Canine Upper Digestive Tract 3D Model: Assessing Its Utility for Anatomy and Upper Endoscopy Learning
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
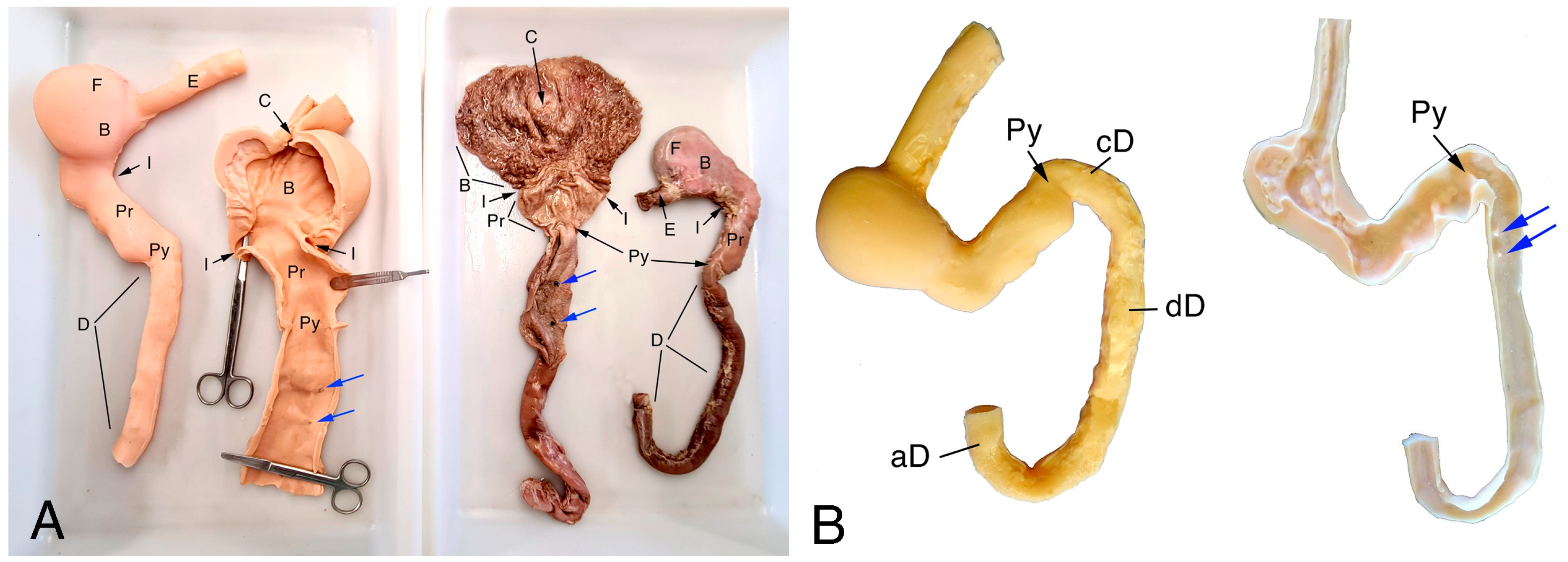
2.1. Samples and Images
2.2. 3D Model Design and Manufacturing
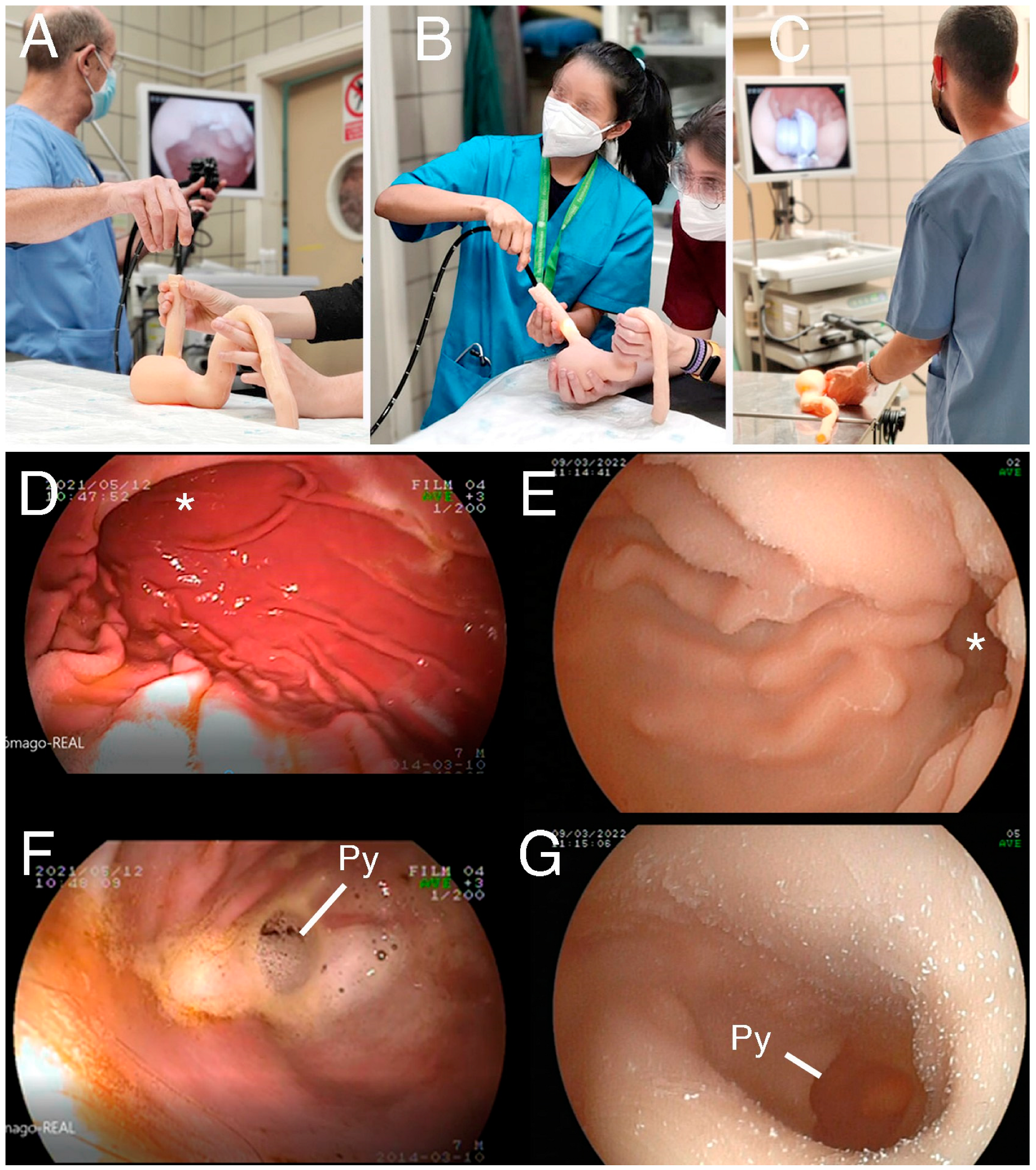
2.3. Participants
2.4. Statistical Analysis
3. Results
3.1. UDT Manufacturing Results and Preliminary Testing
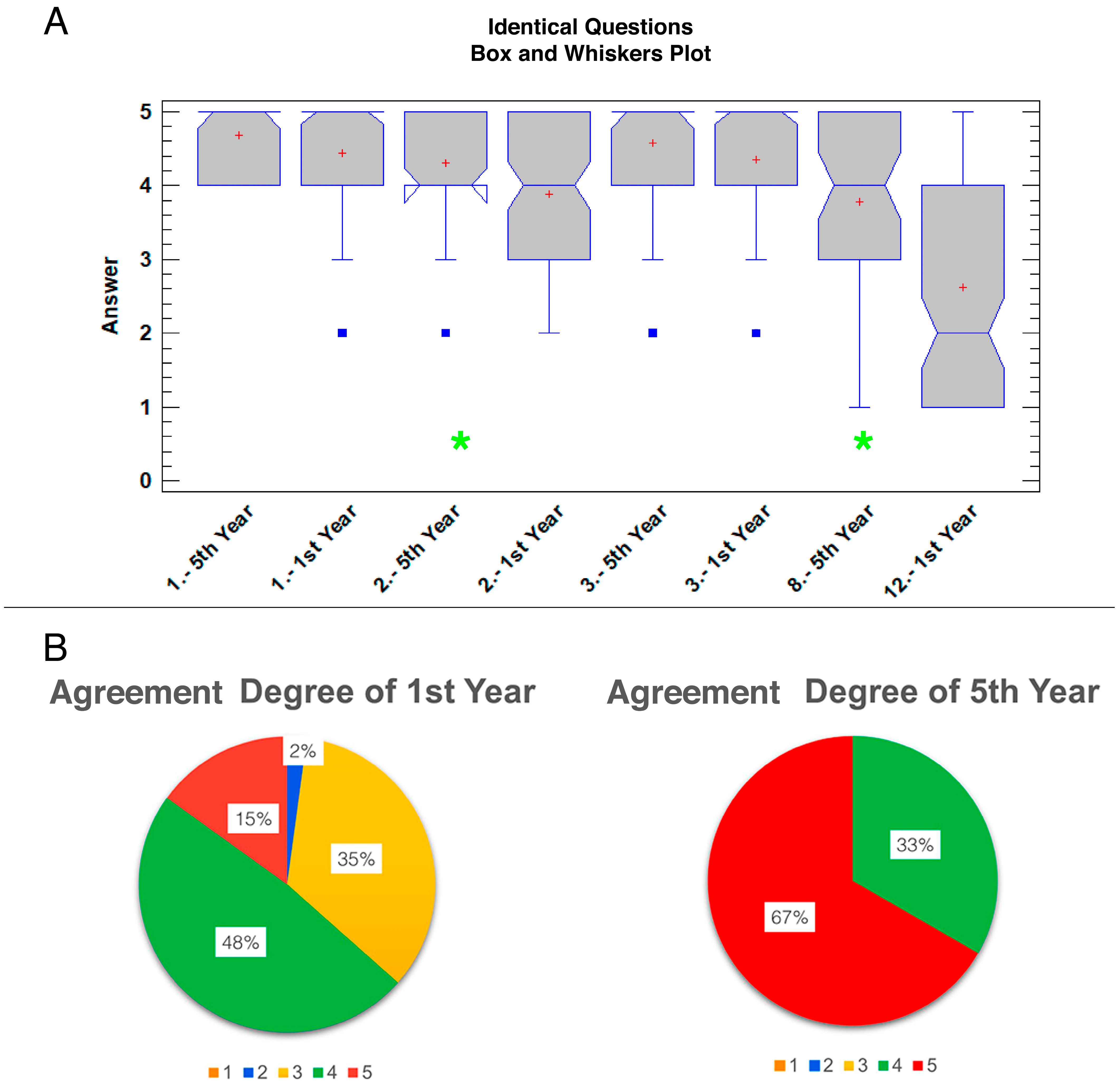
3.2. Survey Analysis
4. Discussion
4.1. High Acceptance of the 3D Model by Students but Not Complete Replacement
4.2. Different Learning Context Makes Differences
4.3. Limitations and Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schoenfeld-Tacher, R.M.; Horn, T.J.; Scheviak, T.A.; Royal, K.D.; Hudson, L.C. Evaluation of 3D additively manufactured canine brain models for teaching veterinary neuroanatomy. J. Vet. Med. Educ. 2017, 44, 612–619. [Google Scholar] [CrossRef]
- Wilhite, R.; Wölfel, I. 3D Printing for veterinary anatomy: An overview. Anat. Histol. Embryol. 2019, 48, 609–620. [Google Scholar] [CrossRef]
- Borgeat, K.; Shearn, A.I.; Payne, J.R.; Hezzell, M.; Biglino, G. Three-Dimensional Printed Models of the Heart Represent an Opportunity for Inclusive Learning. J. Vet. Med. Educ. 2020, 49, 346–352. [Google Scholar] [CrossRef]
- Di-Donato, B.A.; dos-Santos, A.C.; da Silveira, E.E.; Pereira, H.C.S.; da Silva Lisboa-Neto, A.; de Oliveira Alcobaca, M.M.; de de Assis-Neto, A. Three-dimensional digitalized and printed tongue models of the cow, dog, pig and horse for undergraduate veterinary education. Int. J. Morphol. 2021, 39, 436–440. [Google Scholar] [CrossRef]
- Hackmann, C.H.; Dos-Reis, D.d.A.L.; de-Assis-Neto, A.C. Digital revolution in veterinary anatomy: Confection of anatomical models of canine stomach by scanning and three-dimensional printing (3D). Int. J. Morphol. 2019, 37, 486–490. [Google Scholar] [CrossRef]
- Mendaza-DeCal, R.M.; Rojo, C. 3D-Printed Model of the Ovine Stomach by Surface Scanning: Evaluation for Teaching Veterinary Anatomy. Int. J. Morphol. 2021, 39, 1480–1486. [Google Scholar] [CrossRef]
- Altwal, J.; Wilson, C.H.; Griffon, D.J. Applications of 3-dimensional printing in small-animal surgery: A review of current practices. Vet. Surg. 2022, 51, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, A.; Linden, A.z.; Phillips, J.; Arroyo, L.G.; Koenig, J.; Monteith, G. Various 3D printed materials mimic bone ultrasonographically: 3D printed models of the equine cervical articular process joints as a simulator for ultrasound guided intra-articular injections. PLoS ONE 2019, 14, e0220332. [Google Scholar] [CrossRef] [PubMed]
- Barry Issenberg, S.; Mcgaghie, W.C.; Petrusa, E.R.; Lee Gordon, D.; Scalese, R.J. Features and uses of high-fidelity medical simulations that lead to effective learning: A BEME systematic review. Med. Teach. 2005, 27, 10–28. [Google Scholar] [CrossRef]
- Favier, V.; Zemiti, N.; Caravaca Mora, O.; Subsol, G.; Captier, G.; Lebrun, R.; Crampette, L.; Mondain, M.; Gilles, B. Geometric and mechanical evaluation of 3D-printing materials for skull base anatomical education and endoscopic surgery simulation–A first step to create reliable customized simulators. PLoS ONE 2017, 12, e0189486. [Google Scholar] [CrossRef]
- Baribeau, Y.; Sharkey, A.; Mahmood, E.; Feng, R.; Chaudhary, O.; Baribeau, V.; Mahmood, F.; Matyal, R.; Khabbaz, K. Three-dimensional printing and transesophageal echocardiographic imaging of patient-specific mitral valve models in a pulsatile phantom model. J. Cardiothorac. Vasc. Anesth. 2019, 33, 3469–3475. [Google Scholar] [CrossRef] [PubMed]
- Finocchiaro, M.; Cortegoso Valdivia, P.; Hernansanz, A.; Marino, N.; Amram, D.; Casals, A.; Menciassi, A.; Marlicz, W.; Ciuti, G.; Koulaouzidis, A. Training Simulators for Gastrointestinal Endoscopy: Current and Future Perspectives. Cancers 2021, 13, 1427. [Google Scholar] [CrossRef] [PubMed]
- Sakai, D.M.; Skrzypczak, H.; Nejamkin, P.; Clausse, M.; Bulant, C.; José Del Sole, M. Implementation of a Low-Cost 3D-Printed Feline Larynx Model for Veterinary Students. J. Vet. Med. Educ. 2021, 49, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Fransson, B.A.; Ragle, C.A. Assessment of laparoscopic skills before and after simulation training with a canine abdominal model. J. Am. Vet. Med. Assoc. 2010, 236, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Usón-Gargallo, J.; Tapia-Araya, A.E.; Díaz-Güemes Martin-Portugués, I.; Sánchez-Margallo, F.M. Development and evaluation of a canine laparoscopic simulator for veterinary clinical training. J. Vet. Med. Educ. 2014, 41, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, J.J.; Singh, A.; Kerr, C.L.; Khosa, D.K.; Fransson, B.A. Factors associated with simulator-assessed laparoscopic surgical skills of veterinary students. J. Am. Vet. Med. Assoc. 2017, 250, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.D.; Ragle, C.A.; Kinser, M.L.; Coffey, T.; Fransson, B.A. Effect of simulator orientation during skills training on performance of basic laparoscopic tasks by veterinary students. J. Am. Vet. Med. Assoc. 2017, 251, 1196–1201. [Google Scholar] [CrossRef]
- Usón-Gargallo, J.; Usón-Casaús, J.M.; Pérez-Merino, E.M.; Soria-Gálvez, F.; Morcillo, E.; Enciso, S.; Sánchez-Margallo, F.M. Validation of a realistic simulator for veterinary gastrointestinal endoscopy training. J. Vet. Med. Educ. 2014, 41, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Merino, E.M.; Usón-Gargallo, J.; Sánchez-Margallo, F.M.; Usón-Casaús, J.M. Comparison of the use of fresh-frozen canine cadavers and a realistic composite ex vivo simulator for training in small animal flexible gastrointestinal endoscopy. J. Am. Vet. Med. Assoc. 2018, 252, 839–845. [Google Scholar] [CrossRef]
- Suñol, A.; Aige, V.; Morales, C.; López-Beltran, M.; Feliu-Pascual, A.L.; Puig, J. Use of three-dimensional printing models for veterinary medical education: Impact on learning how to identify canine vertebral fractures. J. Vet. Med. Educ. 2018, 46, 523–532. [Google Scholar] [CrossRef]
- Mogali, S.R.; Yeong, W.Y.; Tan, H.K.J.; Tan, G.J.S.; Abrahams, P.H.; Zary, N.; Low-Beer, N.; Ferenczi, M.A. Evaluation by medical students of the educational value of multi-material and multi-colored three-dimensional printed models of the upper limb for anatomical education. Anat. Sci. Educ. 2018, 11, 54–64. [Google Scholar] [CrossRef] [PubMed]
- McMenamin, P.G.; Quayle, M.R.; McHenry, C.R.; Adams, J.W. The production of anatomical teaching resources using three-dimensional (3D) printing technology. Anat. Sci. Educ. 2014, 7, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Chae, M.P.; Rozen, W.M.; McMenamin, P.G.; Findlay, M.W.; Spychal, R.T.; Hunter-Smith, D.J. Emerging applications of bedside 3D printing in plastic surgery. Front. Surg. 2015, 2, 25. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, J.; Lee, A.; Park, N.; Song, S.; Seo, A.; Lee, H.; Kim, J.-I.; Eom, K. Augmented reality intravenous injection simulator based 3D medical imaging for veterinary medicine. Vet. J. 2013, 196, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Azer, S.A.; Azer, S. 3D anatomy models and impact on learning: A review of the quality of the literature. Health Prof. Educ. 2016, 2, 80–98. [Google Scholar] [CrossRef]
- Smith, M.L.; Jones, J.F. Dual-extrusion 3D printing of anatomical models for education. Anat. Sci. Educ. 2018, 11, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen, H.; Hansen, K.; Nørgård, M.Ø.; Wang, T.; Pedersen, M. From tissue to silicon to plastic: Three-dimensional printing in comparative anatomy and physiology. R. Soc. Open. Sci. 2016, 3, 150643. [Google Scholar] [CrossRef] [PubMed]
- Zanchetti, D.J.; Schueler, S.A.; Jacobson, B.C.; Lowe, R.C. Effective teaching of endoscopy: A qualitative study of the perceptions of gastroenterology fellows and attending gastroenterologists. Gastroenterol. Rep. 2016, 4, 125–130. [Google Scholar] [CrossRef]
- Abel, A.; Ziman, R. Learning Dogfish Shark Anatomy Using 3D-Printed Models: A Feasibility Study. Educ. Sci. 2023, 14, 34. [Google Scholar] [CrossRef]


| A. Veterinary Anatomy (First-Year Students) |
| 1. The 3D-printed model reproduces the external morphology realistically 2. The 3D-printed model reproduces the internal morphology realistically 3. Size and volume of the organs are easier understood on the 3D-printed model 4. Topography and orientation of the structures are more readily appreciated with the 3D-printed model 5. Hands-on manipulation is easier with the 3D-printed model 6. Compared with the real organ, the color of the 3D-printed model eases the learning of anatomy 7. Compared with the real organ, the texture of the 3D-printed model eases the learning of anatomy 8. Compared with the real organ, the rigidity of the 3D-printed model eases the learning of anatomy 9. I would use again this 3D-printed model for studying anatomy rather than the real organ 10. The 3D-printed model is preferred to real organs for examination 11. The 3D-printed model is preferred to anatomical images for examination 12. The 3D-printed model can replace real specimens in anatomy learning |
| B. Upper Digestive Endoscopy (Fifth-year students) |
| 1. The 3D-printed model reproduces the external morphology realistically 2. The 3D-printed model reproduces the internal morphology realistically 3. Size and volume of the organs are easier understood on the 3D-printed model 4. Topography and orientation of the structures during the endoscopic procedure are more readily appreciated using the 3D-printed model 5. The use of 3D-printed model allows me to better understand the upper gastrointestinal endoscopic examination and the taking of endoscopic biopsies 6. The use of the 3D-printed model allows me to better understand the use of the instruments used for the extraction of foreign bodies 7. The 3D-printed model is useful for endoscopic training of the students 8. The 3D-printed model can replace real specimens in endoscopy learning 9. Do you consider useful the inclusion of 3D-printed models in endoscopy training programs for vet students before the practice? 10. The 3D-printed model is important to complement endoscopy clinical practices 11. The 3D-printed model should always be used in this type of practice 12. I would use this 3D-printed model again to study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Regañón, D.; Mendaza-De Cal, R.; García-Sancho, M.; Rodríguez-Franco, F.; Sainz, Á.; Rodriguez-Quiros, J.; Rojo, C. Canine Upper Digestive Tract 3D Model: Assessing Its Utility for Anatomy and Upper Endoscopy Learning. Animals 2024, 14, 1070. https://doi.org/10.3390/ani14071070
Díaz-Regañón D, Mendaza-De Cal R, García-Sancho M, Rodríguez-Franco F, Sainz Á, Rodriguez-Quiros J, Rojo C. Canine Upper Digestive Tract 3D Model: Assessing Its Utility for Anatomy and Upper Endoscopy Learning. Animals. 2024; 14(7):1070. https://doi.org/10.3390/ani14071070
Chicago/Turabian StyleDíaz-Regañón, David, Rosa Mendaza-De Cal, Mercedes García-Sancho, Fernando Rodríguez-Franco, Ángel Sainz, Jesus Rodriguez-Quiros, and Concepción Rojo. 2024. "Canine Upper Digestive Tract 3D Model: Assessing Its Utility for Anatomy and Upper Endoscopy Learning" Animals 14, no. 7: 1070. https://doi.org/10.3390/ani14071070
APA StyleDíaz-Regañón, D., Mendaza-De Cal, R., García-Sancho, M., Rodríguez-Franco, F., Sainz, Á., Rodriguez-Quiros, J., & Rojo, C. (2024). Canine Upper Digestive Tract 3D Model: Assessing Its Utility for Anatomy and Upper Endoscopy Learning. Animals, 14(7), 1070. https://doi.org/10.3390/ani14071070









