals subtle new mechanisms of risk management. Anim. Behav. 2010, 79, 1373–1380. [Google Scholar] [CrossRef]
- Tefera, M. Acoustic Signals in Domestic Chicken (Gallus gallus): A Tool for Teaching Veterinary Ethology and Implication for language learning. Ethiop. Vet. J. 2012, 16, 77–84. [Google Scholar] [CrossRef]
- McGrath, N.; Dunlop, R.; Dwyer, C.; Burman, O.; Phillips, C.J. Hens vary their vocal repertoire and structure when anticipating different types of reward. Anim. Behav. 2017, 130, 79–96. [Google Scholar] [CrossRef]
- Marx, G.; Leppelt, J.; Ellendorff, F. Vocalisation in chicks (Gallus gallus dom.) during stepwise social isolation. Appl. Anim. Behav. Sci. 2001, 75, 61–74. [Google Scholar] [CrossRef]
- Jacobsen, K.R.; Kalliokoski, O.; Teilmann, A.C.; Hau, J.; Abelson, K.S.P. The effect of isoflurane anaesthesia and vasectomy on circulating corticosterone and ACTH in BALB/c mice. Gen. Comp. Endocrinol. 2012, 179, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Bekhbat, M.; Merrill, L.; Kelly, S.D.; Lee, V.K.; Neigh, G.N. Brief anesthesia by isoflurane alters plasma corticosterone levels distinctly in male and female rats: Implications for tissue collection methods. Behav. Brain Res. 2016, 305, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Koller, M. robustlmm: An R Package for Robust Estimation of Linear Mixed-Effects Models. J. Stat. Softw. 2016, 75, 1–24. [Google Scholar] [CrossRef]
- Raiola, G.; Di Tore, P.A. Statistical study on bodily communication skills in volleyball to improve teaching methods. J. Hum. Sport Exerc. 2012, 7, 468–488. [Google Scholar] [CrossRef]
- Etches, R.J. Plasma concentrations of progesterone and corticosterone during the ovulation cycle of the hen (Gallus domesticus). Poult. Sci. 1979, 58, 211–216. [Google Scholar] [CrossRef]
- Geng, A.L.; Zhang, J.; Zhang, Y.; Wang, H.H.; Chu, Q.; Yan, Z.X.; Liu, H.G. Effects of lighting regimes on performance, pineal melanopsin expression and melatonin content in native laying hens aged from 19 to 34 weeks. Poult. Sci. 2022, 101, 101567. [Google Scholar] [CrossRef]
- Leliavski, A.; Dumbell, R.; Ott, V.; Oster, H. Adrenal clocks and the role of adrenal hormones in the regulation of circadian physiology. J. Biol. Rhythm. 2015, 30, 20–34. [Google Scholar] [CrossRef]
- Yang, Y.; Han, W.; Zhang, A.; Zhao, M.; Cong, W.; Jia, Y.; Wang, D.; Zhao, R. Chronic corticosterone disrupts the circadian rhythm of CRH expression and m6A RNA methylation in the chicken hypothalamus. J. Anim. Sci. Biotechnol. 2022, 13, 29. [Google Scholar] [CrossRef]
- Baxter, M.; Bédécarrats, G.Y. Evaluation of the Impact of Light Source on Reproductive Parameters in Laying Hens Housed in Individual Cages. J. Poult. Sci. 2019, 56, 148–158. [Google Scholar] [CrossRef]
- Shimizu, M.; Bédécarrats, G.Y. Identification of a novel pituitary-specific chicken gonadotropin-releasing hormone receptor and its splice variants. Biol. Reprod. 2006, 75, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Ubuka, T.; Bentley, G.E.; Tsutsui, K. Neuroendocrine regulation of gonadotropin secretion in seasonally breeding birds. Front. Neurosci. 2013, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, T.K.; Chatterjee, R.N.; Dange, M.; Bhanja, S.K. Polymorphisms in GnRHI and GnRHII genes and their association with egg production and egg quality traits in chicken. Br. Poult. Sci. 2019, 60, 187–194. [Google Scholar] [CrossRef]
- Gwinner, E.; Brandstätter, R. Complex bird clocks. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001, 356, 1801–1810. [Google Scholar] [CrossRef]
- Yang, Y.; Cong, W.; Liu, J.; Zhao, M.; Xu, P.; Han, W.; Wang, D.; Zhao, R. Constant light in early life induces fear-related behavior in chickens with suppressed melatonin secretion and disrupted hippocampal expression of clock- and BDNF-associated genes. J. Anim. Sci. Biotechnol. 2022, 13, 67. [Google Scholar] [CrossRef] [PubMed]
- Tchekalarova, J.; Stoynova, T.; Ilieva, K.; Mitreva, R.; Atanasova, M. Agomelatine treatment corrects symptoms of depression and anxiety by restoring the disrupted melatonin circadian rhythms of rats exposed to chronic constant light. Pharmacol. Biochem. Behav. 2018, 171, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kjaer, J.; Vestergaard, K. Development of feather pecking in relation to light intensity. Appl. Anim. Behav. Sci. 1999, 62, 243–254. [Google Scholar] [CrossRef]
- Fouda, M.; Darwish, R.; Abou-ismail, U.; Saad, A. Comparative effects of natural and artificial light on behaviour, performance and welfare of broiler chickens. Mansoura Vet. Med. J. 2018, 19, 321–333. [Google Scholar]
- Sobotik, E.B.; Nelson, J.R.; Archer, G.S. How does ultraviolet light affect layer production, fear, and stress. Appl. Anim. Behav. Sci. 2020, 223, 104926. [Google Scholar] [CrossRef]
- Proudman, J.A. Daily rhythm of prolactin and corticosterone in unrestrained, incubating turkey hens. Domest. Anim. Endocrinol. 1991, 8, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Scanes, C.G. Biology of stress in poultry with emphasis on glucocorticoids and the heterophil to lymphocyte ratio. Poult. Sci. 2016, 95, 2208–2215. [Google Scholar] [CrossRef] [PubMed]
- Astheimer, L.B.; Buttemer, W.A.; Wingfield, J.C. Interactions of Corticosterone with Feeding, Activity and Metabolism in Passerine Birds. Ornis Scand. 1992, 23, 355. [Google Scholar] [CrossRef]
- Tsipoura, N.; Scanes, C.G.; Burger, J. Corticosterone and growth hormone levels in shorebirds during spring and fall migration stopover. J. Exp. Zool. 1999, 284, 645–651. [Google Scholar] [CrossRef]
- Crossin, G.T.; Trathan, P.N.; Phillips, R.A.; Gorman, K.B.; Dawson, A.; Sakamoto, K.Q.; Williams, T.D. Corticosterone predicts foraging behavior and parental care in Macaroni penguins. Am. Nat. 2012, 180, E31–E41. [Google Scholar] [CrossRef]
- Nasir, A.; Moudgal, R.P.; Singh, N.B. Involvement of corticosterone in food intake, food passage time and in vivo uptake of nutrients in the chicken (Gallus domesticus). Br. Poult. Sci. 1999, 40, 517–522. [Google Scholar] [CrossRef]
- Koch, K.A.; Wingfield, J.C.; Buntin, J.D. Glucocorticoids and parental hyperphagia in ring doves (Streptopelia risoria). Horm. Behav. 2002, 41, 9–21. [Google Scholar] [CrossRef]
- Beuving, G.; Vonder, G.M. The influence of ovulation and oviposition on corticosterone levels in the plasma of laying hens. Gen. Comp. Endocrinol. 1981, 44, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Buntin, J.D. Neural and Hormonal Control of Parental Behavior in Birds. In Parental Care: Evolution, Mechanisms, and Adaptive Significance; Rosenblatt, J.S., Ed.; Academic Press: San Diego, CA, USA, 1996; pp. 161–213. ISBN 9780120045259. [Google Scholar]
- Silver, R.; Reboulleau, C.; Lehrman, D.S.; Feder, H.H. Radioimmunoassay of plasma progesterone during the reproductive cycle of male and female ring doves (Streptopelia risoria). Endocrinology 1974, 94, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Sockman, K.W.; Schwabl, H. Daily estradiol and progesterone levels relative to laying and onset of incubation in canaries. Gen. Comp. Endocrinol. 1999, 114, 257–268. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Takahashi, T.; Kawashima, M. Mesotocin increases the sensitivity of the hen oviduct uterus to arginine vasotocin. Poult. Sci. 2008, 87, 2107–2111. [Google Scholar] [CrossRef] [PubMed]
- Connolly, P.B.; Callard, I.P. Steroids modulate the release of luteinizing hormone from quail pituitary cells. Gen. Comp. Endocrinol. 1987, 68, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Goutte, A.; Angelier, F.; Chastel, C.C.; Trouvé, C.; Moe, B.; Bech, C.; Gabrielsen, G.W.; Chastel, O. Stress and the timing of breeding: Glucocorticoid-luteinizing hormones relationships in an arctic seabird. Gen. Comp. Endocrinol. 2010, 169, 108–116. [Google Scholar] [CrossRef]
- Etches, R.J.; Croze, F. Plasma concentrations of LH, progesterone, and corticosterone during ACTH- and corticosterone-induced ovulation in the hen (Gallus domesticus). Gen. Comp. Endocrinol. 1983, 50, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Etches, R.J.; Petitte, J.N.; Anderson-Langmuir, C.E. Interrelationships between the hypothalamus, pituitary gland, ovary, adrenal gland, and the open period for LH release in the hen (Gallus domesticus). J. Exp. Zool. 1984, 232, 501–511. [Google Scholar] [CrossRef]
- Cotter, P.F. An examination of the utility of heterophil-lymphocyte ratios in assessing stress of caged hens. Poult. Sci. 2015, 94, 512–517. [Google Scholar] [CrossRef]
- Matur, E.; Eraslan, E.; Akyazi, I.; Ergul Ekiz, E.; Eseceli, H.; Keten, M.; Metiner, K.; Aktaran Bala, D. The effect of furnished cages on the immune response of laying hens under social stress. Poult. Sci. 2015, 94, 2853–2862. [Google Scholar] [CrossRef]
- Davis, A.K.; Maney, D.L.; Maerz, J.C. The use of leukocyte profiles to measure stress in vertebrates: A review for ecologists. Funct. Ecol. 2008, 22, 760–772. [Google Scholar] [CrossRef]
- Scanes, C.G. Blood. Chapter 10. In Sturkie’s Avian Physiology, 6th ed.; Scanes, C.G., Sturkie, P.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 167–191. ISBN 9780124071605. [Google Scholar]
- Remage-Healey, L.; Romero, L.M. Daily and seasonal variation in response to stress in captive starlings (Sturnus vulgaris): Glucose. Gen. Comp. Endocrinol. 2000, 119, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, J.W.; Marion, C.J. (Eds.) Exotic Animal Formulary, 5th ed.; Saunders: St. Louis, MO, USA, 2018; ISBN 9780323444507. [Google Scholar]
- Zaytsoff, S.J.M.; Brown, C.L.J.; Montina, T.; Metz, G.A.S.; Abbott, D.W.; Uwiera, R.R.E.; Inglis, G.D. Corticosterone-mediated physiological stress modulates hepatic lipid metabolism, metabolite profiles, and systemic responses in chickens. Sci. Rep. 2019, 9, 19225. [Google Scholar] [CrossRef] [PubMed]
- Cray, C.; Gautier, D.; Harris, D.J.; Arheart, K.L. Changes in clinical enzyme activity and bile acid levels in psittacine birds with altered liver function and disease. J. Avian Med. Surg. 2008, 22, 17–24. [Google Scholar] [CrossRef]
- Hu, Y.; Sun, Q.; Liu, J.; Jia, Y.; Cai, D.; Idriss, A.A.; Omer, N.A.; Zhao, R. In ovo injection of betaine alleviates corticosterone-induced fatty liver in chickens through epigenetic modifications. Sci. Rep. 2017, 7, 40251. [Google Scholar] [CrossRef]
- Hu, Y.; Sun, Q.; Hu, Y.; Hou, Z.; Zong, Y.; Omer, N.A.; Abobaker, H.; Zhao, R. Corticosterone-Induced Lipogenesis Activation and Lipophagy Inhibition in Chicken Liver Are Alleviated by Maternal Betaine Supplementation. J. Nutr. 2018, 148, 316–325. [Google Scholar] [CrossRef]
- Häffelin, K.E.; Lindenwald, R.; Kaufmann, F.; Döhring, S.; Spindler, B.; Preisinger, R.; Rautenschlein, S.; Kemper, N.; Andersson, R. Corticosterone in feathers of laying hens: An assay validation for evidence-based assessment of animal welfare. Poult. Sci. 2020, 99, 4685–4694. [Google Scholar] [CrossRef]



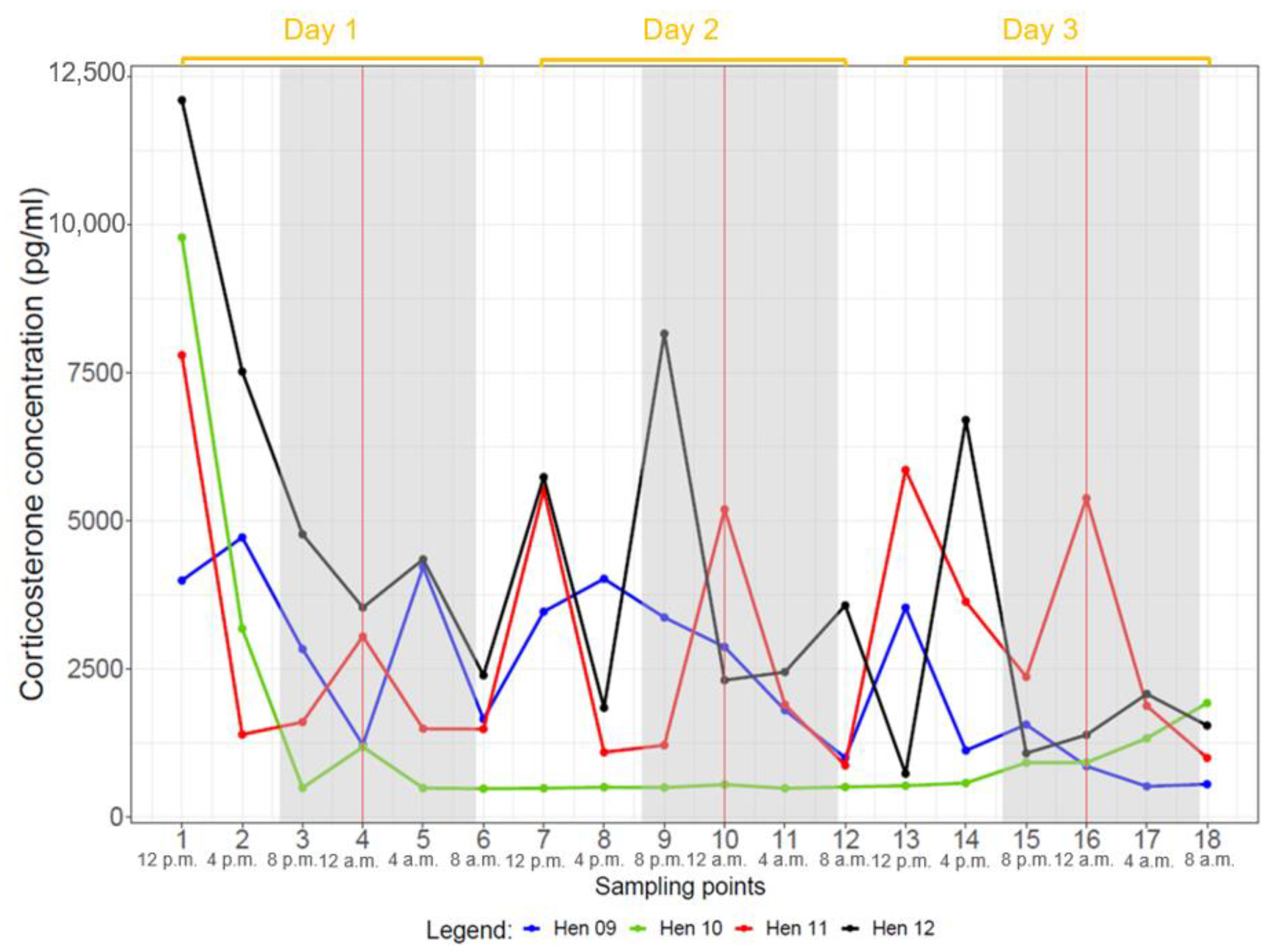
| Day | Sampling Point | Time |
|---|---|---|
| 1 | 1 | 12:00 p.m. |
| 2 | 04:00 p.m. | |
| 3 | 08:00 p.m. | |
| 4 | 12:00 a.m. | |
| 5 | 04:00 a.m. | |
| 6 | 08:00 a.m. | |
| 2 | 7 | 12:00 p.m. |
| 8 | 04:00 p.m. | |
| 9 | 08:00 p.m. | |
| 10 | 12:00 a.m. | |
| 11 | 04:00 a.m. | |
| 12 | 08:00 a.m. | |
| 3 | 13 | 12:00 p.m. |
| 14 | 04:00 p.m. | |
| 15 | 08:00 p.m. | |
| 16 | 12:00 a.m. | |
| 17 | 04:00 a.m. | |
| 18 | 08:00 a.m. |
| Being Pecked (Audio and Video) | Being Chased (Audio and Video) | Blood Sampling-Related Distress during a Test Series (Audio and Video) | ||||
|---|---|---|---|---|---|---|
| Hen | Total/Day | Mean/Day | Total/Day | Mean/Day | Total | Mean/Day |
| 01 | 0 | 0 | 0 | |||
| 02 | 0–26 | 14.8 | 1–28 | 18 | 0 | |
| 03 | 0–2 | 0.3 | 0 | 4 | 1.3 | |
| 04 | 0–4 | 1.3 | 0–1 | 0.2 | 1 | 0.3 |
| 05 | 0–7 | 3.0 | 0–2 | 0.8 | 1 | 0.3 |
| 06 | 0–1 | 0.3 | 0 | 1 | 0.3 | |
| 07 | 0–2 | 0.5 | 0–1 | 0.2 | 0 | |
| 08 | 0–1 | 0.3 | 0 | 0 | ||
| 09 | 0–2 | 0.5 | 0 | 5 | 1.7 | |
| 10 | 0 | 0 | 2 | 0.7 | ||
| 11 | 0–1 | 0.2 | 0 | 1 | 0.3 | |
| 12 | 0–1 | 0.2 | 0 | 0 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hillebrecht, T.; Korbel, R.; Rinder, M.; Gahr, M. Circadian Corticosterone Profile in Laying Hens (Gallus gallus domesticus). Animals 2024, 14, 873. https://doi.org/10.3390/ani14060873
Hillebrecht T, Korbel R, Rinder M, Gahr M. Circadian Corticosterone Profile in Laying Hens (Gallus gallus domesticus). Animals. 2024; 14(6):873. https://doi.org/10.3390/ani14060873
Chicago/Turabian StyleHillebrecht, Theresa, Rüdiger Korbel, Monika Rinder, and Manfred Gahr. 2024. "Circadian Corticosterone Profile in Laying Hens (Gallus gallus domesticus)" Animals 14, no. 6: 873. https://doi.org/10.3390/ani14060873
APA StyleHillebrecht, T., Korbel, R., Rinder, M., & Gahr, M. (2024). Circadian Corticosterone Profile in Laying Hens (Gallus gallus domesticus). Animals, 14(6), 873. https://doi.org/10.3390/ani14060873






