Progesterone-Related Diabetes Mellitus in the Bitch: Current Knowledge, the Role of Pyometra, and Relevance in Practice
Abstract
Simple Summary
Abstract
1. Introduction
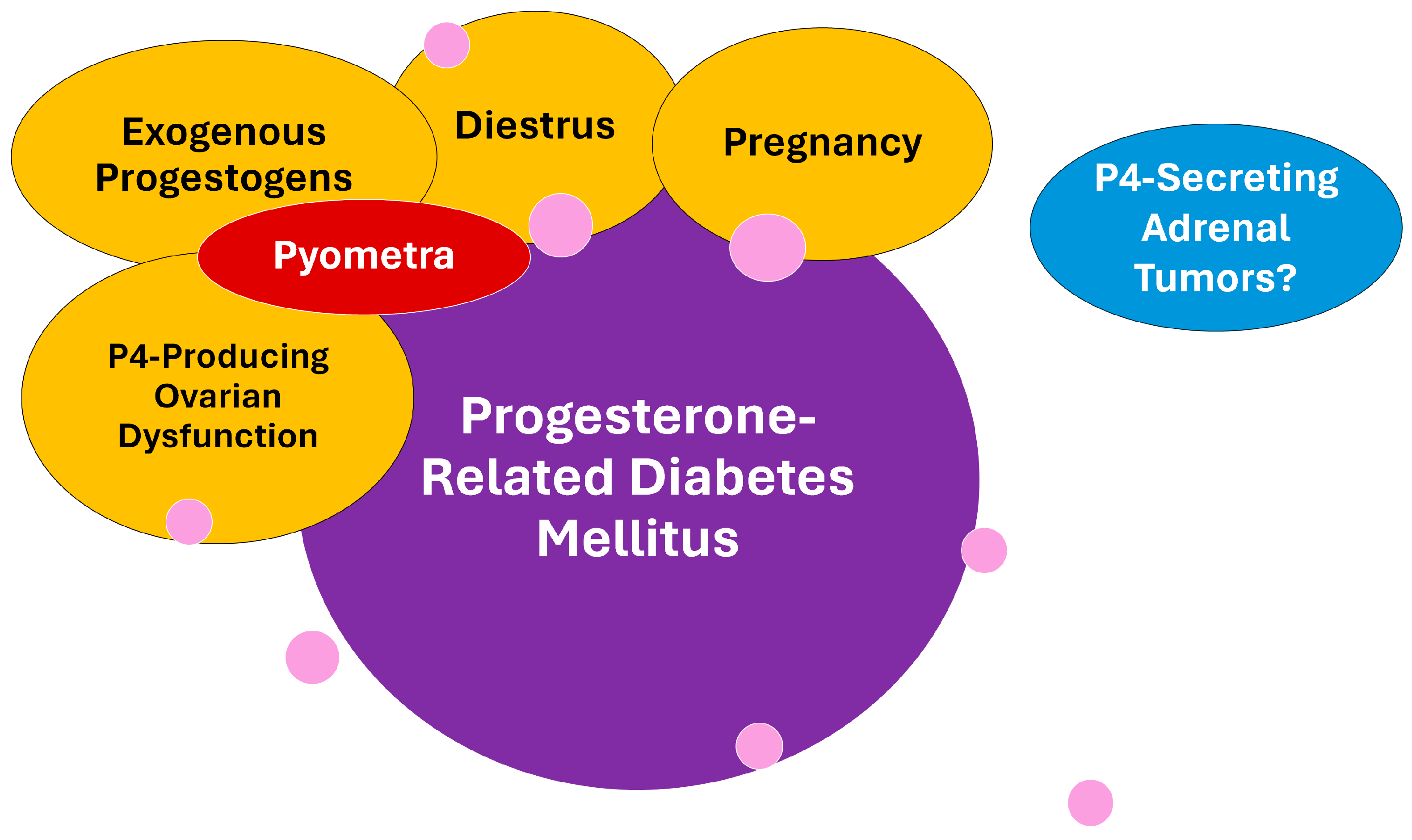
2. The Relevance of Progesterone-Related Diabetes Mellitus Subtype
3. Canine Estrus Cycle: The Progesterone Perspective
Estrus Cycle Effects on Mammary Glands
4. Estrus Cycle Effects on Insulin Sensitivity
4.1. Progesterone: The Evil One?
4.2. How Does Growth Hormone Cause Insulin Resistance?
4.3. Do Estrogens Play a Role in Insulin Sensitivity?
4.4. Estrus Cycle Effects on Markers of Insulin Sensitivity
5. Pyometra and Its Impact on Insulin Sensitivity
6. How to Best Manage Progesterone-Related Diabetes Mellitus?
6.1. Initial Patient Evaluation
6.2. Treatment Goals and Recommendations
6.3. When Is Surgery Indicated?
6.4. What Are the Next Steps after Surgery?
6.5. What to Do When Spaying Is Not an Option?
6.6. Appliable Preventive Measures
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilor, C.; Niessen, S.J.M.; Furrow, E.; DiBartola, S.P. What’s in a name? Classification of diabetes mellitus in veterinary medicine and why it matters. J. Vet. Intern. Med. 2016, 30, 927–940. [Google Scholar] [CrossRef]
- O’Kell, A.L.; Davison, L.J. Etiology and pathophysiology of diabetes mellitus in dogs. Vet. Clin. N. Am. Small Anim. Pract. 2023, 53, 493–510. [Google Scholar] [CrossRef]
- Campbell, E.A. The treatment and control of diabetes in the dog. Aust. Vet. J. 1958, 34, 222–224. [Google Scholar] [CrossRef]
- Wilkinson, J.S. Spontaneous diabetes mellitus. Vet. Rec. 1960, 72, 548–554. [Google Scholar]
- Pöppl, A.G.; de Carvalho, G.L.C.; Vivian, I.F.; Corbellini, L.G.; González, F.H.D. Canine diabetes mellitus risk factors: A matched case-control study. Res. Vet. Sci. 2017, 114, 469–473. [Google Scholar] [CrossRef]
- Heeley, A.M.; Brodbelt, D.C.; O’Neill, D.G.; Church, D.B.; Davison, L.J. Assessment of glucocorticoid and antibiotic exposure as risk factors for diabetes mellitus in selected dog breeds attending UK primary-care clinics. Vet. Rec. 2023, 192, e2785. [Google Scholar] [CrossRef]
- Pöppl, A.G.; González, F.H.D. Epidemiologic and clinical-pathological features of canine diabetes mellitus. Acta Sci. Vet. 2005, 33, 33–40. [Google Scholar] [CrossRef]
- Brito-Casillas, Y.; Melián, C.; Holder, A.; Wiebe, J.C.; Navarro, A.; Quesada-Canales, O.; Expósito-Montesdeoca, A.B.; Catchpole, B.; Wägner, A.M. Studying the heterogeneous pathogenesis of canine diabetes: Observational characterization of an island population. Vet. Med. Sci. 2021, 7, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Fleeman, L.; Barrett, R. Cushing’s syndrome and other causes of insulin resistance in dogs. Vet. Clin. N. Am. Small Anim. Pract. 2023, 53, 711–723. [Google Scholar] [CrossRef]
- Fall, T.; Kreuger, S.J.; Juberget, A.; Bergström, A.; Hedhammar, A. Gestational diabetes mellitus in 13 dogs. J. Vet. Intern. Med. 2008, 22, 1296–1300. [Google Scholar] [CrossRef]
- Fall, T.; Hedhammar, A.; Wallberg, A.; Fall, N.; Ahlgren, K.M.; Hamlin, H.H.; Lindblad-Toh, K.; Andersson, G.; Kämpe, O. Diabetes mellitus in elkhounds is associated with diestrus and pregnancy. J. Vet. Intern. Med. 2010, 24, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Hagman, R. Pyometra in Small Animals 3.0. Vet. Clin. N. Am. Small Anim. Pract. 2023, 53, 1223–1254. [Google Scholar] [CrossRef] [PubMed]
- Krook, L.; Larsson, S.; Rooney, J.R. The interrelationship of diabetes mellitus, obesity, and pyometra in the dog. Am. J. Vet. Res. 1960, 21, 120–124. [Google Scholar] [PubMed]
- Pöppl, A.G.; Mottin, T.S.; González, F.H.D. Diabetes mellitus remission after resolution of inflammatory and P4-related conditions in bitches. Res. Vet. Sci. 2013, 94, 471–473. [Google Scholar] [CrossRef]
- Pöppl, A.G.; Lasta, C.S.; González, F.H.D.; Kucharski, L.C.; Da Silva, R.S.M. Insulin sensitivity indexes in female dogs: Effect of estrus cycle and pyometra. Acta Sci. Vet. 2009, 37, 341–350. [Google Scholar] [CrossRef]
- Pöppl, A.G.; Valle, S.C.; Mottin, T.S.; Leal, J.S.; González, F.H.D.; Kucharski, L.C.; Da Silva, R.S.M. Pyometra-associated insulin resistance assessment by insulin binding assay and tyrosine kinase activity evaluation in canine muscle tissue. Domest. Anim. Endocrinol. 2021, 76, 106626. [Google Scholar] [CrossRef] [PubMed]
- Pöppl, A.G.; Valle, S.C.; González, F.H.D.; Beck, C.A.C.; Kucharski, L.C.; Da Silva, R.S.M. Estrus cycle effect on muscle tyrosine kinase activity in bitches. Vet. Res. Comm. 2012, 36, 81–84. [Google Scholar] [CrossRef]
- Pöppl, A.G.; Valle, S.C.; González, F.H.D.; Kucharski, L.C.; Da Silva, R.S.M. Insulin binding characteristics in canine muscle tissue: Effects of the estrous cycle phases. Pesq. Vet. Bras. 2016, 36, 761–766. [Google Scholar] [CrossRef][Green Version]
- Niessen, S.J.M.; Bjornvad, C.; Church, D.B.; Davison, L.; Esteban-Saltiveri, D.; Fleeman, L.M.; Forcada, Y.; Fracassi, F.; Gilor, C.; Hanson, J.; et al. Agreeing Language in Veterinary Endocrinology (ALIVE): Diabetes mellitus—A modified Delphi-method-based system to create consensus disease definitions. Vet. J. 2022, 289, 105910. [Google Scholar] [CrossRef]
- Yoon, S.; Fleeman, L.M.; Wilson, B.J.; Mansfield, C.S.; McGreevy, P. Epidemiological study of dogs with diabetes mellitus attending primary care veterinary clinics in Australia. Vet. Rec. 2020, 187, e22. [Google Scholar] [CrossRef]
- Davison, L.J.; Herrtage, M.E.; Catchpole, B. Study of 253 dogs in the United Kingdom with diabetes mellitus. Vet. Rec. 2005, 156, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Fracassi, F.; Pietra, M.; Boari, A.; Aste, G.; Giunti, M.; Famigli-Bergamini, P. Breed distribution of canine diabetes mellitus in Italy. Vet. Res. Commun. 2004, 28, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Guptill, L.; Glickman, L.; Glickman, N. Time trends and risk factors for diabetes mellitus in dogs: Analysis of veterinary medical database records (1970–1999). Vet. J. 2003, 165, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Mattin, M.; O’Neill, D.; Church, D.; McGreevy, P.D.; Thomson, P.C.; Brodbelt, D. An epidemiological study of diabetes mellitus in dogs attending first opinion practice in the UK. Vet. Rec. 2014, 174, 349. [Google Scholar] [CrossRef] [PubMed]
- Wiles, B.M.; Llewellyn-Zaidi, A.M.; Evans, K.M.; O’Neill, D.G.; Lewis, T.W. Large-Scale survey to estimate the prevalence of disorders for 192 Kennel Club registered breeds. Canine Genet. Epidemiol. 2017, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Fall, T.; Hamlin, H.H.; Hedhammar, A.; Kampe, O.; Egenvall, A. Diabetes mellitus in a population of 180,000 insured dogs: Incidence, survival, and breed distribution. J. Vet. Intern. Med. 2007, 21, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Fleeman, L.M.; Rand, J.S. Management of canine diabetes. Vet. Clin. N. Am. Small Anim. Pract. 2001, 31, 855–879. [Google Scholar] [CrossRef] [PubMed]
- Catchpole, B.; Ristic, J.M.; Fleeman, L.M.; Davison, L.J. Canine diabetes mellitus: Can old dogs teach-us new tricks? Diabetologia 2005, 48, 1948–1956. [Google Scholar] [CrossRef]
- Nelson, R.W. Canine diabetes mellitus. In Canine and Feline Endocrinology, 4th ed.; Feldman, E.C., Nelson, R.W., Reusch, C.E., Scott-Moncrieff, J.C.R., Behrend, E.N., Eds.; Saunders: St. Louis, MO, USA, 2015; pp. 213–257. [Google Scholar]
- Mui, M.L.; Famula, T.R.; Henthorn, P.S.; Hess, R.S. Heritability and complex segregation analysis of naturally-occurring diabetes in Australian Terrier Dogs. PLoS ONE 2020, 15, e0239542. [Google Scholar] [CrossRef]
- Cai, S.V.; Famula, T.R.; Oberbauer, A.M.; Hess, R.S. Heritability and complex segregation analysis of diabetes mellitus in American Eskimo Dogs. J. Vet. Intern. Med. 2019, 33, 1926–1934. [Google Scholar] [CrossRef]
- Denyer, A.L.; Massey, J.P.; Davison, L.J.; Ollier, W.E.R.; Catchpole, B.; Kennedy, L.J. Dog leucocyte antigen (DLA) class II haplotypes and risk of canine diabetes mellitus in specific dog breeds. Canine Med. Genet. 2020, 7, 15. [Google Scholar] [CrossRef]
- Ringstad, N.K.; Lingaas, F.; Thoresen, S.I. Breed distributions for diabetes mellitus and hypothyroidism in Norwegian dogs. Canine Med. Genet. 2022, 9, 9. [Google Scholar] [CrossRef]
- Imamura, T.; Koffler, M.; Helderman, J.H.; Prince, D.; Thirlby, R.; Inman, L.; Unger, R.H. Severe diabetes induced in subtotally depancreatized dogs by sustained hyperglycemia. Diabetes 1988, 37, 600–609. [Google Scholar] [CrossRef]
- Nelson, R.W.; Reusch, C.E. Animal model of disease Classification and etiology of diabetes in dogs and cats. J. Endocrinol. 2014, 222, T1–T9. [Google Scholar] [CrossRef]
- Renauld, A.; Sverdlik, R.C.; Von Lawzewitsch, I.; Agüero, A.; Pérez, R.L.; Rodríguez, R.R.; Foglia, V.G. Metabolic and histological pancreatic changes induced by ovariectomy in the female dog. Acta Physiol. Pharmacol. Latinoam. 1987, 37, 289–304. [Google Scholar] [PubMed]
- Concannon, P.W. Reproductive cycles of the domestic bitch. Anim. Reprod. Sci. 2011, 124, 200–210. [Google Scholar] [CrossRef]
- Concannon, P.W. Endocrinologic control of normal canine ovarian function. Reprod. Dom. Anim. 2009, 44, 3–15. [Google Scholar] [CrossRef]
- Feldman, E.C.; Nelson, R.W. Canine and Feline Endocrinology and Reproduction, 3rd ed.; Saunders: St. Louis, MO, USA, 2004; pp. 752–774. [Google Scholar]
- Hoffmann, B.; Riesenbeck, A.; Klein, R. Reproductive endocrinology of bitches. Anim. Reprod. Sci. 1996, 42, 275–288. [Google Scholar] [CrossRef]
- Hollinshead, F.K.; Hanlon, D.W. Normal P4 profiles during estrus in the bitch: A prospective analysis of 1420 estrous cycles. Theriogenology 2019, 125, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Post, K. Canine vaginal cytology during the estrous cycle. Can. Vet. J. 1985, 26, 101–104. [Google Scholar]
- Van Cruchten, S.; Van den Broeck, W.; D’haeseleer, M.; Simoens, P. Proliferation patterns in the canine endometrium during the estrous cycle. Theriogenology 2004, 62, 631–641. [Google Scholar] [CrossRef]
- Bouchard, P. P4 and the P4 receptor. J. Reprod. Med. 1999, 44, 153–157. [Google Scholar]
- Thompson, F.N. Reprodução em mamíferos do sexo feminino. In Dukes: Fisiologia dos Animais Domésticos, 2nd ed.; Reece, W.O., Ed.; Guanabara Koogan: Rio de Janeiro, Brasil, 2006; Volume 1, pp. 644–669. [Google Scholar]
- Concannon, P.W.; Hansel, W.; Visek, W.J. The ovarian cycle of the bitch: Plasma estrogen, LH and P4. Biol. Reprod. 1975, 13, 112–121. [Google Scholar] [CrossRef]
- Kooistra, H.S.; Okkens, A.C. Secretion of growth hormone and prolactin during progression of the luteal phase in healthy dogs: A review. Mol. Cell Endocrinol. 2002, 197, 167–172. [Google Scholar] [CrossRef]
- Gobello, C. Revisiting canine pseudocyesis. Theriogenology 2021, 167, 94–98. [Google Scholar] [CrossRef]
- Selman, P.J.; Mol, J.A.; Rutterman, G.R.; Rijnberk, A. Progestin-induced growth hormone excess in the dog originates in the mammary gland. Endocrinology 1994, 134, 287–292. [Google Scholar] [CrossRef]
- Eingenmann, J.E.; Eingenmann, R.Y.; Rijinberk, A.; Van der Gaag, I.; Zapf, J.; Froesch, E.R. P4-controlled growth hormone overproduction and naturally occurring canine diabetes and acromegaly. Acta Endocrinol. 1983, 104, 167–176. [Google Scholar] [CrossRef]
- De Cock, H.; Ducatelle, R.; Tilmant, K.; De Schepper, J. Possible role for insulin-like growth factor-I in the pathogenesis of cystic endometrial hyperplasia pyometra complex in the bitch. Theriogenology 2002, 57, 2271–2287. [Google Scholar] [CrossRef] [PubMed]
- Dominici, F.P.; Argentino, D.P.; Muñoz, M.C.; Miquet, J.G.; Sotelo, A.I.; Turyn, D. Influence of the crosstalk between growth hormone and insulin signaling on the modulation of insulin sensitivity. Growth Horm. IGF Res. 2005, 15, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Gouvêa, F.N.; Pennacchi, C.S.; Assaf, N.D.; Branco, L.O.; Costa, P.B.; Reis, P.A.; Borin-Crivellenti, S. Acromegaly in dogs and cats. Ann. Endocrinol. 2021, 82, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, J.K.; Patra, M.K.; Singh, L.K.; Saxena, A.C.; De, U.K.; Singh, V.; Mathesh, K.; Kumar, H.; Krishnaswamy, N. ovarian cysts in the bitch: An update. Top. Companion Anim. Med. 2021, 43, 100511. [Google Scholar] [CrossRef]
- Farges, A.; Crawford, D.; Silva, C.A.; Ramsey, I. Hyperprogesteronism associated with adrenocortical tumours in two dogs. Vet. Rec. Case Rep. 2024, 1–9. [Google Scholar] [CrossRef]
- Connolly, C.C.; Aglione, L.N.; Smith, M.S.; Lacy, D.B.; Moore, M.C. Insulin action during late pregnancy in the conscious dog. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E909–E9015. [Google Scholar] [CrossRef][Green Version]
- Russell, M.A.; Carpenter, M.W.; Coustan, D.R. Screening and diagnosis of gestacional diabetes mellitus. Clin. Obstet. Gynecol. 2007, 50, 949–958. [Google Scholar] [CrossRef] [PubMed]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Gaglia, J.S.; Hilliard, M.E.; Isaacs, D.; et al. American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46 (Suppl. S1), S19–S40. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, C.; Ursing, D.; Törn, C.; Aberg, A.; Landin-Olsson, M. Presence of GAD antibodies during gestational diabetes mellitus predicts type 1 diabetes. Diabetes Care 2007, 30, 1968–1971. [Google Scholar] [CrossRef] [PubMed]
- DiNinni, A.; Hess, R.S. Development of a requirement for exogenous insulin treatment in dogs with hyperglycemia. J. Vet. Intern. Med. 2024, 1–7. [Google Scholar] [CrossRef]
- Watanake, R.M.; Black, M.H.; Xiang, A.H.; Allayee, H.; Lawrence, J.M.; Buchanan, T.A. Genetics of gestational diabetes mellitus and type 2 diabetes. Diabetes Care 2007, 30 (Suppl. S2), S134–S140. [Google Scholar] [CrossRef] [PubMed]
- Beischer, N.A.; Oats, J.N.; Henry, O.A.; Sheedy, M.T.; Walstab, J.E. Incidence and severity of gestational diabetes mellitus according to country of birth in woman living in Australia. Diabetes 1991, 40 (Suppl. S2), 35–38. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.Y.; Callagan, W.M.; Kim, S.Y.; Schmid, C.H.; Lau, J.; England, L.J.; Dietz, P.M. Maternal obesity and risk of gestacional diabetes mellitus. Diabetes Care 2007, 30, 2070–2076. [Google Scholar] [CrossRef] [PubMed]
- Wejdmark, A.K.; Bonnet, B.; Hedhammar, A.; Fall, T. Lifestyle risk factors for P4-related diabetes mellitus in Elkhounds—A case-control study. J. Small Anim. Pract. 2011, 52, 240–245. [Google Scholar] [CrossRef]
- Kampman, U.; Knorr, S.; Fuglsang, J.; Ovesen, P. Determinants of maternal insulin resistance during pregnancy: An updated overview. J. Diabetes Res. 2019, 2019, 5320156. [Google Scholar] [CrossRef]
- Johnson, C.A. High-risk pregnancy and hypoluteoidism in the bitch. Theriogenology 2008, 70, 1424–1430. [Google Scholar] [CrossRef]
- Johnson, C.A. Glucose homeostasis during canine pregnancy: Insulin resistance, ketosis, and hypoglycemia. Theriogenology 2008, 70, 1418–1423. [Google Scholar] [CrossRef]
- Kim, C.; Berger, D.K.; Chamany, S. Recurrence of gestational diabetes mellitus. Diabetes Care 2007, 30, 1314–1319. [Google Scholar] [CrossRef]
- Philips, P.J. Gestational diabetes: Worth finding and actively treating. Aust. Fam. Physician 2006, 35, 701–703. [Google Scholar]
- Ryan, E.A.; Enns, L. Role of gestational hormones in the induction of insulin resistance. J. Clin. Endocrionol. Metab. 1988, 67, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Hori, S.; Sugiyama, M.; Fujisawa, E.; Nakano, T.; Tsuneki, H.; Nagira, K.; Saito, S.; Sasaoka, T. P4 inhibits glucose uptake by affecting diverse steps of insulin signaling in 3T3-L1 adipocytes. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E881–E888. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Choi, W.; Heo, J.H.; Huh, J.; Kim, G.; Lee, K.; Kwun, H.; Shin, H.; Baek, I.; Hong, E. P4 increases blood glucose via hepatic P4 receptor membrane component 1 under limited or impaired action of insulin. Sci. Rep. 2020, 10, 16316. [Google Scholar] [CrossRef] [PubMed]
- Rand, J.S.; Fleeman, L.M.; Farrow, H.A.; Appleton, D.J.; Lederer, R. Canine and feline diabetes mellitus: Nature or nurture? J. Nutr. 2004, 134, 2072S–2080S. [Google Scholar] [CrossRef] [PubMed]
- Mared, M.; Catchpole, B.; Kämpe, O.; Fall, T. Evaluation of circulating concentrations of glucose homeostasis biomarkers, P4, and growth hormone in healthy Elkhounds during anestrus and diestrus. Am. J. Vet. Res. 2012, 73, 242–247. [Google Scholar] [CrossRef]
- Strage, E.M.; Lewitt, M.S.; Hanson, J.M.; Olsson, U.; Norrvik, F.; Lilliehöök, I.; Holst, B.S.; Fall, T. Relationship among insulin resistance, growth hormone, and insulin-like growth factor I concentrations in diestrous Swedish Elkhounds. J. Vet. Intern. Med. 2014, 28, 419–428. [Google Scholar] [CrossRef]
- Scaramal, J.D.; Renauld, A.; Gómez, N.V.; Garrido, D.; Wanke, M.M.; Marquez, A.G. Natural estrous cycle in normal and diabetic bitches in relation to glucose and insulin tests. Medicina 1997, 57, 169–180. [Google Scholar]
- Renauld, A.; Sverdlik, R.C.; Agüero, A.; Pérez, R.L. Influence of estrogen-P4 sequential administration on pancreas cytology. Serum insulin and metabolic adjustments in female dogs. Acta Diabetol. Lat. 1990, 27, 315–327. [Google Scholar] [CrossRef]
- Rijnberk, A.D.; Kooistra, H.S.; Mol, J.A. Endocrine diseases in dogs and cats: Similarities and differences with endocrine diseases in humans. Growth Horm. IGF Res. 2003, 13, S158–S164. [Google Scholar] [CrossRef]
- Sharma, R.; Kopchick, J.J.; Puri, V.; Sharma, V.M. Effect of growth hormone on insulin signaling. Mol. Cell Endocrinol. 2020, 1, 111038. [Google Scholar] [CrossRef]
- Moller, D.; Flier, J.S. Insulin resistance. Mechanisms, syndromes, and implications. N. Engl. J. Med. 1991, 325, 938–948. [Google Scholar]
- Saltiel, A.R.; Kahn, C.R. Insulin signaling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Kooistra, H.S.; Den Hertog, E.; Okkens, A.C.; Mol, J.A.; Rijnberk, A. Pulsatile secretion pattern of growth hormone during the luteal phase and mild-anoestrus in beagle bitches. J. Reprod. Fertil. 2000, 119, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Godsland, I.F. Oestrogens and insulin secretion. Diabetologia 2005, 48, 2213–2220. [Google Scholar] [CrossRef] [PubMed]
- De Paoli, M.; Zakharia, A.; Werstuck, G.H. The role of estrogen in insulin resistance: A review of clinical and preclinical data. Am. J. Pathol. 2021, 191, 1490–1498. [Google Scholar] [CrossRef]
- Renauld, A.; Von Lawzewitsch, I.; Pérez, R.L.; Sverdlik, R.; Agüero, A.; Foglia, V.G.; Rodríguez, R.R. Effect of estrogens on blood sugar, serum insulin and serum free fatty acids, and pancreas cytology in female dogs. Acta Diabetol. Lat. 1983, 20, 47–56. [Google Scholar] [CrossRef]
- Barros, R.P.A.; Machado, U.F.; Warmer, M.; Gostafsson, J. Muscle GLUT4 regulation by estrogen receptors ERβ and ERα. Proc. Natl. Acad. Sci. USA 2006, 103, 1605–1608. [Google Scholar] [CrossRef]
- Homma, H.; Kurachi, H.; Nishio, Y.; Takeda, T.; Yamamoto, T.; Adachi, K.; Morishige, K.; Ohmichi, M.; Matsuzawa, Y.; Murata, Y. Estrogen suppresses transcription of lipoprotein lipase gene. Existence of a unique estrogen response element on the lipoprotein lipase promoter. J. Biol. Chem. 2000, 275, 11404–11411. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.M.; Barros, R.P.A.; Zampieri, R.A.; Okamoto, M.M.; Carvalho Papa, P.; Machado, U.F. Abnormal subcellular distribution of GLUT4 protein in obese and insulin-treated diabetic female dogs. Braz. J. Med. Biol. Res. 2004, 37, 1095–1101. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Le Roux, P.H.; Van Der Walt, L.A. Ovarian autograft as an alternative to ovariectomy in bitches. J. S. Afr. Vet. Assoc. 1977, 48, 117–123. [Google Scholar]
- Vendramini, T.H.A.; Amaral, A.R.; Pedrinelli, V.; Zafalon, R.V.A.; Rodrigues, R.B.A.; Brunetto, M.A. Neutering in dogs and cats: Current scientific evidence and importance of adequate nutritional management. Nutr. Res. Rev. 2020, 33, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef]
- Ader, M.; Stefanovski, D.; Richey, J.M.; Kim, S.P.; Kolka, C.M.; Ionut, V.; Kabir, M.; Bergman, R.N. Failure of homeostatic model assessment of insulin resistance to detect marked diet-induced insulin resistance in dogs. Diabetes 2014, 63, 1914–1919. [Google Scholar] [CrossRef]
- Koenig, A. Endocrine emergencies in dogs and cats. Vet. Clin. N. Am. Small Anim. Pract. 2013, 43, 869–897. [Google Scholar] [CrossRef]
- Verkest, K.R.; Fleeman, L.M.; Rand, J.S.; Morton, J.M. Basal measures of insulin sensitivity and insulin secretion and simplified glucose tolerance tests in dogs. Domest. Anim. Endocrinol. 2010, 39, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Fukuta, H.; Mori, A.; Urumuhan, N.; Lee, P.; Oda, H.; Saeki, K.; Kurishima, M.; Nozawa, S.; Mizutani, H.; Mishina, S.; et al. Characterization and comparison of insulin resistance induced by Cushing syndrome or diestrus against healthy control dogs as determined by euglycemic-hyperinsulinemic glucose clamp profile glucose in- fusion rate using an artificial pancreas apparatus. J. Vet. Med. Sci. 2012, 74, 1527–1530. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bailhache, E.; Nguyen, P.; Krempf, M.; Siliart, B.; Magot, T.; Ougueram, K. Lipoproteins abnormalities in obese insulin-resistant dogs. Metabolism 2003, 52, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Renauld, A.; Gomez, N.V.; Scaramal, J.D.; Garrido, D.; Wanke, M.M. Natural estrous cycle in normal and diabetic bitches. Basal serum total lipids and cholesterol. Serum tryglicerides profiles during glucose and insulin tests. Acta Physiol. Pharmacol. Ther. Latinoam. 1998, 48, 41–51. [Google Scholar] [CrossRef]
- Lawrence, M.C.; McKern, N.M.; Ward, C.W. Insulin receptor structure and its implications for the IGF-1 receptor. Curr. Opin. Struct. Biol. 2007, 17, 699–705. [Google Scholar] [CrossRef]
- Barbour, L.A.; McCurdy, C.E.; Hernandez, T.L.; Kirwan, J.P.; Catalano, P.M.; Friedman, J.E. Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes Care 2007, 30, 112–119. [Google Scholar] [CrossRef]
- Schäffer, L. A model for insulin binding to the insulin receptor. Eur. J. Biochem. 1994, 221, 1127–1132. [Google Scholar] [CrossRef]
- Johnston, V.; Frazzini, V.; Davidheiser, S.; Przybylski, R.J.; Kliegman, R.M. Insulin binding receptor number and binding affinity in newborn dogs. Pediatr. Res. 1991, 29, 611–614. [Google Scholar] [CrossRef][Green Version]
- Sanvitto, G.L.; Marques, M.; Dellacha, J.M.; Turyn, D. Masked insulin receptors in rat hypothalamus microsomes. Horm. Metab. Res. 1994, 26, 160–161. [Google Scholar] [CrossRef]
- Batista, M.R.; Smith, M.S.; Snead, W.L.; Connolly, C.C.; Lacy, D.B.; Moore, M.C. Chronic estradiol and P4 treatment in conscious dogs: Effects on insulin sensitivity and response to hypoglycemia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R1064–R1073. [Google Scholar] [CrossRef]
- Yu, E.N.; Winnick, J.J.; Edgerton, D.S.; Scott, M.F.; Smith, M.S.; Farmer, B.; Williams, P.E.; Cherrington, A.D.; Moore, M.C. Hepatic and whole-body insulin metabolism during proestrus and estrus in mongrel dogs. Comp. Med. 2016, 66, 235–240. [Google Scholar]
- Hagman, R. Canine pyometra: What is new? Reprod. Domest. Anim. 2017, 52, 288–292. [Google Scholar] [CrossRef]
- Smith, F.O. Canine pyometra. Theriogenology 2006, 66, 610–612. [Google Scholar] [CrossRef]
- De Bosschere, H.; Ducatelle, R.; Vermeirsch, H.; Simoens, P.; Coryn, M. Estrogen-α and P4 receptor expression in cystic endometrial hyperplasia and pyometra in the bitch. Anim. Reprod. Sci. 2002, 70, 251–259. [Google Scholar] [CrossRef]
- Hagman, R. Pyometra in Small Animals. Vet. Clin. N. Am. Small Anim. Pract. 2018, 48, 639–661. [Google Scholar] [CrossRef]
- Chen, L.; Chen, R.; Wang, H.; Liang, F. Mechanisms linking inflammation to insulin resistance. Int. J. Endocrinol. 2015, 2015, 508409. [Google Scholar] [CrossRef]
- Egenvall, A.; Hagman, R.; Bonnett, B.N.; Hedhammar, A.; Olson, P.; Lagerstedt, A.-S. Breed Risk of Pyometra in Insured Dogs in Sweden. J. Vet. Intern. Med. 2001, 15, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Gibson, A.; Dean, R.; Yates, D.; Stavisky, J. A retrospective study of pyometra at five RSPCA hospitals in the UK: 1728 cases from 2006 to 2011. Vet. Rec. 2013, 173, 380–404. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Current advances in sepsis and septic shock with particular emphasis on the role of insulin. Med. Sci. Monit. 2003, 9, RA181–RA192. [Google Scholar] [PubMed]
- Das, U.N. Insulin in sepsis and septic shock. J. Assoc. Physicians India 2003, 51, 695–700. [Google Scholar] [PubMed]
- Grimble, R.F. Inflammatory status and insulin resistance. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Inflammatory mechanisms in the regulation of insulin resistance. Mol. Med. 2008, 14, 222–231. [Google Scholar] [CrossRef] [PubMed]
- German, A.J.; Hervera, M.; Hunter, L.; Holden, S.L.; Morris, P.J.; Biourge, V.; Trayhurn, P. Improvement in insulin resistance and reduction in plasma inflammatory adipokines after weight loss in obese dogs. Domest. Anim. Endocrinol. 2009, 37, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Wakshlag, J.J.; Struble, A.M.; Levine, C.B.; Bushey, J.J.; Laflamme, D.P.; Long, G.M. The effects of weight loss on adipokines and markers of inflammation in Dogs. Br. J. Nutr. 2011, 106, S11–S14. [Google Scholar] [CrossRef] [PubMed]
- Jeremias, J.T.; Takeara, P.; Vendramini, T.H.A.; Rodrigues, R.B.A.; Perini, M.P.; Pedrinelli, V.; Teixeira, F.A.; Brunetto, M.A.; Pontieri, C.F.F. Markers of inflammation and insulin resistance in dogs before and after weight loss versus lean healthy dogs. Pesq. Vet. Bras. 2020, 40, 300–305. [Google Scholar] [CrossRef]
- Marchi, P.H.; Vendramini, T.H.A.; Perini, M.P.; Zafalon, R.V.A.; Amaral, A.R.; Ochamotto, V.A.; Da Silveira, J.C.; Dagli, M.L.Z.; Brunetto, M.A. Obesity, inflammation, and cancer in dogs: Review and perspectives. Front. Vet. Sci. 2022, 9, 1004122. [Google Scholar] [CrossRef] [PubMed]
- Pöppl, A.G.; Müller, F.; Queiroga, L.; González, F.H.D. Insulin resistance due to periodontal disease in an old diabetic female poodle. Diab Res. Treat. 2015, 2, 122. [Google Scholar]
- Nivy, R.; Bar-Am, Y.; Retzkin, H.; Bruchim, Y.; Mazaki-Tovi, M. Preliminary evaluation of the impact of periodontal treatment on markers of glycaemic control in dogs with diabetes mellitus: A prospective, clinical case series. Vet. Rec. 2023, 194, e3310. [Google Scholar] [CrossRef]
- Loste, A.; Marca, M.C. Study of the effect of total serum protein and albumin concentrations on canine fructosamine concentration. Can. J. Vet. Res. 1999, 63, 138–141. [Google Scholar]
- Pöppl, A.G. Canine diabetes mellitus: Assessing risk factors to inform preventive measures. Vet. Rec. 2023, 192, 406–408. [Google Scholar] [CrossRef]
- Behrend, E.; Holford, A.; Lathan, P.; Rucinsky, R.; Schulman, R. 2018 AAHA Diabetes Management Guidelines for Dogs and Cats. J. Am. Anim. Hosp. Assoc. 2018, 54, 1–21. [Google Scholar] [CrossRef]
- Mol, J.A.; Garderen, E.V.; Selman, P.J.; Wolfswinkel, J.; Rijinberk, A.; Rutteman, G.R. Growth hormone mRNA in mammary gland tumors of dogs and cats. J. Clin. Investig. 1995, 95, 2028–2034. [Google Scholar] [CrossRef]
- Murai, A.; Nishii, N.; Morita, T.; Yuki, M. GH-producing mammary tumors in two dogs with acromegaly. J. Vet. Med. Sci. 2012, 74, 771–774. [Google Scholar] [CrossRef]
- Weese, J.S.; Blondeau, J.M.; Boothe, D.; Guardabassi, L.; Gumley, N.; Papich, M.G.; Jessen, L.R.; Lappin, M.; Rankin, S.C.; Westropp, J.L.; et al. International Society for Companion Animal Infectious Diseases (ISCAID) guidelines for the diagnosis and management of bacterial urinary tract infections in dogs and cats. Vet. J. 2019, 247, 8–25. [Google Scholar] [CrossRef]
- Staroňová, R.; Kožár, M.; Horňáková, L.; Fialkovičová, M. Diestrous diabetes mellitus remission in a Yorkshire terrier bitch. Vet. Rec. Case Rep. 2021, 9, e205. [Google Scholar] [CrossRef]
- Jeon, Y.; Bae, H.; Yu, D. A case of transient diabetes mellitus in a dog managed by ovariohysterectomy. J. Biomed. Transl. Res. 2023, 24, 93–99. [Google Scholar] [CrossRef]
- Lee, W.M.; Kooistra, H.S.; Mol, J.A.; Dieleman, S.J.; Schaefers-Okkens, A.C. Ovariectomy during the luteal phase influences secretion of prolactin, growth hormone, and insulin-like growth factor-I in the bitch. Theriogenology 2006, 66, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.S.; Jones, T. Anesthetic considerations in dogs and cats with diabetes mellitus. Vet. Clin. N. Am. Small Anim. Pract. 2023, 53, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Gilor, C.; Fleeman, L.M. One hundred years of insulin: Is it time for smart? J. Small Anim. Pract. 2022, 63, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Shiel, R.E.; Mooney, C.T. Insulins for the long-term management of diabetes mellitus in dogs: A review. Canine Med. Genet. 2022, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, F.A.; Brunetto, M.A. Nutritional factors related to glucose and lipid modulation in diabetic dogs: Literature review. Braz. J. Vet. Res. Anim. Sci. 2017, 54, 330–341. [Google Scholar] [CrossRef][Green Version]
- Teixeira, F.A.; Machado, D.P.; Jeremias, J.T.; Queiroz, M.R.; Pontieri, C.F.F.; Brunetto, M.A. Effects of pea with barley and less-processed maize on glycaemic control in diabetic dogs. Br. J. Nutr. 2018, 120, 777–786. [Google Scholar] [CrossRef]
- Teixeira, F.A.; Machado, D.P.; Jeremias, J.T.; Queiroz, M.R.; Pontieri, C.F.F.; Brunetto, M.A. Starch sources influence lipidaemia of diabetic dogs. BMC Vet. Res. 2020, 16, 2. [Google Scholar] [CrossRef] [PubMed]
- Society for Comparative Endocrinology. Available online: https://www.veterinaryendocrinology.org/ (accessed on 12 January 2024).
- van Bokhorst, K.L.; Galac, S.; Kooistra, H.S.; Valtolina, C.; Fracassi, F.; Rosenberg, D.; Meij, B.P. Evaluation of hypophysectomy for treatment of hypersomatotropism in 25 cats. J. Vet. Intern. Med. 2021, 35, 834–842. [Google Scholar] [CrossRef]
- Fenn, J.; Kenny, P.J.; Scudder, C.J.; Hazuchova, K.; Gostelow, R.; Fowkes, R.C.; Forcada, Y.; Church, D.B.; Niessen, S.J.M. Efficacy of hypophysectomy for the treatment of hypersomatotropism-induced diabetes mellitus in 68 cats. J. Vet. Intern. Med. 2021, 35, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Gostelow, R.; Scudder, C.J.; Keyte, S.; Forcada, Y.; Fowkes, R.C.; Schmid, D.B.; Church, D.B.; Niessen, S.J.M. Pasireotide long-acting release treatment for diabetic cats with underlying hypersomatotropism. J. Vet. Intern. Med. 2017, 31, 355–364. [Google Scholar] [CrossRef]
- Miceli, D.D.; García, J.D.; Pompili, G.A.; Amunategui, J.P.R.; Ferraris, S.; Pignataro, O.M.; Guitelman, M. Cabergoline treatment in cats with diabetes mellitus and hypersomatotropism. J. Feline Med. Surg. 2022, 24, 1238–1244. [Google Scholar] [CrossRef]
- Aptekmann, K.P.; Schwartz, D.S. A survey of owner attitudes and experiences in managing diabetic dogs. Vet. J. 2011, 190, e122–e124. [Google Scholar] [CrossRef]
- Aptekmann, K.P.; Armstrong, J.; Coradini, M.; Rand, J. Owner experiences in treating dogs and cats diagnosed with diabetes mellitus in the United States. J. Am. Anim. Hosp. Assoc. 2014, 50, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Tardo, A.M.; Baldo, D.F.; Dondi, F.; Pietra, M.; Chiocchetti, R.; Fracassi, F. Survival estimates and outcome predictors in dogs with newly diagnosed diabetes mellitus treated in a veterinary teaching hospital. Vet. Rec. 2019, 185, 692. [Google Scholar] [CrossRef]
- Gal, A.; Odunayo, A. Diabetes Ketoacidosis and Hyperosmolar Hyperglycemic Syndrome in Companion Animals. Vet. Clin. N. Am. Small Anim. Pract. 2023, 53, 531–550. [Google Scholar] [CrossRef]
- Corradini, S.; Pilosio, B.; Dondi, F.; Linari, G.; Testa, S.; Brugnoli, F.; Gianella, P.; Pietra, M.; Fracassi, F. Accuracy of a flash glucose monitoring system in diabetic dogs. J. Vet. Intern. Med. 2016, 30, 983–988. [Google Scholar] [CrossRef]
- Baldo, D.F.; Fracassi, F. Continuous glucose monitoring in dogs and cats. Application of new technology to an old problem. Vet. Clin. N. Am. Small Anim. Pract. 2023, 53, 591–613. [Google Scholar] [CrossRef]
- Fink, H.; Herbert, C.; Gilor, C. Pharmacodynamics and pharmacokinetics of insulin detemir and insulin glargine 300 U/mL in healthy dogs. Domest. Anim. Endocrinol. 2018, 64, 17–30. [Google Scholar] [CrossRef]
- Harris-Samson, A.R.; Rand, J.; Ford, S.L. Detemir improves diabetic regulation in poorly controlled diabetic dogs with concurrent diseases. J. Am. Vet. Med. Assoc. 2023, 261, 237–335. [Google Scholar] [CrossRef]
- Miceli, D.D.; Pignataro, O.P.; Castillo, V.A. Concurrent hyperadrenocorticism and diabetes mellitus in dogs. Res. Vet. Sci. 2017, 115, 425–431. [Google Scholar] [CrossRef]
- Fleeman, L.; Gilor, C. Insulin Therapy in Small Animals, Part 3: Dogs. Vet. Clin. N. Am. Small Anim. Pract. 2023, 53, 645–656. [Google Scholar] [CrossRef]
- Kuzi, S.; Mazaki-Tovi, M.; Hershkovitz, S.; Yas, E.; Hess, R.S. Long-term field study of lispro and neutral protamine Hagedorn insulins treatment in dogs with diabetes mellitus. Vet. Med. Sci. 2023, 9, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Niessen, S.J.M.; Powney, S.; Guitian, J.; Niessen, A.P.M.; Pion, P.D.; Shaw, J.A.M.; Church, D.B. Evaluation of a quality-of-life tool for dogs with diabetes mellitus. J. Vet. Intern. Med. 2012, 26, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Baan, M.; Taverne, M.A.M.; Kooistra, H.S.; Gier, J.D.; Dieleman, S.J.; Okkens, A.C. Induction of parturition in the bitch with the P4-receptor blocker aglépristone. Theriogenology 2005, 63, 1958–1972. [Google Scholar] [CrossRef] [PubMed]
- Galac, S.; Kooistra, H.S.; Butinar, J.; Bevers, M.M.; Dieleman, S.J.; Voorhout, G.; Okkens, A.C. Termination of mid-gestation pregnancy in bitches with aglepristone, a P4 receptor antagonist. Theriogenology 2000, 53, 941–950. [Google Scholar] [CrossRef]
- Binli, F.; Inan, I.; Büyükbudak, F.; Gram, A.; Kaya, D.; Liman, N.; Aslan, S.; Fındık, M.; Ay, S.S. The Efficacy of a 3-Hydroxysteroid Dehydrogenase Inhibitor for the Termination of Mid-Term Pregnancies in Dogs. Animals 2022, 12, 2475. [Google Scholar] [CrossRef]
- Galac, S.; Kooistra, H.S.; Dieleman, S.J.; Cestnik, V.; Okkens, A.C. Effects of aglépristone, a P4 receptor antagonist, administered during the early luteal phase in non-pregnant bitches. Theriogenology 2004, 62, 494–500. [Google Scholar] [CrossRef]
- Bigliardi, E.; Bresciani, C.; Callegari, D.; Ianni, F.D.; Morini, G.; Parmigiani, E.; Bianchi, E. Use of aglepristone for the treatment of P4 induced insulin resistance in dogs. J. Vet. Sci. 2014, 15, 267–271. [Google Scholar] [CrossRef]
- Bhatti, S.F.M.; Duchateau, L.; Okkens, A.C.; Vanham, L.M.L.; Mol, J.A.; Kooistra, H.S. Treatment of growth hormone excess in dogs with the P4 receptor antagonist aglépristone. Theriogenology 2006, 66, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Lemetayer, J.; Blois, S. Update on the use of trilostane in dogs. Can. Vet. J. 2018, 59, 397–407. [Google Scholar] [PubMed]
- Sanders, K.; Kooistra, H.S.; Galac, S. Treating canine Cushing’s syndrome: Current options and future prospects. Vet. J. 2018, 241, 42–51. [Google Scholar] [CrossRef]
- Gier, J.D.; Wolthers, C.H.J.; Galac, S.; Okkens, A.C.; Kooistra, H.S. Effects of the 3-hydroxysteroid dehydrogenase inhibitor trilostane on luteal P4 production in the dog. Theriogenology 2011, 75, 1271–1279. [Google Scholar] [CrossRef]
- Jeukenne, P.; Verstegen, J. Termination of dioestrus and induction of oestrus in dioestrous nonpregnant bitches by the prolactin antagonist cabergoline. J. Reprod. Fertil. Suppl. 1997, 51, 59–66. [Google Scholar] [PubMed]
- Romagnoli, S.; Stelletta, C.; Milani, C.; Gelli, D.; Falomo, M.E.; Mollo, A. Clinical use of deslorelin for the control of reproduction in the bitch. Reprod. Dom. Anim. 2009, 44 (Suppl. S2), 36–39. [Google Scholar] [CrossRef]
- Hadd, M.J.; Bienhoff, S.E.; Little, S.E.; Geller, S.; Ogne-Stevenson, J.; Dupree, T.J.; Scott-Moncrieff, J.C. Safety and effectiveness of the sodium-glucose cotransporter inhibitor bexagliflozin in cats newly diagnosed with diabetes mellitus. J. Vet. Intern. Med. 2023, 37, 915–924. [Google Scholar] [CrossRef]
- Reinhart, M.R.; Graves, T.K. The Future of Diabetes Therapies New Insulins and Insulin Delivery Systems, Glucagon-Like Peptide 1 Analogs, Sodium-Glucose Cotransporter Type 2 Inhibitors, and Beta Cell Replacement Therapy. Vet. Clin. N. Am. Small Anim. Pract. 2023, 53, 675–690. [Google Scholar] [CrossRef]
- Oyama, M.; Mosenco, A.; Hess, R. Effect of sodium-glucose cotransporter-2 inhibitor, canagliflozin, on interstitial glucose in diabetic insulin treated dog. In Proceedings of the American College of Veterinary Internal Medicine Forum 2023, Philadelphia, PA, USA, 15–17 June 2023. [Google Scholar]
- Beauvais, W.; Cardwell, J.M.; Brodbelt, D.C. The effect of neutering on the risk of mammary tumours in dogs—A systematic review. J. Small Anim. Pract. 2012, 53, 314–322. [Google Scholar] [CrossRef]
- Edmunds, G.; Beck, S.; Kale, U.K.; Spasic, I.; O’Neill, D.; Brodbelt, B.; Smalley, M.J. Associations between dog breed and clinical features of mammary epithelial neoplasia in bitches: An Epidemiological Study of Submissions to a Single Diagnostic Pathology Centre Between 2008–2021. J. Mammary Gland Biol. Neoplasia 2023, 28, 6. [Google Scholar] [CrossRef]
- Sundburg, C.R.; Belanger, J.M.; Bannasch, D.L.; Famula, T.R.; Oberbauer, A.M. Gonadectomy effects on the risk of immune disorders in the dog: A retrospective study. BMC Vet. Res. 2016, 12, 278. [Google Scholar] [CrossRef] [PubMed]
- Urfer, S.R.; Kaeberlein, M. Desexing dogs: A review of the current literature. Animals 2019, 9, 1086. [Google Scholar] [CrossRef] [PubMed]
- Palestrini, C.; Mazzola, S.; Caione, B.; Groppetti, D.; Pecile, A.; Minero, M.; Cannas, S. Influence of Gonadectomy on Canine Behavior. Animals 2021, 11, 553. [Google Scholar] [CrossRef] [PubMed]
- Pegram, C.; Raffan, E.; White, E.; Ashworth, A.H.; Brodbelt, D.C.; Church, D.B.; O’Neill, D.G. Frequency, breed predisposition and demographic risk factors for overweight status in dogs in the UK. J. Small Anim. Pract. 2021, 62, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Hart, B.L.; Hart, L.A.; Thigpen, A.P.; Willits, N.H. Assisting decision-making on age of neutering for 35 breeds of dogs: Associated joint disorders, cancers, and urinary incontinence. Front. Vet. Sci. 2020, 7, 388. [Google Scholar] [CrossRef] [PubMed]
- Pegram, C.; O’Neill, D.G.; Church†, D.B.; Hall, J.; Owen, L.; Brodbelt, D.C. Spaying and urinary incontinence in bitches under UK primary veterinary care: A case–control study. J. Small Anim. Pract. 2019, 60, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Espiñeira, I.M.; Vidal, P.N.; Ghersevich, M.C.; Arias, E.A.S.; Bosetti, F.; Blatter, M.F.C.; Miceli, D.D.; Castillo, V.A. Adrenal cortex stimulation with hCG in spayed female dogs with Cushing’s syndrome: Is the LH-dependent variant possible? Open Vet. J. 2021, 11, 319–329. [Google Scholar] [CrossRef]
- Kutzler, M.A. Possible relationship between long-term adverse health effects of gonad-removing surgical sterilization and luteinizing hormone in dogs. Animals 2020, 10, 599. [Google Scholar] [CrossRef]
- Meij, B.P.; Kooistra, H.S.; Rijnberk, A. Hypothalamus-Pituitary System. In Clinical Endocrinology of Dogs and Cats an Illustrated Text, 2nd ed.; Rijnberk, A., Kooistra, H.S., Eds.; Schlütersche: Hannover, Germany, 2010; Volume 1, pp. 25–34. [Google Scholar]
- Fracassi, F.; Gandini, G.; Diana, A.; Preziosi, R.; van den Ingh, T.S.G.A.M.; Famigli-Bergamini, P.; Kooistra, H.S. Acromegaly due to a somatroph adenoma in a dog. Domest. Anim. Endocrinol. 2007, 32, 43–54. [Google Scholar] [CrossRef]
- Steele, M.M.E.; Lawson, J.S.; Scudder, C.; Watson, A.H.; Ho, N.T.Z.; Yaffy, D.; Batchelor, D.; Fenn, J. Transsphenoidal hypophysectomy for the treatment of hypersomatotropism secondary to a pituitary somatotroph adenoma in a dog. J. Vet. Intern. Med. 2024, 38, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.M.; Diaz-Espineira, M.; Mol, J.A.; Rijnberk, A.; Kooistra, H.S. Primary hypothyroidism in dogs is associated with elevated GH release. J. Endocrinol. 2001, 168, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Espiñeira, M.M.; Galac, S.; Mol, J.A.; Rijnberk, A.; Kooistra, H.S. Thyrotropin-releasing hormone-induced growth hormone secretion in dogs with primary hypothyroidism. Domest. Anim. Endocrinol. 2008, 34, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, T.; Mooney, C.T. Hypothyroidism associated with acromegaly and insulin-resistant diabetes mellitus in a Samoyed. Aust. Vet. J. 2004, 92, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Rasotto, R.; Berlato, D.; Goldschmidt, M.H.; Zappulli, V. Prognostic Significance of Canine Mammary Tumor Histologic Subtypes: An Observational Cohort Study of 229 Cases. Vet. Pathol. 2017, 54, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Klinkenberg, H.; Sallander, M.H.; Hedhammar, A. Feeding, exercise and weight identified as risk factors in canine diabetes mellitus. J. Nutr. 2006, 136, 1985–1987. [Google Scholar] [CrossRef] [PubMed]
- Kipperman, B.S.; German, A.J. The responsibility of veterinarians to address companion animal obesity. Animals 2018, 8, 143. [Google Scholar] [CrossRef]
- Rak, M.B.; Gilor, C.; Niessen, S.J.M.; Furrow, E. Spontaneous remission and relapse of diabetes mellitus in a male dog. J. Vet. Intern. Med. 2024; online ahead of print. [Google Scholar] [CrossRef]



| Increased Risk for Pyometra [12,13,110,111] | Increased Risk for Diabetes Mellitus [20,21,22,23,24,25,26] |
|---|---|
| Airedale Terrier | Australian Terrier |
| Bernese Mountain Dog | Bichon Frise |
| Bouvier de Flandres | Border Collie |
| Boxer | Border Terrier |
| Bull Terrier | Cairn Terrier |
| Bullmastiff | Cavalier King Charles Spaniel 1 |
| Cavalier King Charles Spaniel 1 | English Setter |
| Dogue de Bordeaux | English Springer Spaniel 1 |
| Drever | Finish Spitz |
| English Cocker Spaniel | Fox Terrier |
| English Springer Spaniel 1 | Irish Setter |
| German Shepherd Dog | Keeshond 1 |
| Golden Retriever | Miniature Schnauzer 1 |
| Great Dane | Poodle (toy/miniature) 1 |
| Irish Terrier | Samoyed |
| Irish Wolfhound | Siberian Husky |
| Jämthund | Standard Schnauzer |
| Keeshond 1 | Swedish Elkhound (female) |
| Labrador Retriever | Swedish Lapphund |
| Leonberger | Tibetan Terrier |
| Miniature Schnauzer1 | Toy Poodle |
| Newfoundland | West Highland White Terrier 1 |
| Poodle (toy/miniature) 1 | Yorkshire Terrier |
| Rottweiler | |
| Rough Collie | |
| Saint Bernard | |
| Shetland Sheepdog | |
| Staffordshire Bull Terrier | |
| West Highland White Terrier 1 |
| Decreased Risk for Pyometra [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110] | Decreased Risk for Diabetes Mellitus [20,21,22,23,24,25,26] |
|---|---|
| Coton de Tulear | Airedale Terrier |
| Dachshund | Basset Hound |
| Finnish Spitz | Beagle |
| Gordon Setter | Boston Terrier |
| Laika | Boxer |
| Lancashire Bull Terrier | Brittany Spaniel |
| Maltese | Bulldog |
| Norrbotten Spitz | Cocker Spaniel |
| Norwich Terrier | Collie |
| Saluki | Dalmatian |
| Tibetan Terrier | Doberman Pinscher |
| English Pointer | |
| English Setter | |
| German Sheperd Dog | |
| German Short-Hair Pointer | |
| Golden Retriever | |
| Great Dane | |
| Greyhound | |
| Irish Setter | |
| Norwegian Elkhound | |
| Old English Sheepdog | |
| Pekingese | |
| Shetland Sheepdog | |
| Shih Tzu |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pöppl, Á.G.; Lopes, J.L.X.; Nogueira, T.B.; da Silva, D.I.; Machado, B.d.S. Progesterone-Related Diabetes Mellitus in the Bitch: Current Knowledge, the Role of Pyometra, and Relevance in Practice. Animals 2024, 14, 890. https://doi.org/10.3390/ani14060890
Pöppl ÁG, Lopes JLX, Nogueira TB, da Silva DI, Machado BdS. Progesterone-Related Diabetes Mellitus in the Bitch: Current Knowledge, the Role of Pyometra, and Relevance in Practice. Animals. 2024; 14(6):890. https://doi.org/10.3390/ani14060890
Chicago/Turabian StylePöppl, Álan Gomes, José Lucas Xavier Lopes, Taís Bock Nogueira, Denise Iparraguirre da Silva, and Bruna dos Santos Machado. 2024. "Progesterone-Related Diabetes Mellitus in the Bitch: Current Knowledge, the Role of Pyometra, and Relevance in Practice" Animals 14, no. 6: 890. https://doi.org/10.3390/ani14060890
APA StylePöppl, Á. G., Lopes, J. L. X., Nogueira, T. B., da Silva, D. I., & Machado, B. d. S. (2024). Progesterone-Related Diabetes Mellitus in the Bitch: Current Knowledge, the Role of Pyometra, and Relevance in Practice. Animals, 14(6), 890. https://doi.org/10.3390/ani14060890






