Strain-Specific Benefits of Bacillus Probiotics in Hybrid Grouper: Growth Enhancement, Metabolic Health, Immune Modulation, and Vibrio harveyi Resistance
Abstract
:Simple Summary
Abstract
1. Introduction
2. Experimental Design and Methods
2.1. Experimental Location and Holding Preparation
2.2. Fish Growth Parameters Measurement
2.3. Preparation of Probiotic Diet
2.4. Fish Rearing and Experimental Design
2.5. Sampling Process
2.6. Analysis of Liver Health-Related Enzyme Activities
2.7. Antioxidant Capacity Analysis
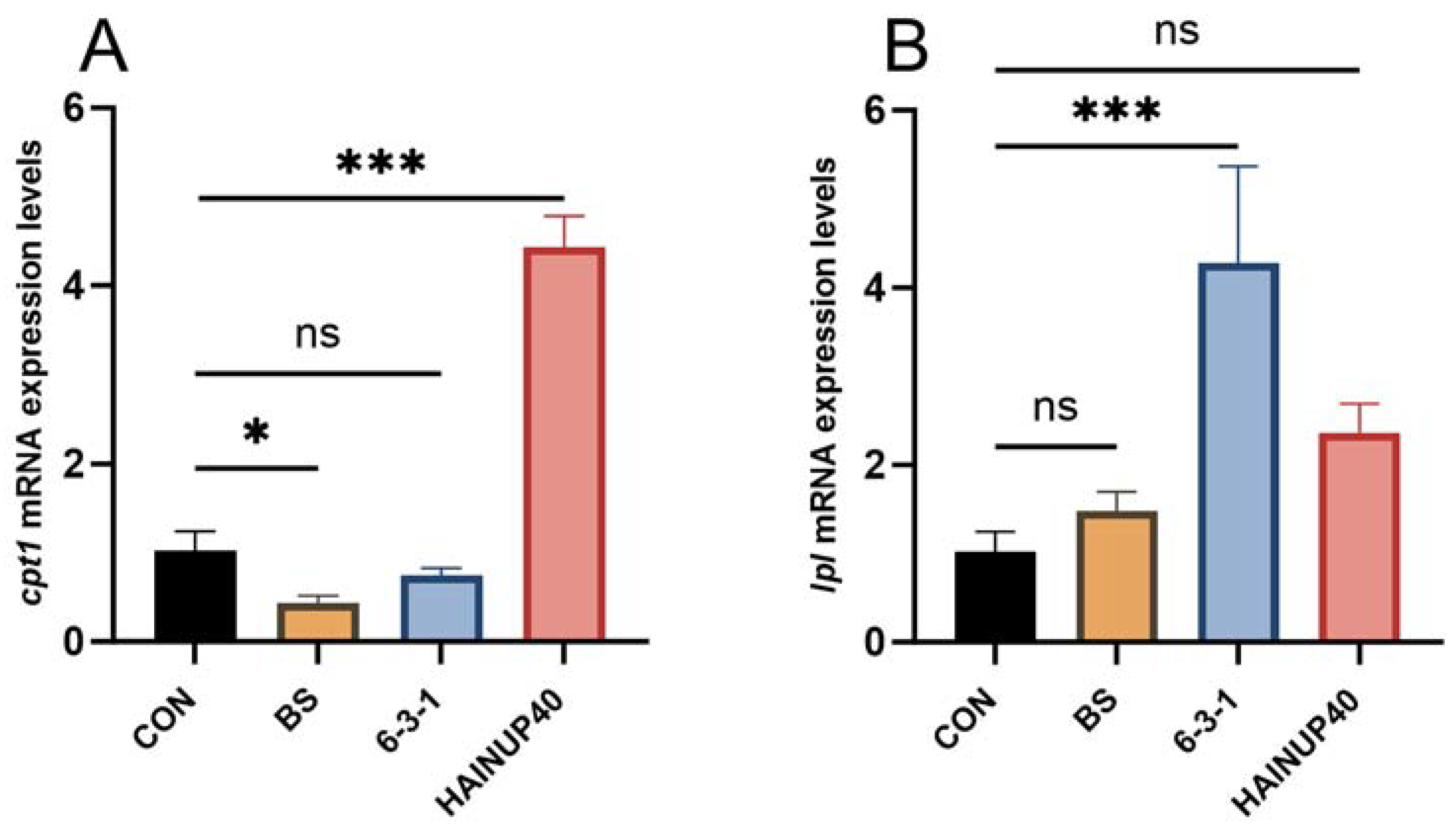
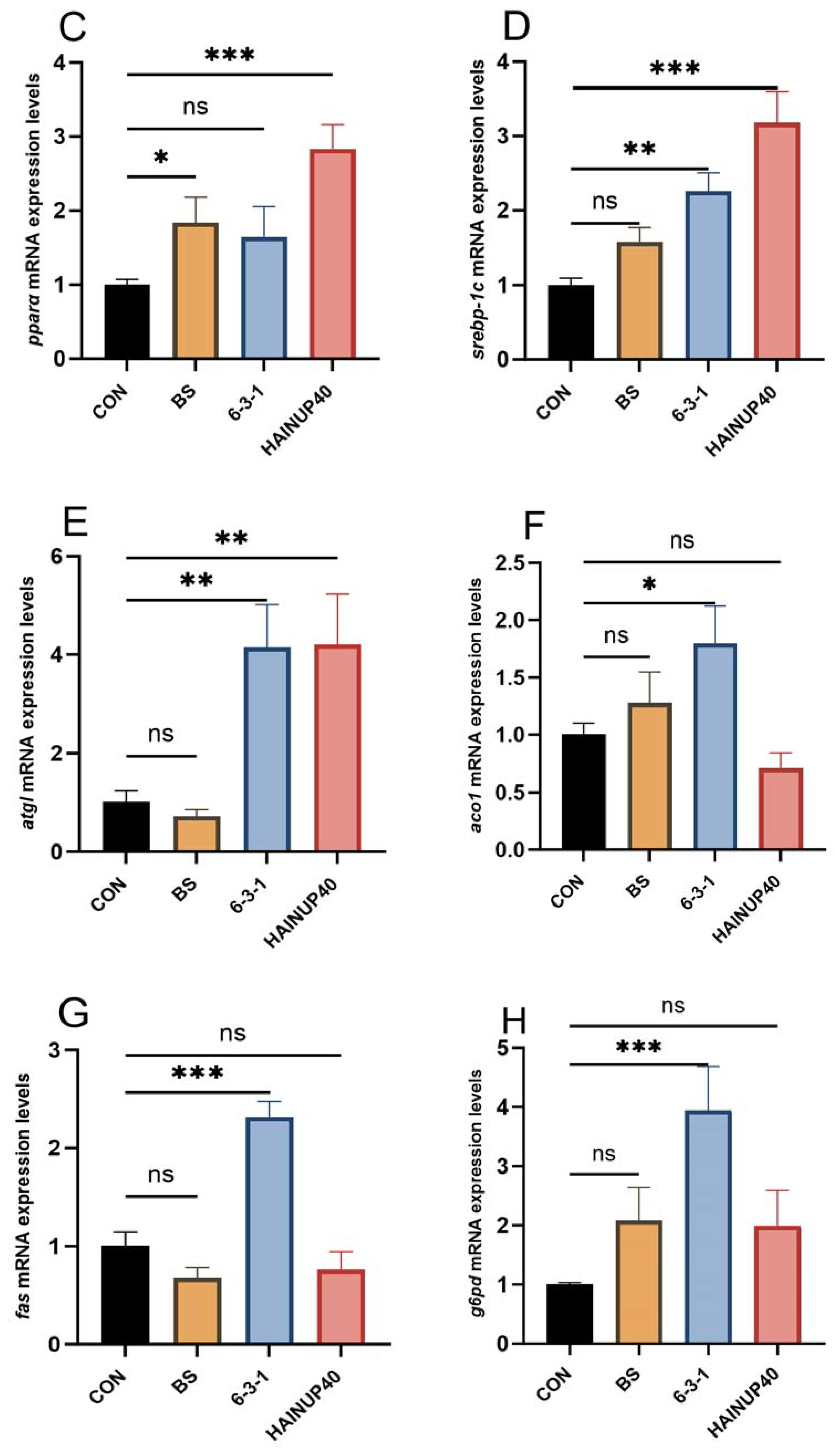
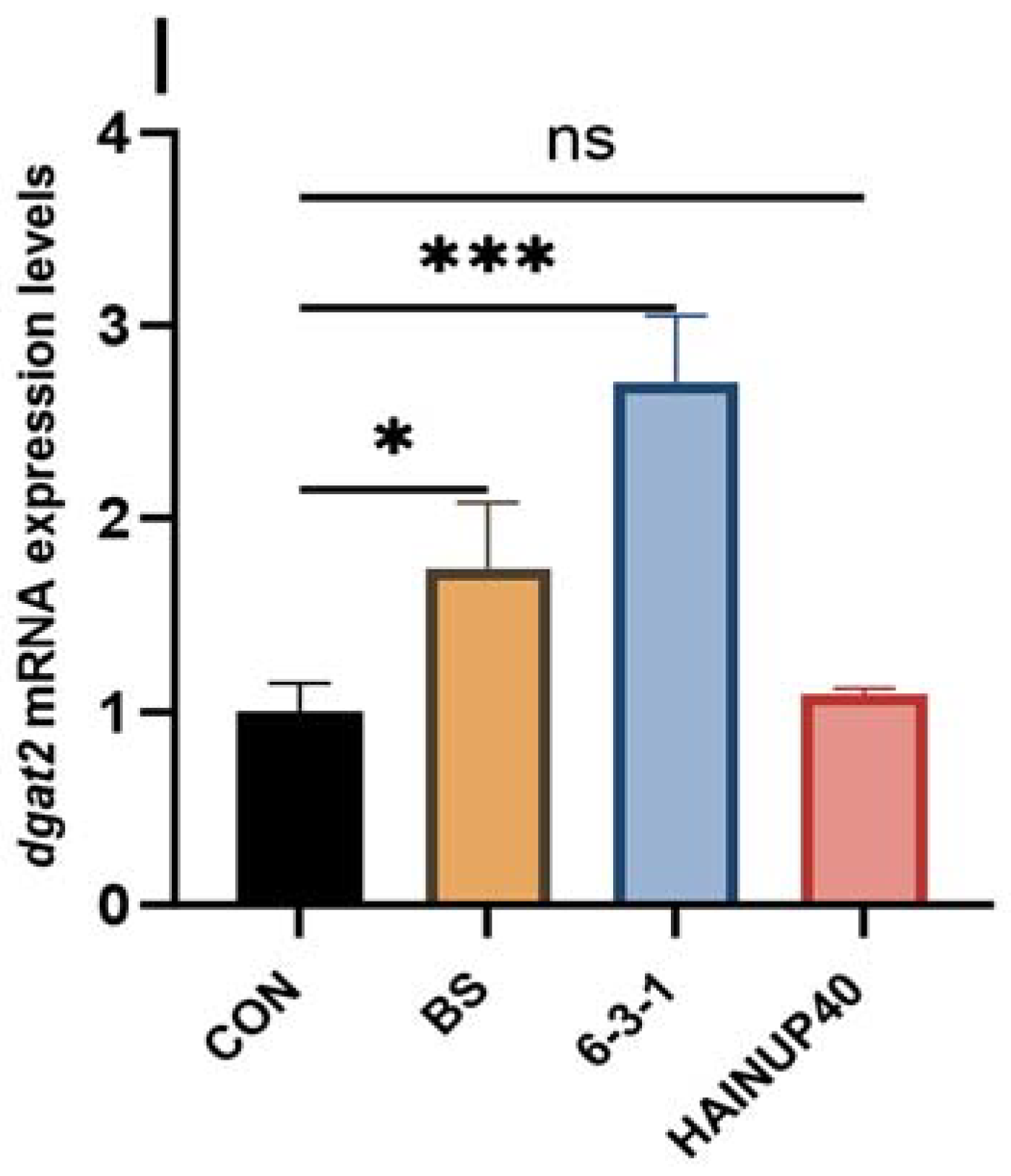
2.8. Expression of Immune/Lipid Metabolism-Related Genes in Liver
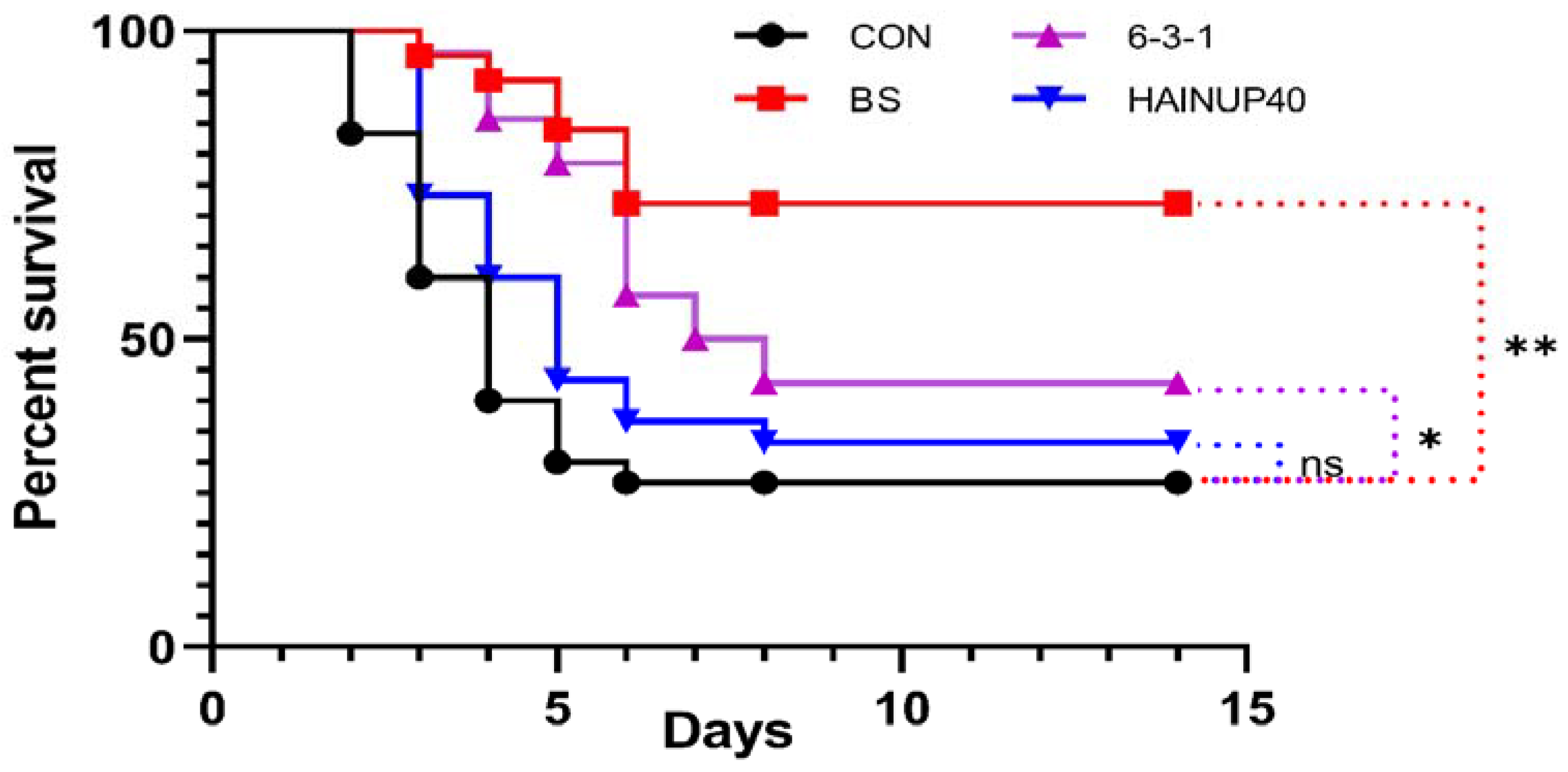
2.9. Challenge Test
2.10. Data Analysis
3. Results
3.1. Fish growth parameters
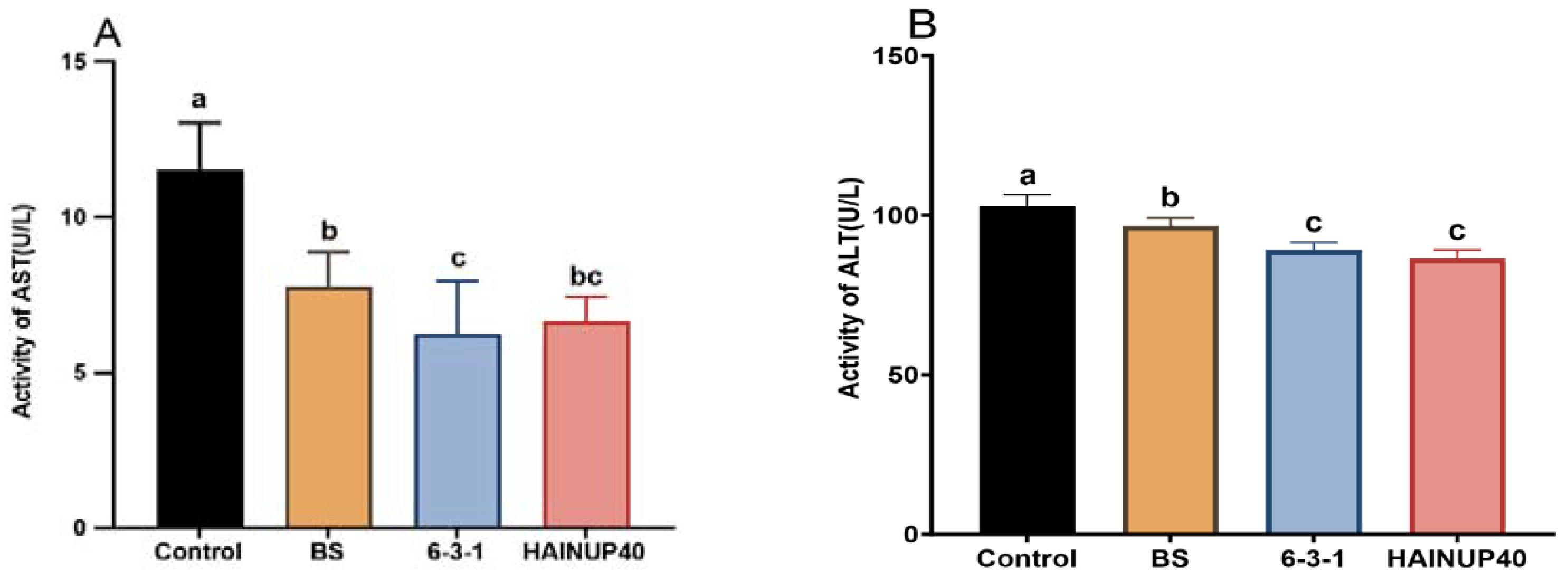
3.2. Liver Function Enzyme Activity Analysis
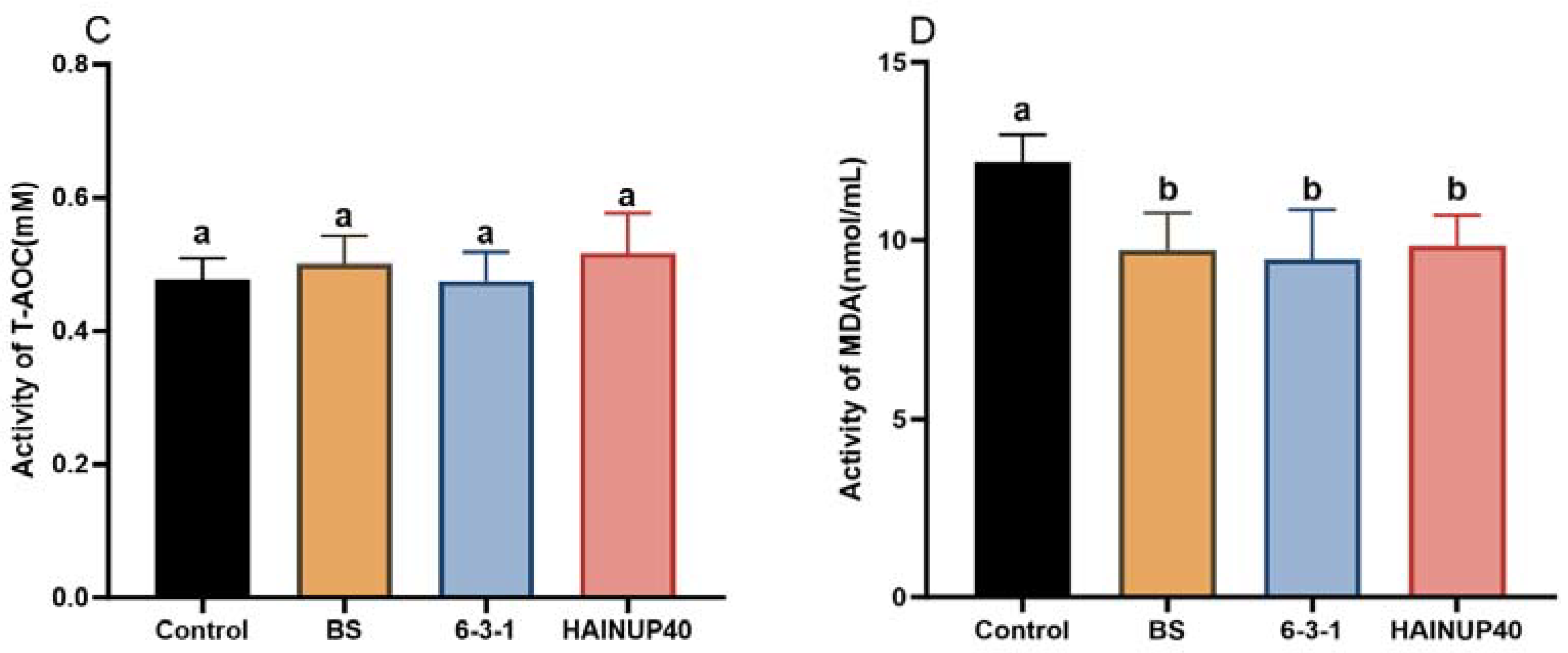
3.3. Antioxidant Capacity Analysis
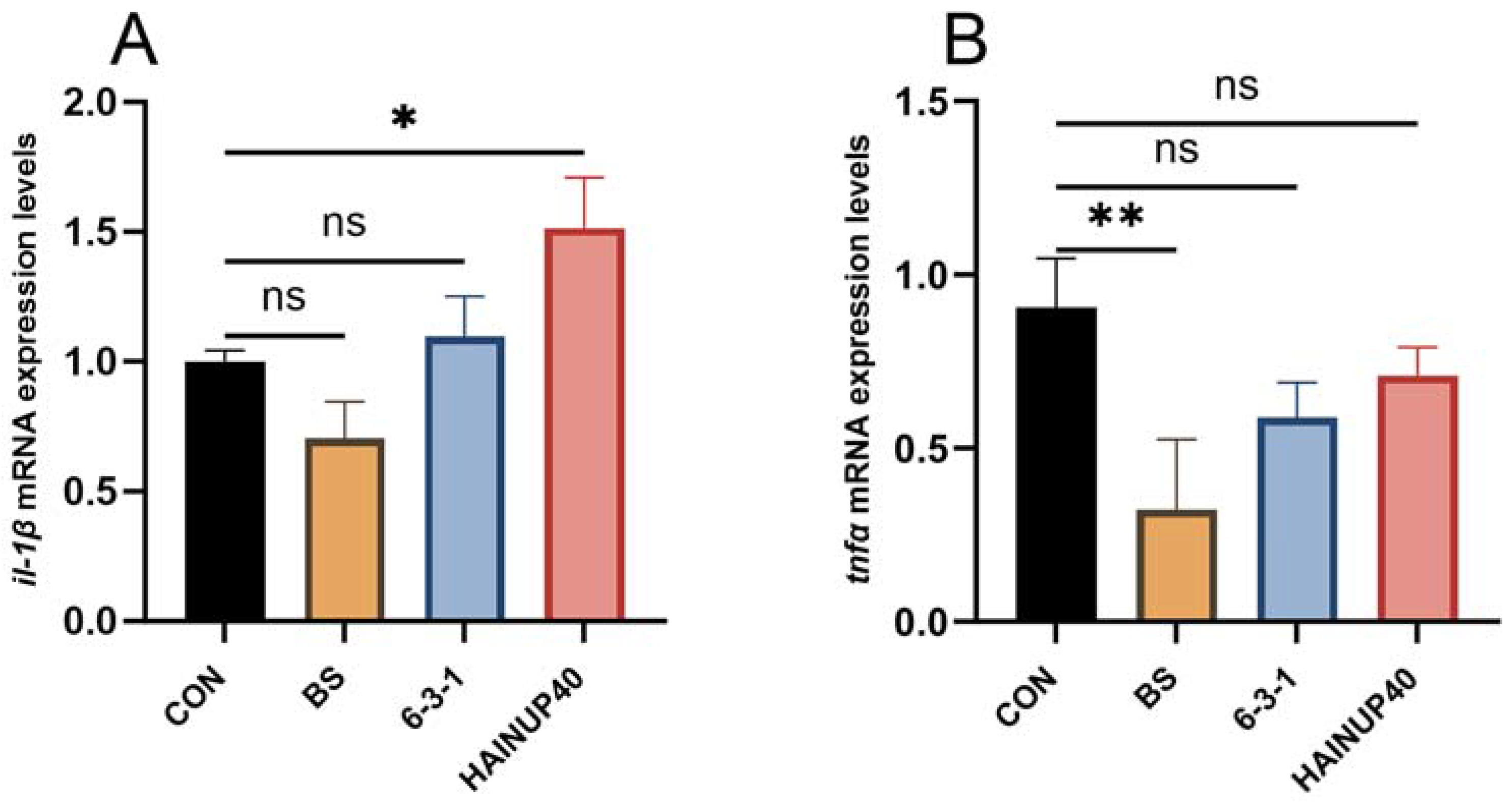
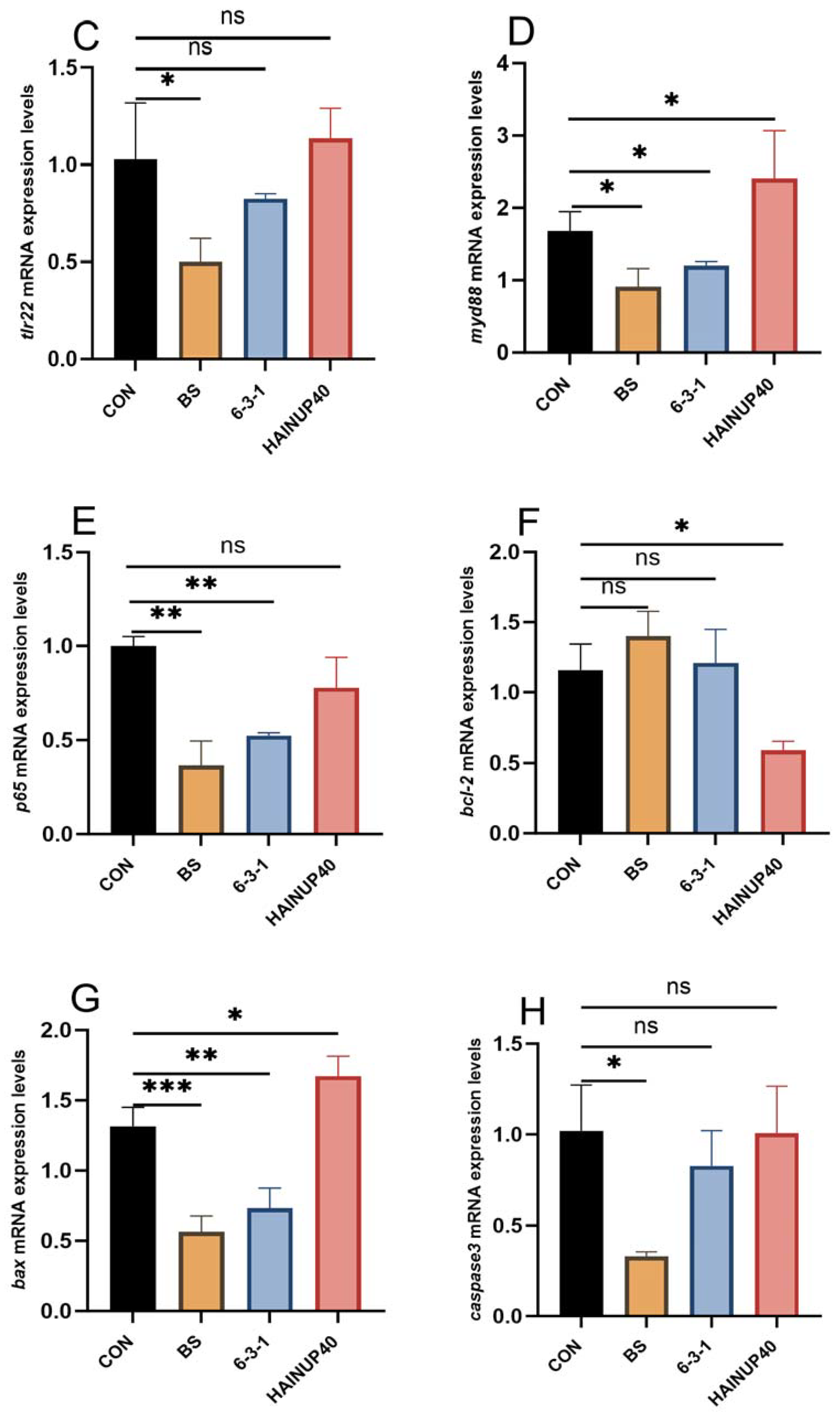
3.4. Expression of Immunity-Related Genes in Liver
3.5. Expression of Lipid Metabolism-Related Genes in Liver
3.6. Challenge Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IBW | initial body weight | ALT | alanine aminotransferase |
| FBW | final body weight | AST | aspartate aminotransferase |
| FI | feed intake | CAT | catalase |
| FER | feed efficiency rate | T-AOC | total antioxidant capacity |
| FCR | feed conversion rate | GSH-Px | glutathione peroxidase |
| WGR | weight gain rate | MDA | malondialdehyde |
| ucp2 | mitochondrial uncoupling protein 2 | il-1β | interleukin-1 beta |
| acc1 | acetyl coenzyme a carboxylase 1 | bcl-2 | b-cell lymphoma 2 |
| atgl | adipose triglyceride lipase | tlr22 | toll-like receptor 22 |
| aco1 | acyl-CoA oxidase1 | V. harveyi | Vibrio harveyi |
| fas | fatty acid synthase | O. niloticus | Oreochromis niloticus |
| g6pd | glucose 6-phosphate dehydrogenase | B. subtilis | Bacillus subtilis |
| dgat2 | diacylgycerol acyltransferase 2 | ||
| c/ebpα | CCAAT/enhancer binding protein α | ||
| tgfβ | transforming growth factor beta | ||
| myd88 | myeloid differentiation primary response 88 | ||
| p65 | nuclear factor kappa b subunit p65 | ||
| tnfα | tumor necrosis factor alpha | ||
| bax | bcl2 associated x, apoptosis regulator | ||
| caspase3 | apoptosis-related cysteine peptidase | ||
| caspase9 | apoptosis-related cysteine peptidase | ||
| cpt1 | carnitine palmitoyltransferase1 | ||
| lpl | lipoprotein lipase | ||
| pparα | peroxisome proliferator-activated receptor alpha | ||
| srebp-1c | sterol regulatory element binding protein-1 |
References
- Pauly, D.; Zeller, D. Comments on FAOs state of world fisheries and aquaculture (SOFIA 2016). Mar. Policy 2017, 77, 176–181. [Google Scholar] [CrossRef]
- Zhang, W.; Belton, B.; Edwards, P.; Henriksson, P.J.; Little, D.C.; Newton, R.; Troell, M. Aquaculture will continue to depend more on land than sea. Nature 2022, 603, E2–E4. [Google Scholar] [CrossRef] [PubMed]
- Naylor, R.L.; Hardy, R.W.; Buschmann, A.H.; Bush, S.R.; Cao, L.; Klinger, D.H.; Little, D.C.; Lubchenco, J.; Shumway, S.E.; Troell, M. A 20-year retrospective review of global aquaculture. Nature 2021, 591, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Loh, J.Y.; Chan, H.K.; Yam, H.C.; In, L.L.A.; Lim, C.S.Y. An overview of the immunomodulatory effects exerted by probiotics and prebiotics in grouper fish. Aquac. Int. 2019, 28, 729–750. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, H.; Xu, L.; Zhang, K.; Mei, Y.; Chen, J.; Wang, M.; Guan, Y.; Pang, H.; Wang, Y. The effect of dietary lactic acid bacteria on intestinal microbiota and immune responses of crucian carp (Carassius auratus) under water temperature decrease. Front. Microbiol. 2022, 13, 847167. [Google Scholar] [CrossRef] [PubMed]
- Rohani, M.F.; Islam, S.M.; Hossain, M.K.; Ferdous, Z.; Siddik, M.A.; Nuruzzaman, M.; Padeniya, U.; Brown, C.; Shahjahan, M. Probiotics, prebiotics and synbiotics improved the functionality of aquafeed: Upgrading growth, reproduction, immunity and disease resistance in fish. Fish Shellfish Immunol. 2022, 120, 569–589. [Google Scholar] [CrossRef] [PubMed]
- Mohd Faudzi, N.; Yong, A.S.K.; Shapawi, R.; Senoo, S.; Biswas, A.; Takii, K. Soy protein concentrate as an alternative in replacement of fish meal in the feeds of hybrid grouper, brown-marbled grouper (Epinephelus fuscoguttatus) × giant grouper (E. lanceolatus) juvenile. Aquac. Res. 2018, 49, 431–441. [Google Scholar] [CrossRef]
- Bunlipatanon, P.; U-taynapun, K. Growth performance and disease resistance against Vibrio vulnificus infection of novel hybrid grouper (Epinephelus lanceolatus × Epinephelus fuscoguttatus). Aquac. Res. 2017, 48, 1711–1723. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, C.Y.; Wang, D.D.; Li, X.F.; Xiao, L.; Zhang, X.; You, X.; Shi, Q.; Hu, G.J.; Fang, C.; et al. Transcriptome analysis reveals the molecular mechanisms underlying growth superiority in a novel grouper hybrid (Epinephelus fuscogutatusfemale symbol × E. lanceolatusmale symbol). BMC Genet. 2016, 17, 24. [Google Scholar] [CrossRef]
- Hai, N.V. The use of probiotics in aquaculture. J. Appl. Microbiol. 2015, 119, 917–935. [Google Scholar] [CrossRef]
- Van Doan, H.; Hoseinifar, S.H.; Khanongnuch, C.; Kanpiengjai, A.; Unban, K.; Van Kim, V.; Srichaiyo, S. Host-associated probiotics boosted mucosal and serum immunity, disease resistance and growth performance of Nile tilapia (Oreochromis niloticus). Aquaculture 2018, 491, 94–100. [Google Scholar] [CrossRef]
- Kavitha, M.; Raja, M.; Perumal, P. Evaluation of probiotic potential of Bacillus spp. isolated from the digestive tract of freshwater fish Labeo calbasu (Hamilton, 1822). Aquac. Rep. 2018, 11, 59–69. [Google Scholar] [CrossRef]
- Kuebutornye, F.K.A.; Abarike, E.D.; Lu, Y. A review on the application of Bacillus as probiotics in aquaculture. Fish Shellfish Immunol. 2019, 87, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Loh, J.Y.; Lim, Y.Y.; Harmin, S.A.; Ting, A.S.Y. In vitro assessment on intestinal microflora from commonly farmed fishes for control of the fish pathogen Edwardsiella tarda. Turk. J. Vet. Anim. Sci. 2014, 38, 257–263. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Kim, J.-S.; Kim, M.-C.; Balasundaram, C.; Heo, M.-S. Lactuca indica extract as feed additive enhances immunological parameters and disease resistance in Epinephelus bruneus to Streptococcus iniae. Aquaculture 2011, 318, 43–47. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S.; Abdel-Daim, M.M.; Van Doan, H. Probiotic application for sustainable aquaculture. Rev. Aquac. 2019, 11, 907–924. [Google Scholar] [CrossRef]
- Hasan, M.T.; Je Jang, W.; Lee, J.M.; Lee, B.-J.; Hur, S.W.; Gu Lim, S.; Kim, K.W.; Han, H.-S.; Kong, I.-S. Effects of Immunostimulants, Prebiotics, Probiotics, Synbiotics, and Potentially Immunoreactive Feed Additives on Olive Flounder (Paralichthys olivaceus): A Review. Rev. Fish. Sci. Aquac. 2019, 27, 417–437. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Zhang, E.; Zhou, M.; Lin, J.; Yang, Q. Intranasal administration with recombinant Bacillus subtilis induces strong mucosal immune responses against pseudorabies. Microb. Cell Factories 2019, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Docando, F.; Nuñez-Ortiz, N.; Gonçalves, G.; Serra, C.R.; Gomez-Casado, E.; Martín, D.; Abós, B.; Oliva-Teles, A.; Tafalla, C.; Díaz-Rosales, P. Bacillus subtilis Expressing the Infectious Pancreatic Necrosis Virus VP2 Protein Retains Its Immunostimulatory Properties and Induces a Specific Antibody Response. Front. Immunol. 2022, 13, 888311. [Google Scholar] [CrossRef]
- Zhang, W.; Tong, Q.; You, J.; Lv, X.; Liu, Z.; Ni, L. The application of Bacillus subtilis for adhesion inhibition of Pseudomonas and preservation of fresh fish. Foods 2021, 10, 3093. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.; Li, S.; Chen, S.; Liu, G.; Deng, X.; Chang, W.; Cai, H. Bacillus subtilis Protects the Ducks from Oxidative Stress Induced by Escherichia coli: Efficacy and Molecular Mechanism. Antioxidants 2022, 11, 1951. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yuan, J.; Chen, X.; Zhang, S.; Xie, M.; Chen, C.; Wu, Z. Screening of intestinal probiotics and the effects of feeding probiotics on the digestive enzyme activity, immune, intestinal flora and WSSV resistance of Procambarus clarkii. Aquaculture 2021, 540, 736748. [Google Scholar] [CrossRef]
- Butt, U.D.; Lin, N.; Akhter, N.; Siddiqui, T.; Li, S.; Wu, B. Overview of the latest developments in the role of probiotics, prebiotics and synbiotics in shrimp aquaculture. Fish Shellfish Immunol. 2021, 114, 263–281. [Google Scholar] [CrossRef] [PubMed]
- El-Saadony, M.T.; Alagawany, M.; Patra, A.K.; Kar, I.; Tiwari, R.; Dawood, M.A.O.; Dhama, K.; Abdel-Latif, H.M.R. The functionality of probiotics in aquaculture: An overview. Fish Shellfish Immunol. 2021, 117, 36–52. [Google Scholar] [CrossRef] [PubMed]
- van Baarlen, P.; Troost, F.; van der Meer, C.; Hooiveld, G.; Boekschoten, M.; Brummer, R.J.; Kleerebezem, M. Human mucosal in vivo transcriptome responses to three lactobacilli indicate how probiotics may modulate human cellular pathways. Proc. Natl. Acad. Sci. USA 2011, 108, 4562–4569. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, M.R.; Kragelund, C.; Jensen, P.Ø.; Keller, M.K.; Twetman, S. Probiotic Lactobacillus reuteri has antifungal effects on oral Candida species in vitro. J. Oral Microbiol. 2017, 9, 1274582. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, B. Probiotic-induced changes in the intestinal epithelium: Implications in gastrointestinal disease. Trop. Gastroenterol. 2009, 30, 76–85. [Google Scholar] [PubMed]
- Drago, L.; Nicola, L.; Iemoli, E.; Banfi, G.; De Vecchi, E. Strain-dependent release of cytokines modulated by Lactobacillus salivarius human isolates in an in vitro model. BMC Res. Notes 2010, 3, 44. [Google Scholar] [CrossRef] [PubMed]
- Susanti, D.; Volland, A.; Tawari, N.; Baxter, N.; Gangaiah, D.; Plata, G.; Nagireddy, A.; Hawkins, T.; Mane, S.P.; Kumar, A. Multi-Omics characterization of host-derived Bacillus spp. probiotics for improved growth performance in poultry. Front. Microbiol. 2021, 12, 747845. [Google Scholar] [CrossRef]
- Tian, L.J.; Zhu, W.W.; Cai, Y.; Zhang, D.D.; Jiang, L.; Zhang, Q.; Guan, B.Y.; Wang, S.F. Screening and potential probiotic effects of Bacillus from shrimp paste. J. Hainan Univ. 2021, 39, 570228. (In Chinese) [Google Scholar] [CrossRef]
- Liao, J.; Cai, Y.; Wang, X.; Shang, C.; Zhang, Q.; Shi, H.; Wang, S.; Zhang, D.; Zhou, Y. Effects of a Potential Host Gut-Derived Probiotic, Bacillus subtilis 6-3-1, on the Growth, Non-specific Immune Response and Disease Resistance of Hybrid Grouper (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂). Probiotics Antimicrob. Proteins 2021, 13, 1119–1137. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.; Cai, Y.; Guo, X.; Cao, Z.; Zhang, Y.; Liu, S.; Yuan, W.; Zhu, W.; Zheng, Y.; et al. Dietary administration of Bacillus subtilis HAINUP40 enhances growth, digestive enzyme activities, innate immune responses and disease resistance of tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2017, 60, 326–333. [Google Scholar] [CrossRef]
- Xu, X.; Liu, K.; Wang, S.; Guo, W.; Xie, Z.; Zhou, Y. Identification of pathogenicity, investigation of virulent gene distribution and development of a virulent strain-specific detection PCR method for Vibrio harveyi isolated from Hainan Province and Guangdong Province, China. Aquaculture 2017, 468, 226–234. [Google Scholar] [CrossRef]
- Abdelhamid, A.F.; Ayoub, H.F.; Abd El-Gawad, E.A.; Abdelghany, M.F.; Abdel-Tawwab, M. Potential effects of dietary seaweeds mixture on the growth performance, antioxidant status, immunity response, and resistance of striped catfish (Pangasianodon hypophthalmus) against Aeromonas hydrophila infection. Fish Shellfish Immunol. 2021, 119, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Farsani, M.N.; Hoseinifar, S.H.; Rashidian, G.; Farsani, H.G.; Ashouri, G.; Van Doan, H. Dietary effects of Coriandrum sativum extract on growth performance, physiological and innate immune responses and resistance of rainbow trout (Oncorhynchus mykiss) against Yersinia ruckeri. Fish Shellfish Immunol. 2019, 91, 233–240. [Google Scholar] [CrossRef]
- Ahmadifar, E.; Pourmohammadi Fallah, H.; Yousefi, M.; Dawood, M.A.; Hoseinifar, S.H.; Adineh, H.; Yilmaz, S.; Paolucci, M.; Doan, H.V. The gene regulatory roles of herbal extracts on the growth, immune system, and reproduction of fish. Animals 2021, 11, 2167. [Google Scholar] [CrossRef]
- Ghafarifarsani, H.; Hoseinifar, S.H.; Javahery, S.; Van Doan, H. Effects of dietary vitamin C, thyme essential oil, and quercetin on the immunological and antioxidant status of common carp (Cyprinus carpio). Aquaculture 2022, 553, 738053. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.; Shukry, M.; Noreldin, A.E.; Ahmed, H.A.; El-Bahrawy, A.; Ghetas, H.A.; Khalifa, E. Milk thistle (Silybum marianum) extract improves growth, immunity, serum biochemical indices, antioxidant state, hepatic histoarchitecture, and intestinal histomorphometry of striped catfish, Pangasianodon hypophthalmus. Aquaculture 2023, 562, 738761. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.; Yilmaz, E.; Dawood, M.A.; Ringø, E.; Ahmadifar, E.; Yilmaz, S. Shrimp vibriosis and possible control measures using probiotics, postbiotics, prebiotics, and synbiotics: A review. Aquaculture 2022, 551, 737951. [Google Scholar] [CrossRef]
- Yilmaz, S.; Yilmaz, E.; Dawood, M.A.; Ringø, E.; Ahmadifar, E.; Abdel-Latif, H.M. Probiotics, prebiotics, and synbiotics used to control vibriosis in fish: A review. Aquaculture 2022, 547, 737514. [Google Scholar] [CrossRef]
- Kim, S.; Shin, Y.-C.; Kim, T.-Y.; Kim, Y.; Lee, Y.-S.; Lee, S.-H.; Kim, M.-N.; O, E.; Kim, K.S.; Kweon, M.-N. Mucin degrader Akkermansia muciniphila accelerates intestinal stem cell-mediated epithelial development. Gut Microbes 2021, 13, 1892441. [Google Scholar] [CrossRef]
- Jin, W.; Jiang, L.; Hu, S.; Zhu, A. Effects of Lactobacillus plantarum and Bacillus subtilis on growth, immunity and intestinal flora of largemouth bass (Micropterus salmoides). Aquaculture 2024, 583, 740581. [Google Scholar] [CrossRef]
- Ji, Z.; Zhu, C.; Zhu, X.; Ban, S.; Yu, L.; Tian, J.; Dong, L.; Wen, H.; Lu, X.; Jiang, M. Dietary host-associated Bacillus subtilis supplementation improves intestinal microbiota, health and disease resistance in Chinese perch (Siniperca chuatsi). Anim. Nutr. 2023, 13, 197–205. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Xing, K.; Jiang, P.; Wang, J. Dietary cinnamaldehyde and Bacillus subtilis improve growth performance, digestive enzyme activity, and antioxidant capability and shape intestinal microbiota in tongue sole, Cynoglossus semilaevis. Aquaculture 2021, 531, 735798. [Google Scholar] [CrossRef]
- Ai, Q.; Xu, H.; Mai, K.; Xu, W.; Wang, J.; Zhang, W. Effects of dietary supplementation of Bacillus subtilis and fructooligosaccharide on growth performance, survival, non-specific immune response and disease resistance of juvenile large yellow croaker, Larimichthys crocea. Aquaculture 2011, 317, 155–161. [Google Scholar] [CrossRef]
- Shi, F.; Zi, Y.; Lu, Z.; Li, F.; Yang, M.; Zhan, F.; Li, Y.; Li, J.; Zhao, L.; Lin, L. Bacillus subtilis H2 modulates immune response, fat metabolism and bacterial flora in the gut of grass carp (Ctenopharyngodon idellus). Fish Shellfish Immunol. 2020, 106, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Liu, Y.; Wei, H.; He, X.; Wang, Z.; Zhuang, Z.; Zhao, W.; Masagounder, K.; He, J.; Niu, J. Effects of dietary supplementation of Bacillus subtilis DSM 32315 on growth, immune response and acute ammonia stress tolerance of Nile tilapia (Oreochromis niloticus) fed with high or low protein diets. Anim. Nutr. 2023, 15, 375–385. [Google Scholar] [CrossRef]
- Amoah, K.; Tan, B.; Zhang, S.; Chi, S.; Yang, Q.; Liu, H.; Yang, Y.; Zhang, H.; Dong, X. Host gut-derived Bacillus probiotics supplementation improves growth performance, serum and liver immunity, gut health, and resistive capacity against Vibrio harveyi infection in hybrid grouper (♀ Epinephelus fuscoguttatus×♂ E. lanceolatus). Anim. Nutr. 2023, 14, 163–184. [Google Scholar] [CrossRef] [PubMed]
- Thy, H.T.T.; Tri, N.N.; Quy, O.M.; Fotedar, R.; Kannika, K.; Unajak, S.; Areechon, N. Effects of the dietary supplementation of mixed probiotic spores of Bacillus amyloliquefaciens 54A, and Bacillus pumilus 47B on growth, innate immunity and stress responses of striped catfish (Pangasianodon hypophthalmus). Fish Shellfish Immunol. 2017, 60, 391–399. [Google Scholar]
- Adams, S.; Ham, K.; Greeley, M.; LeHew, R.; Hinton, D.; Saylor, C. Downstream gradients in bioindicator responses: Point source contaminant effects on fish health. Can. J. Fish. Aquat. Sci. 1996, 53, 2177–2187. [Google Scholar] [CrossRef]
- Gao, Y.-J.; Yang, H.-J.; Liu, Y.-J.; Chen, S.-J.; Guo, D.-Q.; Yu, Y.-Y.; Tian, L.-X. Effects of graded levels of threonine on growth performance, biochemical parameters and intestine morphology of juvenile grass carp Ctenopharyngodon idella. Aquaculture 2014, 424–425, 113–119. [Google Scholar] [CrossRef]
- Gabriel, U.; George, A. Plasma enzymes in Clarias gariepinus exposed to chronic levels of round up (glyphosate). Environ. Ecol. 2005, 23, 271–276. [Google Scholar]
- Sagada, G.; Gray, N.; Wang, L.; Xu, B.; Zheng, L.; Zhong, Z.; Ullah, S.; Tegomo, A.F.; Shao, Q. Effect of dietary inactivated Lactobacillus plantarum on growth performance, antioxidative capacity, and intestinal integrity of black sea bream (Acanthopagrus schlegelii) fingerlings. Aquaculture 2021, 535, 736370. [Google Scholar] [CrossRef]
- Chillemi, G.; De Santis, S.; Falconi, M.; Mancini, G.; Migliorati, V.; Battistoni, A.; Pacello, F.; Desideri, A.; D’Angelo, P. Carbon monoxide binding to the heme group at the dimeric interface modulates structure and copper accessibility in the Cu, Zn superoxide dismutase from Haemophilus ducreyi: In silico and in vitro evidences. J. Biomol. Struct. Dyn. 2012, 30, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P. Oxidative Damage to Plants: Antioxidant Networks and Signaling; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Brigelius-Flohe, R.; Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta 2013, 1830, 3289–3303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.; Gong, X.; Chen, L.; Zhang, B.; Wang, Q.; Han, B.; Zhang, N.; Xue, F.; Vedal, S. Ambient PM2. 5 exposures and systemic biomarkers of lipid peroxidation and total antioxidant capacity in early pregnancy. Environ. Pollut. 2020, 266, 115301. [Google Scholar] [CrossRef]
- Zhou, S.; Song, D.; Zhou, X.; Mao, X.; Zhou, X.; Wang, S.; Wei, J.; Huang, Y.; Wang, W.; Xiao, S.-M. Characterization of Bacillus subtilis from gastrointestinal tract of hybrid Hulong grouper (Epinephelus fuscoguttatus × E. lanceolatus) and its effects as probiotic additives. Fish Shellfish Immunol. 2019, 84, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liao, B.; Wang, C.; Zhong, O.; Lei, X.; Yang, Y. Effects of Antioxidant Supplementation on Metabolic Disorders in Obese Patients from Randomized Clinical Controls: A Meta-Analysis and Systematic Review. Oxidative Med. Cell. Longev. 2022, 2022, 7255413. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, J.; Sun, P.; Yi, R.; Han, X.; Zhao, X. Raw bowl tea (Tuocha) polyphenol prevention of nonalcoholic fatty liver disease by regulating intestinal function in mice. Biomolecules 2019, 9, 435. [Google Scholar] [CrossRef]
- Hu, J.-P.; Zheng, T.-T.; Zeng, B.-F.; Wu, M.-L.; Shi, R.; Zhang, Y.; Chen, L.-J.; Cheng, W.-J.; Liang, P. Effects of Lactobacillus plantarum FZU3013-fermented Laminaria japonica on lipid metabolism and gut microbiota in hyperlipidaemic rats. Front. Nutr. 2021, 8, 786571. [Google Scholar] [CrossRef]
- Qian, Y.; Li, X.-F.; Zhang, D.-D.; Cai, D.-S.; Tian, H.-Y.; Liu, W.-B. Effects of dietary pantothenic acid on growth, intestinal function, anti-oxidative status and fatty acids synthesis of juvenile blunt snout bream Megalobrama amblycephala. PLoS ONE 2015, 10, e0119518. [Google Scholar] [CrossRef]
- Madduma Hewage, S.; Au-Yeung, K.K.; Prashar, S.; Wijerathne, C.U.; O, K.; Siow, Y.L. Lingonberry Improves Hepatic Lipid Metabolism by Targeting Notch1 Signaling. Antioxidants 2022, 11, 472. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.-L.; Xu, W.-N.; Wang, L.-N.; Zhang, D.-D.; Zhang, C.-N.; Liu, W.-B. Hepatic β-oxidation and regulation of carnitine palmitoyltransferase (CPT) I in blunt snout bream Megalobrama amblycephala fed a high fat diet. PLoS ONE 2014, 9, e93135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Bu, S.Y.; Mashek, M.T.; InSug, O.; Sibai, Z.; Khan, S.A.; Ilkayeva, O.; Newgard, C.B.; Mashek, D.G.; Unterman, T.G. Integrated regulation of hepatic lipid and glucose metabolism by adipose triacylglycerol lipase and FoxO proteins. Cell Rep. 2016, 15, 349–359. [Google Scholar] [CrossRef]
- Wang, A.; Meng, D.; Hao, Q.; Xia, R.; Zhang, Q.; Ran, C.; Yang, Y.; Li, D.; Liu, W.; Zhang, Z. Effect of supplementation of solid-state fermentation product of Bacillus subtilis HGcc-1 to high-fat diet on growth, hepatic lipid metabolism, epidermal mucus, gut and liver health and gut microbiota of zebrafish. Aquaculture 2022, 560, 738542. [Google Scholar] [CrossRef]
- Saera-Vila, A.; Calduch-Giner, J.A.; Gómez-Requeni, P.; Médale, F.; Kaushik, S.; Pérez-Sánchez, J. Molecular characterization of gilthead sea bream (Sparus aurata) lipoprotein lipase. Transcriptional regulation by season and nutritional condition in skeletal muscle and fat storage tissues. Comp. Biochem. Physiol. Part. B Biochem. Mol. Biol. 2005, 142, 224–232. [Google Scholar] [CrossRef]
- Guo, Q.; Zhang, L.; Yin, Y.; Gong, S.; Yang, Y.; Chen, S.; Han, M.; Duan, Y. Taurine Attenuates Oxidized Fish Oil-Induced Oxidative Stress and Lipid Metabolism Disorder in Mice. Antioxidants 2022, 11, 1391. [Google Scholar] [CrossRef]
- Zou, C.; Fang, Y.; Lin, N.; Liu, H. Polysaccharide extract from pomelo fruitlet ameliorates diet-induced nonalcoholic fatty liver disease in hybrid grouper (Epinephelus lanceolatus♂ × Epinephelus fuscoguttatus♀). Fish Shellfish Immunol. 2021, 119, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Du, L.; Wu, J.; Gan, S.; Li, Q.; Babu, V.S.; Wu, Y.; Lin, L. Saikosaponin d alleviates high-fat-diet induced hepatic steatosis in hybrid grouper (Epinephelus lanceolatus♂ × Epinephelus fuscoguttatus♀) by targeting AMPK/PPARα pathway. Aquaculture 2022, 553, 738088. [Google Scholar] [CrossRef]
- Barisón, M.J.; Pereira, I.T.; Waloski Robert, A.; Dallagiovanna, B. Reorganization of Metabolism during Cardiomyogenesis Implies Time-Specific Signaling Pathway Regulation. Int. J. Mol. Sci. 2021, 22, 1330. [Google Scholar] [CrossRef]
- Berrocal, A.; Lammers, N.C.; Garcia, H.G.; Eisen, M.B. Kinetic sculpting of the seven stripes of the Drosophila even-skipped gene. eLife 2020, 9, e61635. [Google Scholar] [CrossRef] [PubMed]
- Hoermann, R.; Pekker, M.J.; Midgley, J.E.; Larisch, R.; Dietrich, J.W. Principles of endocrine regulation: Reconciling tensions between robustness in performance and adaptation to change. Front. Endocrinol. 2022, 13, 825107. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Liu, T.; Piao, C.; Wang, Y.; Wang, K.; Zhou, Y.; Cai, L.; Zheng, S.; Lan, F.; Du, J. AMPKα2 knockout enhances tumour inflammation through exacerbated liver injury and energy deprivation-associated AMPKα1 activation. J. Cell. Mol. Med. 2019, 23, 1687–1697. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, Y.; Chen, H.; Xu, L.; Wang, Q.; Feng, J. Gut–liver immune response and gut microbiota profiling reveal the pathogenic mechanisms of vibrio harveyi in pearl gentian grouper (Epinephelus lanceolatus♂ × E. fuscoguttatus♀). Front. Immunol. 2020, 11, 607754. [Google Scholar] [CrossRef]
- Guicciardi, M.; Gores, G. Apoptosis: A mechanism of acute and chronic liver injury. Gut 2005, 54, 1024–1033. [Google Scholar] [CrossRef]


| Ingredients | % |
|---|---|
| Red fishmeal | 40.00 |
| Casein | 11.54 |
| Gelatine | 2.85 |
| Wheat flour | 20.00 |
| Fish oil | 4.66 |
| Soy lecithin | 2.00 |
| Calcium monophosphate | 1.00 |
| Vitamin premix a | 0.22 |
| Mineral premix b | 0.56 |
| Antioxidants | 0.06 |
| Choline chloride | 0.50 |
| Vitamin C | 0.09 |
| Cellulose microcrystalline | 16.52 |
| Proximate composition (in dry matter) | |
| Crude protein | 48.86 |
| Crude lipid | 10.75 |
| Moisture | 9.58 |
| Gene | Primer Name | Primer Sequence 5′–3′ |
|---|---|---|
| atgl | atgl-F | ATTGAGCACCTTCCACCCA |
| atgl-R | CCGAATCCATCCCACATCTT | |
| aco-1 | aco1-F | CGGCATGGACTTCCTGTATG |
| aco1-R | CCTGGTGTGCGTGTTGTGTT | |
| fas | fas-F | CGGGTGTCTACATTGGGGTG |
| fas-R | GAATAGCGTGGAAGGCGTTT | |
| dgat2 | dgat2-F | CATCTTCTGCTTTGGTGCTTTC |
| dgat2-R | GCATTTCCCGTCCCGTTA | |
| g6pd | g6pd-F | GCTTCACATCCTTGTATCTGCTC |
| g6pd-R | GCGTTCCTTTCATTCTCCG | |
| pparα | pparα-F | CATCGACAATGACGCCCTC |
| pparα-R | GCCGCTATCCCGTAAACAAC | |
| srebp-1c | srebp1c-F | AGCTGTTTACACACTCCACTCG |
| srebp1c-R | ACTGTGGAAGCTACAGGGCA | |
| cpt1 | cpt1-F | TCCTTACCGTTGGTCCCTCT |
| cpt1-R | CTTTCCATCTGCTGCTCTATCTC | |
| lpl | lpl-F | TTCAACAGCACCTCCAAAACC |
| lpl-R | GTGAGCCAGTCCACCACGAT | |
| il-1β | il-1β-F | CGACATGGTGCGGTTTC |
| il-1β-R | TCTGTAGCGGCTGGTGG | |
| tnfα | tnfα-F | GTGGCCTACACGACTGCACC |
| tnfα-R | TACAAAGGGCCACAGTGAGA | |
| tlt22 | tlr22-F | CGAGCCAGGTAAACCCATCA |
| tlr22-R | CTCATCAAACAGGCGGAAGC | |
| myd88 | myd88-F | CGAGCCAGGTAAACCCATCA |
| myd88-R | CTCATCAAACAGGCGGAAGC | |
| p65 | p65-F | GGGTGTGTATGGATGGGG |
| p65-R | TGGCTGGGTGGGTCTTAG | |
| bcl-2 | bcl-2-F | TTAGGTCGCAGTGAGT |
| bcl-2-R | CATAGATGGGGAAGAG | |
| bax | bax-F | GCCAGCAGCACATCACTTCC |
| bax-R | AGCCACCGTAGTTCAAGCAGTT | |
| caspase3 | caspase3-F | CGCAAAGAGTAGCGACGGA |
| caspase3-R | CGATGCTGGGGAAATTCAGAC | |
| caspase9 | caspase9-F | TTTTCCTGGTTATGTTTCGTGG |
| caspase9-R | TTGCTTGTAGAGCCCTTTTGC | |
| β-actin | β-actin-F | TACGAGCTGCCTGACGGACA |
| β-actin-R | GGCTGTGATCTCCTTCTGC |
| Parameter | Control | BS | 6-3-1 | HAINUP40 | p-Value |
|---|---|---|---|---|---|
| IBW, g | 540.80 ± 23.17 | 531.67 ± 37.51 | 531.33 ± 20.50 | 525.33 ± 28.07 | 0.198 |
| FBW, g | 1344.00 ± 39.40 a | 1323.33 ± 24.34 a | 1422.00 ± 14.11 b | 1435.33 ± 21.55 b | <0.001 |
| FI, % | 755.91 ± 28.64 a | 592.84 ± 8.88 b | 547.04 ± 8.19 b | 553.56 ± 21.57 b | <0.001 |
| FER, % | 106.23 ± 1.08 a | 133.54 ± 3.60 b | 162.83 ± 1.81 c | 164.53 ± 5.75 c | <0.001 |
| FCR, % | 94.00 ± 1.0 a | 75.00 ± 2.0 b | 58.00 ± 1.0 c | 61.00 ± 2.0 c | <0.001 |
| WGR, % | 148.59 ± 8.29 a | 148.92 ± 4.85 a | 167.66 ± 2.69 b | 173.24 ± 1.40 b | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, C.; Song, S.; Cui, C.; Cai, Y.; Zhou, Y.; Wang, J.; Bei, W.; Zhang, D.; Guo, W.; Wang, S. Strain-Specific Benefits of Bacillus Probiotics in Hybrid Grouper: Growth Enhancement, Metabolic Health, Immune Modulation, and Vibrio harveyi Resistance. Animals 2024, 14, 1062. https://doi.org/10.3390/ani14071062
Han C, Song S, Cui C, Cai Y, Zhou Y, Wang J, Bei W, Zhang D, Guo W, Wang S. Strain-Specific Benefits of Bacillus Probiotics in Hybrid Grouper: Growth Enhancement, Metabolic Health, Immune Modulation, and Vibrio harveyi Resistance. Animals. 2024; 14(7):1062. https://doi.org/10.3390/ani14071062
Chicago/Turabian StyleHan, Congjie, Shizhen Song, Congcong Cui, Yan Cai, Yongcan Zhou, Jiawen Wang, Weilie Bei, Dongdong Zhang, Weiliang Guo, and Shifeng Wang. 2024. "Strain-Specific Benefits of Bacillus Probiotics in Hybrid Grouper: Growth Enhancement, Metabolic Health, Immune Modulation, and Vibrio harveyi Resistance" Animals 14, no. 7: 1062. https://doi.org/10.3390/ani14071062






