Harnessing Vaginal Probiotics for Enhanced Management of Uterine Disease and Reproductive Performance in Dairy Cows: A Conceptual Review
Abstract
Simple Summary
Abstract
1. Introduction
2. The Role of Uterine Microbiota in Bovine Reproductive Health
2.1. Normal Uterine Microbiota
2.2. Dysbiosis and the Development of Uterine Inflammation
3. Overview of Probiotics
3.1. Classification of Probiotics
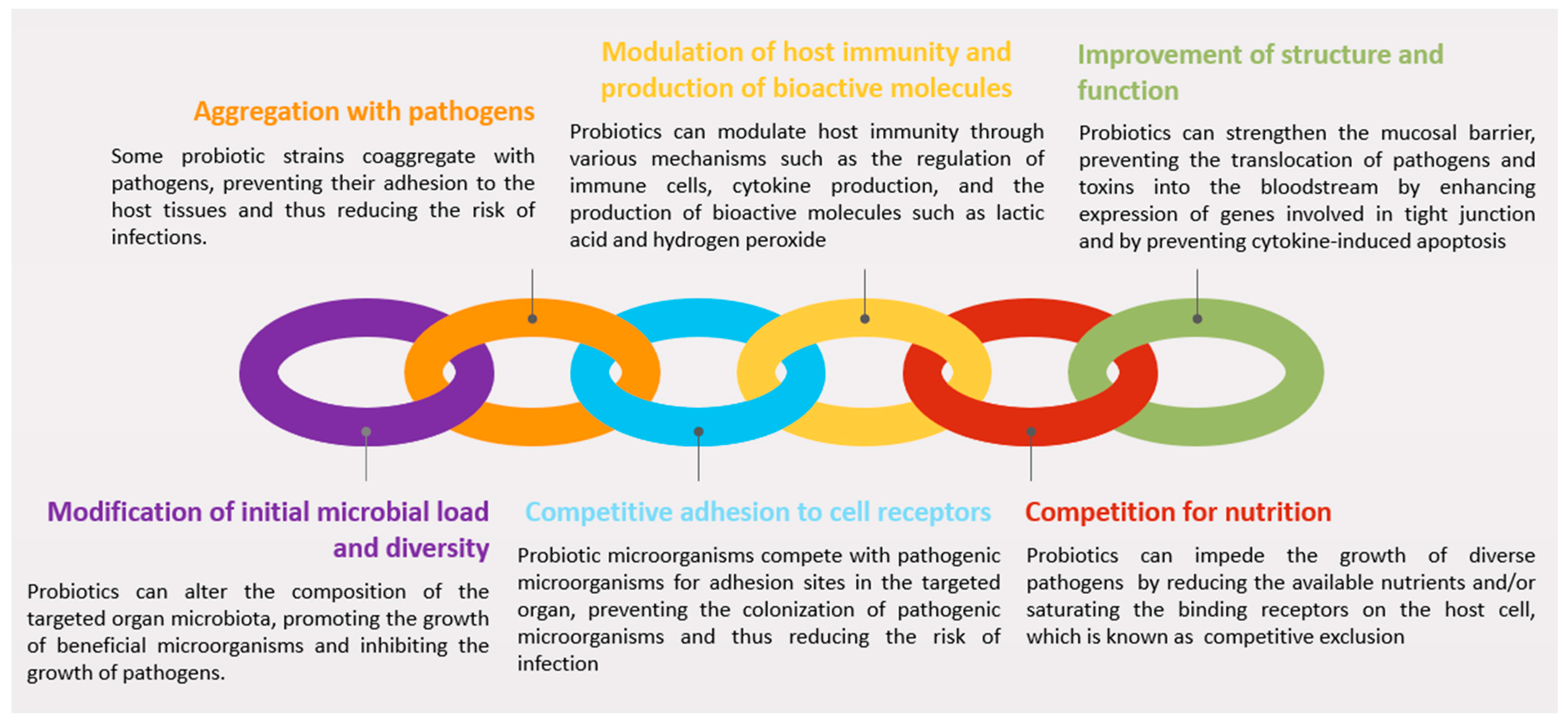
3.2. Mechanisms of Action
3.2.1. Modification of Initial Microbial Load and Diversity
3.2.2. Aggregation with Pathogens
3.2.3. Competitive Adhesion to Cell Receptors
3.2.4. Modulation of Host Immunity and Production of Bioactive Molecules
3.2.5. Competition for Nutrients
3.2.6. Improvement of Structure and Function
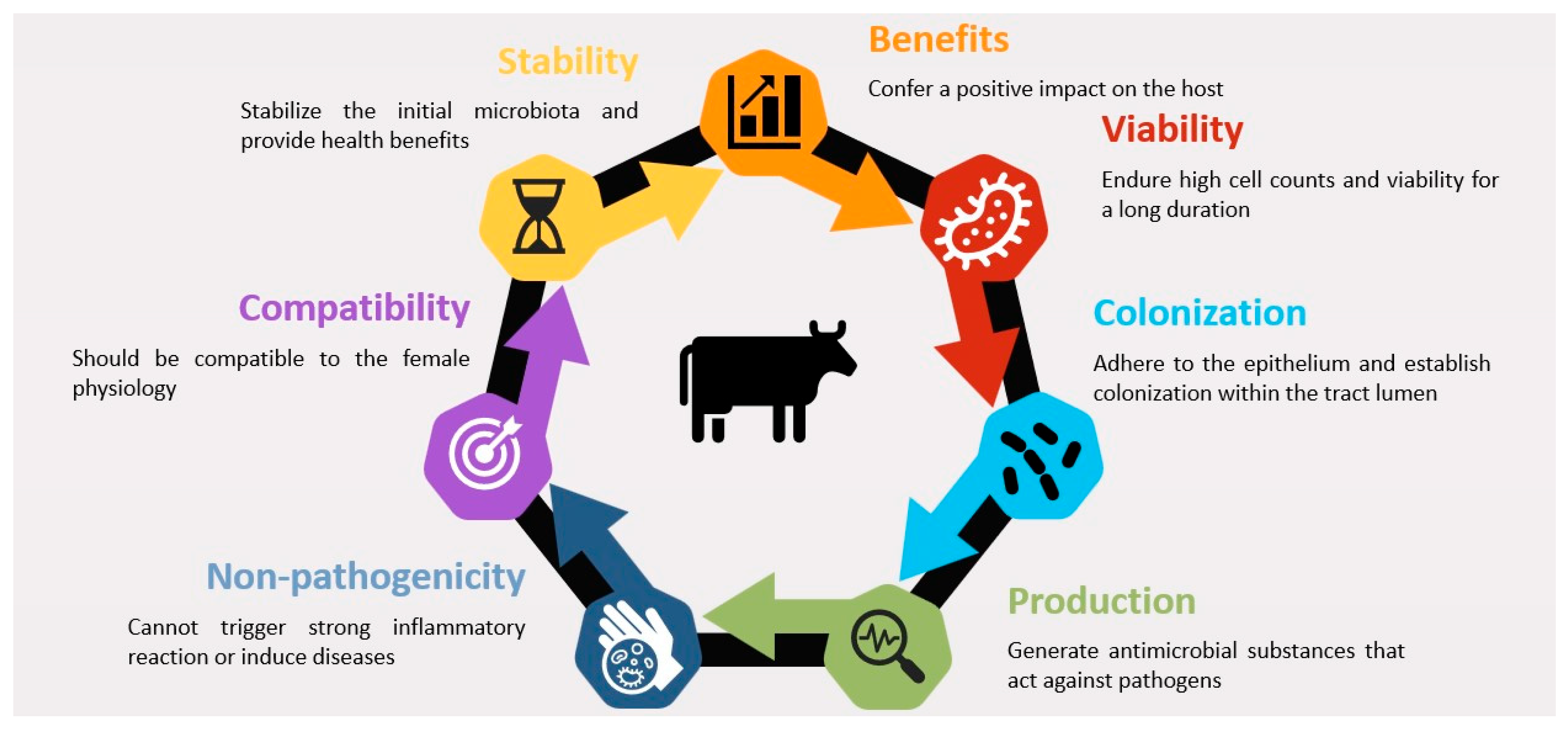
3.3. Factors Affecting Probiotic Efficacy
4. Probiotics for the Management of Uterine Disease in Cattle
4.1. Overview of Research on the Use of Probiotics for Uterine Health in Cattle
4.2. Probiotic Strains and Formulations Used in Research
4.3. Obtaining Probiotic Strains and Formulations Used in Research
4.4. Effects of Probiotics on Uterine Health and Fertility Outcomes
5. Factors Affecting the Efficacy of Probiotics for Uterine Inflammation
5.1. Timing of Probiotic Administration
5.2. Route of Administration
5.3. Dosage of Probiotics
6. Current Gaps and Future Directions in Probiotics Research for Mitigating Uterine Disease in Cattle
6.1. Identification of Optimal Probiotic Strains and Formulations
6.2. Development of Targeted Probiotic Delivery Methods
6.3. Exploring Probiotic Interactions with Other Interventions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cattaneo, L.; Piccioli-Cappelli, F.; Minuti, A.; Trevisi, E. Metabolic and physiological adaptations to first and second lactation in Holstein dairy cows. J. Dairy Sci. 2023, 106, 3559–3575. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, R.O.; Shin, S.T.; Guard, C.L.; Erb, H.N.; Frajblat, M. Prevalence of endometritis and its effects on reproductive performance of dairy cows. Theriogenology 2005, 64, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Nyabinwa, P.; Kashongwe, O.B.; Habimana, J.P.; Hirwa, C.D.; Bebe, B.O. Estimating prevalence of endometritis in smallholder zero-grazed dairy cows in Rwanda. Trop. Anim. Health Prod. 2020, 52, 3135–3145. [Google Scholar] [CrossRef] [PubMed]
- Földi, J.; Kulcsár, M.; Pécsi, A.; Huyghe, B.; de Sa, C.; Lohuis, J.A.C.M.; Cox, P.; Huszenicza, G. Bacterial complications of postpartum uterine involution in cattle. Anim. Reprod. Sci. 2006, 96, 265–281. [Google Scholar] [CrossRef]
- Crowe, M.; Williams, E. Triennial lactation symposium: Effects of stress on postpartum reproduction in dairy cows. J. Anim. Sci. 2012, 90, 1722–1727. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.M.; Lewis, G.S.; LeBlanc, S.; Gilbert, R.O. Defining postpartum uterine disease in cattle. Theriogenology 2006, 65, 1516–1530. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, S.J.; Duffield, T.F.; Leslie, K.E.; Bateman, K.G.; Keefe, G.P.; Walton, J.S.; Johnson, W.H. Defining and diagnosing postpartum clinical endometritis and its impact on reproductive performance in dairy cows. J. Dairy Sci. 2002, 85, 2223–2236. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yang, H.; Ahmad, M.J.; Yang, Y.; Yang, W.; Riaz, H.; Abulaiti, A.; Zhang, S.; Yang, L.; Hua, G. Postpartum Uterine Involution and Embryonic Development Pattern in Chinese Holstein Dairy Cows. Front. Vet. Sci. 2020, 7, 604729. [Google Scholar] [CrossRef]
- Kim, I.H.; Kang, H.G. Risk factors for postpartum endometritis and the effect of endometritis on reproductive performance in dairy cows in Korea. J. Reprod. Dev. 2003, 49, 485–491. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Cronin, J.; Goetze, L.; Donofrio, G.; Schuberth, H.J. Defining postpartum uterine disease and the mechanisms of infection and immunity in the female reproductive tract in cattle. Biol. Reprod. 2009, 81, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, L.C.; Cronin, J.G.; Sheldon, I.M. Mechanisms linking bacterial infections of the bovine endometrium to disease and infertility. Reprod. Biol. 2016, 16, 1–7. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Williams, E.J.; Miller, A.N.; Nash, D.M.; Herath, S. Uterine diseases in cattle after parturition. Vet. J. 2008, 176, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Manimaran, A.; Kumaresan, A.; Jeyakumar, S.; Mohanty, T.K.; Sejian, V.; Kumar, N.; Sreela, L.; Prakash, M.A.; Mooventhan, P.; Anantharaj, A.; et al. Potential of acute phase proteins as predictor of postpartum uterine infections during transition period and its regulatory mechanism in dairy cattle. Vet. World 2016, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Dubuc, J.; Duffield, T.F.; Leslie, K.E.; Walton, J.S.; Leblanc, S.J. Effects of postpartum uterine diseases on milk production and culling in dairy cows. J. Dairy Sci. 2011, 94, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Boudelal, S.; Adnane, M.; Niar, A. Cefacetrile and Rifaximin association might improve first service conception rate and reduce the number of services per conception in cows with clinical endometritis. Vet. Stanica 2021. [Google Scholar] [CrossRef]
- Kaufmann, T.B.; Westermann, S.; Drillich, M.; Plontzke, J.; Heuwieser, W. Systemic antibiotic treatment of clinical endometritis in dairy cows with ceftiofur or two doses of cloprostenol in a 14-d interval. Anim. Reprod. Sci. 2010, 121, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, S.H.; de Zeeuw, A.C. A comparison of two intrauterine antibiotic treatments for endometritis in dairy cows by means of survival analysis. BSAP Occas. Publ. 2018, 26, 417–421. [Google Scholar] [CrossRef]
- Denis-Robichaud, J.; Dubuc, J. Randomized clinical trial of intrauterine cephapirin infusion in dairy cows for the treatment of purulent vaginal discharge and cytological endometritis. J. Dairy Sci. 2015, 98, 6856–6864. [Google Scholar] [CrossRef]
- Oliver, S.P.; Murinda, S.E. Antimicrobial resistance of mastitis pathogens. Vet. Clin. North. Am. Food Anim. Pract. 2012, 28, 165–185. [Google Scholar] [CrossRef] [PubMed]
- Dzieciol, M.; Nizanski, W.; Stanczyk, E.; Kozdrowski, R.; Najder-Kozdrowska, L.; Twardon, J. The influence of antibiotic treatment of bitches in oestrus on their attractiveness to males during mating. Pol. J. Vet. Sci. 2013, 16, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Bajagai, Y.S.; Klieve, A.V.; Dart, P.J.; Bryden, W.L. Probiotics in Animal Nutrition—Production, Impact and Regulation; Makkar, H.P.S., Ed.; FAO Animal Production and Health Paper No. 179; FAO: Rome, Italy, 2016. [Google Scholar]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Odhiambo, J.F.; Farooq, U.; Lam, T.; Dunn, S.M.; Ametaj, B.N. Intravaginal lactic Acid bacteria modulated local and systemic immune responses and lowered the incidence of uterine infections in periparturient dairy cows. PLoS ONE 2014, 10, e0124167. [Google Scholar] [CrossRef]
- Madureira, A.M.L.; Burnett, T.A.; Boyd, C.T.; Baylao, M.; Cerri, R.L.A. Use of intravaginal lactic acid bacteria prepartum as an approach for preventing uterine disease and its association with fertility of lactating dairy cows. J. Dairy Sci. 2023, 106, 4860–4873. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.M.; Dobson, H. Postpartum uterine health in cattle. Anim. Reprod. Sci. 2004, 82–83, 295–306. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, S.J. Interactions of metabolism, inflammation, and reproductive tract health in the postpartum period in dairy cattle. Reprod. Domest. Anim. 2012, 47 (Suppl. S5), 18–30. [Google Scholar] [CrossRef]
- Adnane, M.; Chapwanya, A. A Review of the Diversity of the Genital Tract Microbiome and Implications for Fertility of Cattle. Animals 2022, 12, 460. [Google Scholar] [CrossRef]
- Adnane, M.; Chapwanya, A. Role of Genital Tract Bacteria in Promoting Endometrial Health in Cattle. Microorganisms 2022, 10, 2238. [Google Scholar] [CrossRef]
- Rodrigues, N.; Kästle, J.; Coutinho, T.; Amorim, A.; Campos, G.; Santos, V.; Marques, L.; Timenetsky, J.; de Farias, S. Qualitative analysis of the vaginal microbiota of healthy cattle and cattle with genital-tract disease. Genet. Mol. Res. 2015, 14, 6518–6528. [Google Scholar] [CrossRef]
- Moore, S.G.; Ericsson, A.C.; Poock, S.E.; Melendez, P.; Lucy, M.C. Hot topic: 16S rRNA gene sequencing reveals the microbiome of the virgin and pregnant bovine uterus. J. Dairy Sci. 2017, 100, 4953–4960. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Cronin, J.G.; Bromfield, J.J. Tolerance and innate immunity shape the development of postpartum uterine disease and the impact of endometritis in dairy cattle. Annu. Rev. Anim. Biosci. 2019, 7, 361–384. [Google Scholar] [CrossRef]
- Nicholas, R.; Ayling, R.; McAuliffe, L. Mycoplasma Diseases of Ruminants; CAB International: Wallingford, UK, 2008. [Google Scholar]
- Van der Burgt, G.; Clark, W.; Knight, R.; Colles, K. Cattle fertility problems and Histophilus somni. Vet. Rec. 2007, 160, 600. [Google Scholar] [CrossRef] [PubMed]
- Genis, S.; Bach, A.; Aris, A. Effects of intravaginal lactic acid bacteria on bovine endometrium: Implications in uterine health. Vet. Microbiol. 2017, 204, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.; Sapra, L.; Verma, B.; Srivastava, R.K. Immunomodulatory Potential of Lactobacillus acidophilus: Implications in Bone Health. In Acidophiles—Fundamentals and Applications; Lin, J., Chen, L., Lin, J., Eds.; IntechOpen: Rijeka, Croatia, 2021; p. 6. [Google Scholar]
- Silva, D.R.; Sardi, J.d.C.O.; Pitangui, N.d.S.; Roque, S.M.; da Silva, A.C.B.; Rosalen, P.L. Probiotics as an alternative antimicrobial therapy: Current reality and future directions. J. Funct. Foods 2020, 73, 104080. [Google Scholar] [CrossRef]
- Fulop, V.; Demeter, J.; Cseh, A. Significance and effects of prenatal and postnatal microbiome in the period of early individual development and options for interventional treatment. Orv. Hetil. 2021, 162, 731–740. [Google Scholar] [PubMed]
- Becker, A.; Munden, S.; McCabe, E.; Hurley, D.; Fanning, S.; Chapwanya, A.; Butaye, P. The Endometrial Microbiota-16S rRNA Gene Sequence Signatures in Healthy, Pregnant and Endometritis Dairy Cows. Vet. Sci. 2023, 10, 215. [Google Scholar] [CrossRef] [PubMed]
- Galvao, K.N.; Bicalho, R.C.; Jeon, S.J. Symposium review: The uterine microbiome associated with the development of uterine disease in dairy cows. J. Dairy Sci. 2019, 102, 11786–11797. [Google Scholar] [CrossRef] [PubMed]
- Karstrup, C.C.; Klitgaard, K.; Jensen, T.K.; Agerholm, J.S.; Pedersen, H.G. Presence of bacteria in the endometrium and placentomes of pregnant cows. Theriogenology 2017, 99, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; McClure, M.; Rorie, R.; Wang, X.; Chai, J.; Wei, X.; Lai, S.; Zhao, J. The vaginal and fecal microbiomes are related to pregnancy status in beef heifers. J. Anim. Sci. Biotechnol. 2019, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.M.; Bicalho, R.C. Diversity and succession of bacterial communities in the uterine fluid of postpartum metritic, endometritic and healthy dairy cows. PLoS ONE 2012, 7, e53048. [Google Scholar] [CrossRef] [PubMed]
- Tachedjian, G.; Aldunate, M.; Bradshaw, C.S.; Cone, R.A. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res. Microbiol. 2017, 168, 782–792. [Google Scholar] [CrossRef]
- Swartz, J.D.; Lachman, M.; Westveer, K.; O’Neill, T.; Geary, T.; Kott, R.W.; Berardinelli, J.G.; Hatfield, P.G.; Thomson, J.M.; Roberts, A.; et al. Characterization of the Vaginal Microbiota of Ewes and Cows Reveals a Unique Microbiota with Low Levels of Lactobacilli and Near-Neutral pH. Front. Vet. Sci. 2014, 1, 19. [Google Scholar] [CrossRef] [PubMed]
- Otero, M.C.; Morelli, L.; Nader-Macias, M.E. Probiotic properties of vaginal lactic acid bacteria to prevent metritis in cattle. Lett. Appl. Microbiol. 2006, 43, 91–97. [Google Scholar] [CrossRef]
- Sakai, M.; Ishiyama, A.; Tabata, M.; Sasaki, Y.; Yoneda, S.; Shiozaki, A.; Saito, S. Relationship between cervical mucus interleukin-8 concentrations and vaginal bacteria in pregnancy. Am. J. Reprod. Immunol. 2004, 52, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Miranda-CasoLuengo, R.; Lu, J.; Williams, E.J.; Miranda-CasoLuengo, A.A.; Carrington, S.D.; Evans, A.C.O.; Meijer, W.G. Delayed differentiation of vaginal and uterine microbiomes in dairy cows developing postpartum endometritis. PLoS ONE 2019, 14, e0200974. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.M.; Cronin, J.G.; Healey, G.D.; Gabler, C.; Heuwieser, W.; Streyl, D.; Bromfield, J.J.; Miyamoto, A.; Fergani, C.; Dobson, H. Innate immunity and inflammation of the bovine female reproductive tract in health and disease. Reproduction 2014, 148, R41–R51. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.M.; Noakes, D.E.; Rycroft, A.N.; Pfeiffer, D.U.; Dobson, H. Influence of uterine bacterial contamination after parturition on ovarian dominant follicle selection and follicle growth and function in cattle. Reproduction 2002, 123, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Ruder, C.; Sasser, R.; Williams, R.; Ely, J.; Bull, R.; Butler, J. Uterine infections in the postpartum cow: II. Possible synergistic effect of Fusobacteriumnecrophorum and Corynebacteriumpyogenes. Theriogenology 1981, 15, 573–580. [Google Scholar] [CrossRef]
- Olson, J.D.; Ball, L.; Mortimer, R.G.; Farin, P.W.; Adney, W.S.; Huffman, E.M. Aspects of bacteriology and endocrinology of cows with pyometra and retained fetal membranes. Am. J. Vet. Res. 1984, 45, 2251–2255. [Google Scholar] [PubMed]
- Bromfield, J.J.; Santos, J.E.; Block, J.; Williams, R.S.; Sheldon, I.M. PHYSIOLOGY AND ENDOCRINOLOGY SYMPOSIUM: Uterine infection: Linking infection and innate immunity with infertility in the high-producing dairy cow. J. Anim. Sci. 2015, 93, 2021–2033. [Google Scholar] [CrossRef] [PubMed]
- Amos, M.R.; Healey, G.D.; Goldstone, R.J.; Mahan, S.M.; Duvel, A.; Schuberth, H.J.; Sheldon, I.M. Differential endometrial cell sensitivity to a cholesterol-dependent cytolysin links Trueperella pyogenes to uterine disease in cattle. Biol. Reprod. 2014, 90, 54. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.M.; Rycroft, A.N.; Zhou, C. Association between postpartum pyrexia and uterine bacterial infection in dairy cattle. Vet. Rec. 2004, 154, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Lee, Y.; Kim, E.; Kwak, A.; Ryoo, S.; Bae, S.H.; Azam, T.; Kim, S.; Dinarello, C.A. The Interleukin-1alpha Precursor is Biologically Active and is Likely a Key Alarmin in the IL-1 Family of Cytokines. Front. Immunol. 2013, 4, 391. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Raffrenato, E.; Lukamba, S.D.; Adnane, M.; Irons, P.C.; Cormican, P.; Tasara, T.; Chapwanya, A. Characterization of metabolic and inflammatory profiles of transition dairy cows fed an energy-restricted diet. J. Anim. Sci. 2020, 98. [Google Scholar] [CrossRef] [PubMed]
- Vlasova, A.N.; Saif, L.J. Bovine Immunology: Implications for Dairy Cattle. Front. Immunol. 2021, 12, 643206. [Google Scholar] [CrossRef] [PubMed]
- Adnane, M.; Kaidi, R.; Hanzen, C.; England, G.C.W. Risk factors of clinical and subclinical endometritis in cattle: A review. Turk. J. Vet. Anim. Sci. 2017, 41, 1–11. [Google Scholar] [CrossRef]
- Galvao, K.N.; Santos, N.R.; Galvao, J.S.; Gilbert, R.O. Association between endometritis and endometrial cytokine expression in postpartum Holstein cows. Theriogenology 2011, 76, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Adnane, M.; Kelly, P.; Chapwanya, A.; Meade, K.G.; O’Farrelly, C. Improved detection of biomarkers in cervico-vaginal mucus (CVM) from postpartum cattle. BMC Vet. Res. 2018, 14, 297. [Google Scholar] [CrossRef] [PubMed]
- Adnane, M.; Chapwanya, A.; Kaidi, R.; Meade, K.G.; O’Farrelly, C. Profiling inflammatory biomarkers in cervico-vaginal mucus (CVM) postpartum: Potential early indicators of bovine clinical endometritis? Theriogenology 2017, 103, 117–122. [Google Scholar] [CrossRef]
- Ault, T.B.; Clemmons, B.A.; Reese, S.T.; Dantas, F.G.; Franco, G.A.; Smith, T.P.; Edwards, J.L.; Myer, P.R.; Pohler, K.G. Bacterial taxonomic composition of the postpartum cow uterus and vagina prior to artificial insemination1. J. Anim. Sci. 2019, 97, 4305–4313. [Google Scholar] [CrossRef] [PubMed]
- Fuller, R. Probiotics in man and animals. J. Appl. Bacteriol. 1989, 66, 365–378. [Google Scholar] [PubMed]
- Parvez, S.; Malik, K.A.; Ah Kang, S.; Kim, H.Y. Probiotics and their fermented food products are beneficial for health. J. Appl. Microbiol. 2006, 100, 1171–1185. [Google Scholar] [CrossRef]
- Gill, H.S. Probiotics to enhance anti-infective defences in the gastrointestinal tract. Best. Pract. Res. Clin. Gastroenterol. 2003, 17, 755–773. [Google Scholar] [CrossRef] [PubMed]
- Ametaj, B.; Iqbal, S.; Selami, F.; Odhiambo, J.; Wang, Y.; Gänzle, M.; Dunn, S.; Zebeli, Q. Intravaginal administration of lactic acid bacteria modulated the incidence of purulent vaginal discharges, plasma haptoglobin concentrations, and milk production in dairy cows. Res. Vet. Sci. 2014, 96, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, G.; Tiwari, R.; Pandey, S.; Pandey, P. Promising future of probiotics for human health: Current scenario. Chron. Young Sci. 2012, 3, 17. [Google Scholar] [CrossRef]
- Izuddin, W.I.; Loh, T.C.; Samsudin, A.A.; Foo, H.L.; Humam, A.M.; Shazali, N. Effects of postbiotic supplementation on growth performance, ruminal fermentation and microbial profile, blood metabolite and GHR, IGF-1 and MCT-1 gene expression in post-weaning lambs. BMC Vet. Res. 2019, 15, 315. [Google Scholar] [CrossRef] [PubMed]
- Mansilla, F.I.; Ficoseco, C.A.; Miranda, M.H.; Puglisi, E.; Nader-Macias, M.E.F.; Vignolo, G.M.; Fontana, C.A. Administration of probiotic lactic acid bacteria to modulate fecal microbiome in feedlot cattle. Sci. Rep. 2022, 12, 12957. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Henry, K.C.; Donato, K.A.; Shen-Tu, G.; Gordanpour, M.; Sherman, P.M. Lactobacillus rhamnosus strain GG prevents enterohemorrhagic Escherichia coli O157:H7-induced changes in epithelial barrier function. Infect. Immun. 2008, 76, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.M.; Aiba, Y.; Takagi, A.; Kamiya, S.; Miwa, T.; Koga, Y. Prevention of Helicobacter pylori infection by lactobacilli in a gnotobiotic murine model. Gut 1997, 41, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Neeser, J.R.; Granato, D.; Rouvet, M.; Servin, A.; Teneberg, S.; Karlsson, K.A. Lactobacillus johnsonii La1 shares carbohydrate-binding specificities with several enteropathogenic bacteria. Glycobiology 2000, 10, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, S.; Hashiba, H.; Hirota, T.; Forstner, J.F. Inhibition of the binding of enterotoxigenic Escherichia coli Pb176 to human intestinal epithelial cell line HCT-8 by an extracellular protein fraction containing BIF of Bifidobacterium longum SBT2928: Suggestive evidence of blocking of the binding receptor gangliotetraosylceramide on the cell surface. Int. J. Food Microbiol. 2001, 67, 97–106. [Google Scholar]
- Coconnier, M.H.; Bernet, M.F.; Chauviere, G.; Servin, A.L. Adhering heat-killed human Lactobacillus acidophilus, strain LB, inhibits the process of pathogenicity of diarrhoeagenic bacteria in cultured human intestinal cells. J. Diarrhoeal Dis. Res. 1993, 11, 235–242. [Google Scholar] [PubMed]
- Hirano, J.; Yoshida, T.; Sugiyama, T.; Koide, N.; Mori, I.; Yokochi, T. The effect of Lactobacillus rhamnosus on enterohemorrhagic Escherichia coli infection of human intestinal cells in vitro. Microbiol. Immunol. 2003, 47, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Brito, M.; Plaza-Diaz, J.; Munoz-Quezada, S.; Gomez-Llorente, C.; Gil, A. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Makras, L.; Triantafyllou, V.; Fayol-Messaoudi, D.; Adriany, T.; Zoumpopoulou, G.; Tsakalidou, E.; Servin, A.; De Vuyst, L. Kinetic analysis of the antibacterial activity of probiotic lactobacilli towards Salmonella enterica serovar Typhimurium reveals a role for lactic acid and other inhibitory compounds. Res. Microbiol. 2006, 157, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Greene, J.D.; Klaenhammer, T.R. Factors involved in adherence of lactobacilli to human Caco-2 cells. Appl. Environ. Microbiol. 1994, 60, 4487–4494. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Kjos, M.; Nes, I.F.; Diep, D.B.; Lotfipour, F. Natural antimicrobial peptides from bacteria: Characteristics and potential applications to fight against antibiotic resistance. J. Appl. Microbiol. 2012, 113, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, Z.; Winters, D.K.; Johnson, M.G. Purification, amino acid sequence and mode of action of bifidocin B produced by Bifidobacterium bifidum NCFB 1454. J. Appl. Microbiol. 1999, 86, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Furrie, E.; Macfarlane, S.; Kennedy, A.; Cummings, J.H.; Walsh, S.V.; O’Neil, D.A.; Macfarlane, G.T. Synbiotic therapy (Bifidobacterium longum/Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: A randomised controlled pilot trial. Gut 2005, 54, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Prema, P.; Smila, D.; Palavesam, A.; Immanuel, G. Production and Characterization of an Antifungal Compound (3-Phenyllactic Acid) Produced by Lactobacillus plantarum Strain. Food Bioprocess. Technol. 2008, 3, 379–386. [Google Scholar] [CrossRef]
- Niku-Paavola, M.L.; Laitila, A.; Mattila-Sandholm, T.; Haikara, A. New types of antimicrobial compounds produced by Lactobacillus plantarum. J. Appl. Microbiol. 1999, 86, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Bello, F.D.; Clarke, C.; Ryan, L.; Ulmer, H.; Schober, T.; Ström, K.; Sjögren, J.; van Sinderen, D.; Schnürer, J.; Arendt, E. Improvement of the quality and shelf life of wheat bread by fermentation with the antifungal strain Lactobacillus plantarum FST 1.7. J. Cereal Sci. 2007, 45, 309–318. [Google Scholar] [CrossRef]
- Nakamura, S.; Kuda, T.; An, C.; Kanno, T.; Takahashi, H.; Kimura, B. Inhibitory effects of Leuconostoc mesenteroides 1RM3 isolated from narezushi, a fermented fish with rice, on Listeria monocytogenes infection to Caco-2 cells and A/J mice. Anaerobe 2012, 18, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Zareie, M.; Johnson-Henry, K.; Jury, J.; Yang, P.-C.; Ngan, B.-Y.; McKay, D.M.; Soderholm, J.D.; Perdue, M.H.; Sherman, P.M. Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut 2006, 55, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.C.; Cookson, A.L.; McNabb, W.C.; Park, Z.; McCann, M.J.; Kelly, W.J.; Roy, N.C. Lactobacillus plantarum MB452 enhances the function of the intestinal barrier by increasing the expression levels of genes involved in tight junction formation. BMC Microbiol. 2010, 10, 316. [Google Scholar] [CrossRef] [PubMed]
- Hummel, S.; Veltman, K.; Cichon, C.; Sonnenborn, U.; Schmidt, M.A. Differential targeting of the E-Cadherin/beta-Catenin complex by gram-positive probiotic lactobacilli improves epithelial barrier function. Appl. Environ. Microbiol. 2012, 78, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Polk, D.B. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J. Biol. Chem. 2002, 277, 50959–50965. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Cao, H.; Cover, T.L.; Whitehead, R.; Washington, M.K.; Polk, D.B. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology 2007, 132, 562–575. [Google Scholar] [CrossRef] [PubMed]
- Sartor, R.B. Mechanisms of disease: Pathogenesis of Crohn’s disease and ulcerative colitis. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006, 3, 390–407. [Google Scholar] [CrossRef] [PubMed]
- Yarbrough, V.L.; Winkle, S.; Herbst-Kralovetz, M.M. Antimicrobial peptides in the female reproductive tract: A critical component of the mucosal immune barrier with physiological and clinical implications. Hum. Reprod. Update 2015, 21, 353–377. [Google Scholar] [CrossRef] [PubMed]
- Valenti, P.; Rosa, L.; Capobianco, D.; Lepanto, M.S.; Schiavi, E.; Cutone, A.; Paesano, R.; Mastromarino, P. Role of Lactobacilli and Lactoferrin in the Mucosal Cervicovaginal Defense. Front. Immunol. 2018, 9, 376. [Google Scholar] [CrossRef] [PubMed]
- Mack, D.R.; Ahrne, S.; Hyde, L.; Wei, S.; Hollingsworth, M.A. Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro. Gut 2003, 52, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Mattar, A.F.; Teitelbaum, D.H.; Drongowski, R.A.; Yongyi, F.; Harmon, C.M.; Coran, A.G. Probiotics up-regulate MUC-2 mucin gene expression in a Caco-2 cell-culture model. Pediatr. Surg. Int. 2002, 18, 586–590. [Google Scholar] [PubMed]
- Kim, Y.; Kim, S.H.; Whang, K.Y.; Kim, Y.J.; Oh, S. Inhibition of Escherichia coli O157:H7 attachment by interactions between lactic acid bacteria and intestinal epithelial cells. J. Microbiol. Biotechnol. 2008, 18, 1278–1285. [Google Scholar] [PubMed]
- Buck, B.L.; Altermann, E.; Svingerud, T.; Klaenhammer, T.R. Functional analysis of putative adhesion factors in Lactobacillus acidophilus NCFM. Appl. Environ. Microbiol. 2005, 71, 8344–8351. [Google Scholar] [CrossRef] [PubMed]
- Kankainen, M.; Paulin, L.; Tynkkynen, S.; von Ossowski, I.; Reunanen, J.; Partanen, P.; Satokari, R.; Vesterlund, S.; Hendrickx, A.P.A.; Lebeer, S.; et al. Comparative genomic analysis of Lactobacillus rhamnosus GG reveals pili containing a human- mucus binding protein. Proc. Natl. Acad. Sci. USA 2009, 106, 17193–17198. [Google Scholar] [CrossRef] [PubMed]
- Kovachev, S. Defence factors of vaginal lactobacilli. Crit. Rev. Microbiol. 2018, 44, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Juntunen, M.; Kirjavainen, P.V.; Ouwehand, A.C.; Salminen, S.J.; Isolauri, E. Adherence of probiotic bacteria to human intestinal mucus in healthy infants and during rotavirus infection. Clin. Diagn. Lab. Immunol. 2001, 8, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Schiffrin, E.J.; Brassart, D.; Servin, A.L.; Rochat, F.; Donnet-Hughes, A. Immune modulation of blood leukocytes in humans by lactic acid bacteria: Criteria for strain selection. Am. J. Clin. Nutr. 1997, 66, 515S–520S. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ametaj, B.N.; Ambrose, D.J.; Ganzle, M.G. Characterisation of the bacterial microbiota of the vagina of dairy cows and isolation of pediocin-producing Pediococcus acidilactici. BMC Microbiol. 2013, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, L.R.; Karstrup, C.C.; Pedersen, H.G.; Angen, Ø.; Agerholm, J.S.; Rasmussen, E.L.; Jensen, T.K.; Klitgaard, K. An investigation of the microbiota in uterine flush samples and endometrial biopsies from dairy cows during the first 7 weeks postpartum. Theriogenology 2016, 86, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Adnane, M.; Meade, K.G.; O’Farrelly, C. Cervico-vaginal mucus (CVM)—An accessible source of immunologically informative biomolecules. Vet. Res. Commun. 2018, 42, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.T.; Turni, C.; Blackall, P.J.; Boe-Hansen, G.; Hayes, B.J.; Tabor, A.E. Interrogating the bovine reproductive tract metagenomes using culture-independent approaches: A systematic review. Anim. Microbiome 2021, 3, 41. [Google Scholar] [CrossRef]
- Bernardeau, M.; Vernoux, J.P. Overview of differences between microbial feed additives and probiotics for food regarding regulation, growth promotion effects and health properties and consequences for extrapolation of farm animal results to humans. Clin. Microbiol. Infect. 2013, 19, 321–330. [Google Scholar] [CrossRef]
- Peter, S.; Gärtner, M.A.; Michel, G.; Ibrahim, M.; Klopfleisch, R.; Lübke-Becker, A.; Jung, M.; Einspanier, R.; Gabler, C. Influence of intrauterine administration of Lactobacillus buchneri on reproductive performance and pro-inflammatory endometrial mRNA expression of cows with subclinical endometritis. Sci. Rep. 2018, 8, 5473. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.E.; Kim, G.M.; Lee, S.K.; Yang, C.J. Growth performance, meat yield, oxidative stability, and Fatty Acid composition of meat from broilers fed diets supplemented with a medicinal plant and probiotics. Asian-Australas. J. Anim. Sci. 2012, 25, 1159–1168. [Google Scholar] [CrossRef]
- Genis, S.; Bach, A.; Fabregas, F.; Aris, A. Potential of lactic acid bacteria at regulating Escherichia coli infection and inflammation of bovine endometrium. Theriogenology 2016, 85, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Atshan, S.S.; Shamsudin, M.N.; Lung, L.T.; Sekawi, Z.; Ghaznavi-Rad, E.; Pei, C.P. Comparative characterisation of genotypically different clones of MRSA in the production of biofilms. J. Biomed. Biotechnol. 2012, 2012, 417247. [Google Scholar] [CrossRef] [PubMed]
- Genis, S.; Sanchez-Chardi, A.; Bach, A.; Fabregas, F.; Aris, A. A combination of lactic acid bacteria regulates Escherichia coli infection and inflammation of the bovine endometrium. J. Dairy Sci. 2017, 100, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, J.; Yang, Y.; Li, X.; Sun, C. In vitroassessment of probiotic properties of lactic acid bacteria isolated from vaginas of healthy cows. Indian J. Anim. Res. 2015, 49, 355. [Google Scholar] [CrossRef]
- Deng, Q.; Odhiambo, J.F.; Farooq, U.; Lam, T.; Dunn, S.M.; Ametaj, B.N. Intravaginal probiotics modulated metabolic status and improved milk production and composition of transition dairy cows. J. Anim. Sci. 2016, 94, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Hearps, A.; Gugasyan, R.; Srbinovski, D.; Tyssen, D.; Aldunate, M.; Anderson, D.J.; Cone, R.; Tachedjian, G. Lactic Acid, a Vaginal Microbiota Metabolite, Elicits an Anti-inflammatory Response from Vaginal and Cervical Epithelial Cells. AIDS Res. Hum. Retroviruses 2014, 30, A238–A239. [Google Scholar] [CrossRef]
- Potter, T.J.; Guitian, J.; Fishwick, J.; Gordon, P.J.; Sheldon, I.M. Risk factors for clinical endometritis in postpartum dairy cattle. Theriogenology 2010, 74, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Whiteford, L.C.; Sheldon, I.M. Association between clinical hypocalcaemia and postpartum endometritis. Vet. Rec. 2005, 157, 202–203. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.M. The postpartum uterus. Vet. Clin. North. Am. Food Anim. Pract. 2004, 20, 569–591. [Google Scholar] [CrossRef]
- Aagaard, K.; Riehle, K.; Ma, J.; Segata, N.; Mistretta, T.-A.; Coarfa, C.; Raza, S.; Rosenbaum, S.; Veyver, I.V.D.; Milosavljevic, A.; et al. A metagenomic approach to characterization of the vaginal microbiome signature in pregnancy. PLoS ONE 2012, 7, e36466. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
| Probiotic Strains | Dosage | Study Type | Timing of Treatment | Route of Administration | Main Results | References |
|---|---|---|---|---|---|---|
| Mixture of 3 probiotic bacteria, Lactobacillus sakei FUA 3089, Pediococcus acidilactici FUA 3140, and P. acidilactici FUA 3138 | 1010–1012 CFUs | In vivo | 2 weeks prepartum–4 weeks postpartum | Intravaginal | - Decreased purulent vaginal discharge - Decreased plasma haptoglobin - Increased pregnancy rate - Increased milk production | [66] |
| Mixture composed of Lactobacillus sakei FUA3089, Pediococcus acidilactici FUA3138, and Pediococcus acidilactici FUA3140 | 108–109 CFUs | In vivo | 2 weeks prepartum and 1 week postpartum. or 2 weeks prepartum only | Intravaginal | - Lowered the incidence of metritis and total uterine infections - Lowered concentrations of systemic lbp - Increased vaginal mucus IgA | [23] |
| Lactobacillus buchneri DSM 32407 | 1.5–2 × 1010 CFUs | In vivo | 24–30 days postpartum | Intrauterine | - Higher first-service conception rate - Shorter calving–conception interval - Lower expression of pro-inflammatory genes | [107] |
| Lactobacillus rhamnosus, Pediococcus acidilactici, Lactobacillus reuteri, and Lactobacillus sakei individually or in different combinations | Different doses | In vitro | Endometrial epithelial cells co-cultured with E. coli | - | - Reduced E. coli infection in vitro dependent on the dose and strain - Decreasing expression of IL8 and IL1β | [109] |
| Combination of Lactobacillus rhamnosus, Pediococcus acidilactici, and Lactobacillus reuteri | At a ratio of 25:25:2 | In vitro—Ex vivo | Endometrial epithelial cells co-cultured with E. coli | - | - E. coli infection in vitro reduced by 89.77% - Decreasing IL-8, IL-1β, and IL-6 | [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adnane, M.; Whiston, R.; Tasara, T.; Bleul, U.; Chapwanya, A. Harnessing Vaginal Probiotics for Enhanced Management of Uterine Disease and Reproductive Performance in Dairy Cows: A Conceptual Review. Animals 2024, 14, 1073. https://doi.org/10.3390/ani14071073
Adnane M, Whiston R, Tasara T, Bleul U, Chapwanya A. Harnessing Vaginal Probiotics for Enhanced Management of Uterine Disease and Reproductive Performance in Dairy Cows: A Conceptual Review. Animals. 2024; 14(7):1073. https://doi.org/10.3390/ani14071073
Chicago/Turabian StyleAdnane, Mounir, Ronan Whiston, Taurai Tasara, Ulrich Bleul, and Aspinas Chapwanya. 2024. "Harnessing Vaginal Probiotics for Enhanced Management of Uterine Disease and Reproductive Performance in Dairy Cows: A Conceptual Review" Animals 14, no. 7: 1073. https://doi.org/10.3390/ani14071073
APA StyleAdnane, M., Whiston, R., Tasara, T., Bleul, U., & Chapwanya, A. (2024). Harnessing Vaginal Probiotics for Enhanced Management of Uterine Disease and Reproductive Performance in Dairy Cows: A Conceptual Review. Animals, 14(7), 1073. https://doi.org/10.3390/ani14071073





