Effects of Heat Stress on the Laying Performance, Egg Quality, and Physiological Response of Laying Hens
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Performance and Physiology Parameters
2.3. Egg Quality
2.4. Blood Sampling and Measurements
2.5. Feather Sampling and Corticosterone Measurement
2.6. Statistical Analysis
3. Results and Discussion
3.1. Laying Performance
3.2. Physiological Response
3.3. Egg Quality
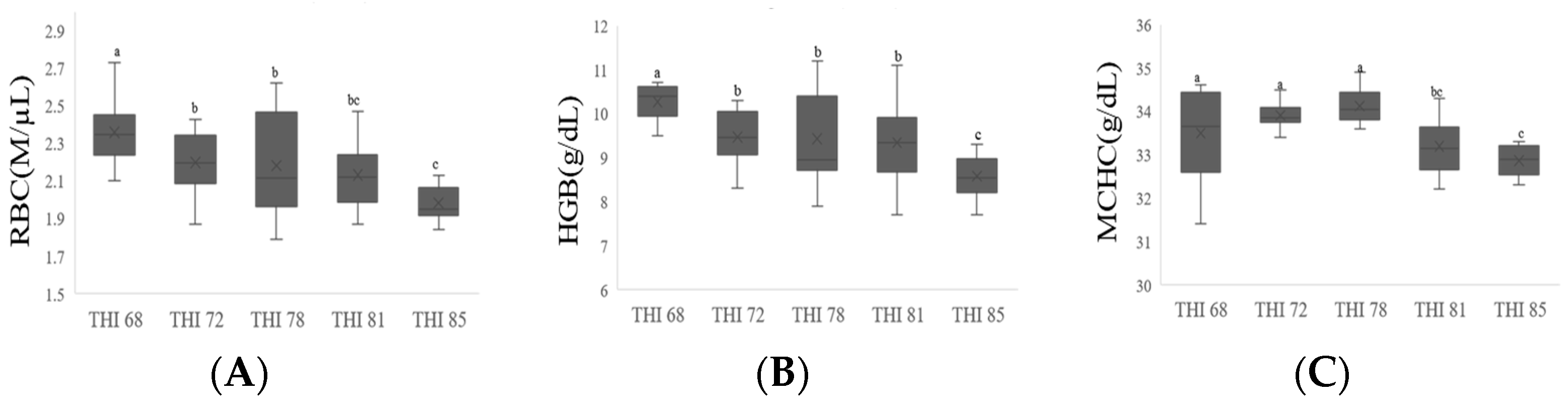
3.4. Blood Parameters
3.5. Corticosterone in Feathers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nawab, A.; Ibtisham, F.; Li, G.; Kieser, B.; Wu, J.; Liu, W. Heat stress in poultry production: Mitigation strategies to overcome the future challenges facing the global poultry industry. J. Therm. Biol. 2018, 78, 131–139. [Google Scholar] [CrossRef]
- Ekine-dzivenu, C.C.; Mrode, R.; Oyieng, E.; Komwihangilo, D.; Lyatuu, E.; Msuta, G. Evaluating the impact of heat stress as measured by temperature-humidity index (THI) on test-day milk yield of small holder dairy cattle in a sub-Sahara African climate. Livest. Sci. 2020, 242, 104314. [Google Scholar] [CrossRef]
- Mashaly, M.M.; Hendricks, G.L.; Kalama, M.A.; Gehad, A.E.; Abbas, A.O.; Patterson, P.H. Effect of heat stress on production parameters and immune responses of commercial laying hens. Poult. Sci. 2004, 83, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Quinteiro-Filho, W.M.; Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Sakai, M.; Sá, L.R.M.; Ferreira, A.J.P.; Palermo-Neto, J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010, 89, 1905–1914. [Google Scholar] [CrossRef] [PubMed]
- Barrett, N.W.; Rowland, K.; Schmidt, C.J.; Lamont, S.J.; Rothschild, M.F.; Ashwell, C.M.; Persia, M.E. Effects of acute and chronic heat stress on the performance, egg quality, body temperature, and blood gas parameters of laying hens. Poult. Sci. 2019, 98, 6684–6692. [Google Scholar] [CrossRef] [PubMed]
- Borzouie, S.; Rathgeber, B.M.; Stupart, C.M.; Macisaac, J.; Maclaren, L.A. Effects of Dietary Inclusion of Seaweed, Heat Stress and Genetic Strain on Performance, Plasma Biochemical and Hematological Parameters in Laying Hens. Animals 2020, 10, 1570. [Google Scholar] [CrossRef]
- Kim, D.; Lee, Y.; Lee, S.; Lee, K. Impact of relative humidity on the laying performance, egg quality, and physiological stress responses of laying hens exposed to high ambient temperature. J. Therm. Biol. 2022, 103, 103167. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, K.Z.; Beck, M.M.; Scheideler, S.E.; Forman, M.F.; Anderson, K.P.; Kachman, S.D. Acute high environmental temperature and calcium-estrogen relationships in the hen. Poult. Sci. 1996, 75, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Allahverdi, A.; Feizi, A.; Takhtfooladi, H.A.; Nikpiran, H. Effects of heat stress on acid-base imbalance, plasma calcium concentration, egg production and egg quality in commercial layers. Glob. Vet. 2013, 10, 203–207. [Google Scholar]
- Moller, A.P. The allometry of number of feathers in birds changes seasonally. Avian Res. 2015, 6, 2. [Google Scholar] [CrossRef]
- Deeb, N.; Cahaner, A. Genotype-by-environment interaction with broiler genotypes differing in growth rate. 3. Growth rate and water consumption of broiler progeny from weight-selected versus nonselected parents under normal and high ambient temperatures. Poult. Sci. 2002, 81, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.S.; Sajjanar, B.; Lonkar, V.D.; Kurade, N.P.; Kadam, A.S.; Nirmal, A.V.; Brahmane, M.P.; Bal, S.K. Assessing and mitigating the impact of heat stress in poultry. Adv. Anim. Vet. Sci. 2016, 4, 332–341. [Google Scholar] [CrossRef]
- Tao, X.; Xin, H. Acute synergistic effects of air temperature, humidity, and velocity on homeostasis of market-size broilers. Trans. Am. Soc. Agric. Eng. 2003, 46, 491–497. [Google Scholar]
- Igbokwe, N.A.R. Effects of Environmental Heat Stress on Reproduction and its Management in Chickens. Niger. Vet. J. 2018, 39, 101–114. [Google Scholar] [CrossRef]
- Borges, S.A.; Fischer da Silva, A.V.; Majorka, A.; Hooge, D.M.; Cummings, K.R. Physiological responses of broiler chickens to heat stress and dietary electrolyte balance (sodium plus potassium minus chloride, milliequivalents per kilogram). Poult. Sci. 2004, 83, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Hy-Line, Int. Understanding Heat Stress in Layers: Management Tips to Improve Hot Weather Flock Performance; Hy-Line, Int.: West Dse Moines, IA, USA, 2016; pp. 1–8. Available online: www.hyline.com (accessed on 20 October 2019).
- Belay, T.; Teeter, R.G. Broiler water balance and thermohalance during Thermoneutral and high ambient temperature exposure. Poult. Sci. J. 1993, 72, 116–124. [Google Scholar] [CrossRef]
- Teeter, R.G.; Smith, M.O.; Owens, F.N.; Arp, S.C.; Sangiah, S.; Breazile, J.E. Chronic heat stress and respiratory alkalosis: Occurrence and treatment in broiler chicks. Poult. Sci. 1985, 64, 1060–1064. [Google Scholar] [CrossRef]
- Ahmad, T.; Sarwar, M. Dietary electrolyte balance: Implications in heat stressed broilers. World’s Poult. Sci. J. 2006, 62, 638–653. [Google Scholar]
- Toyomizu, M.; Tokuda, M.; Mujahid, A.; Akiba, Y. Progressive Alteration to Core Temperature, Respiration and Blood Acid-Base Balance in Broiler Chickens Exposed to Acute Heat Stress. J. Poult. Sci. 2005, 42, 110–118. [Google Scholar] [CrossRef]
- Saki, A.A.; Maleckey, M.; Johari, R.; Goudarzi, S.M.; Abdolmaleki, M. The effects of protein, amino acid, and dietary electrolyte balance on broiler chicken performance and blood parameters under heat stress. Acta Sci.-Anim. Sci. 2016, 38, 285–292. [Google Scholar] [CrossRef]
- Gamba, J.P.; Rodrigues, M.M.; Garcia Neto, M.; Perri, S.H.V.; Faria Júnior, M.D.A.; Pinto, M.F. The strategic application of electrolyte balance to minimize heat stress in broilers. Braz. J. Poult. Sci. 2015, 17, 237–245. [Google Scholar] [CrossRef]
- Khan, R.U.; Naz, S.; Ullah, H.; Ullah, Q.; Laudadio, V.; Qudratullah, B.G.; Tufarelli, V. Physiological dynamics in broiler chickens under heat stress and possible mitigation strategies. Anim. Biotechnol. 2021, 34, 438–447. [Google Scholar] [CrossRef]
- Abu-Dieyeh, Z.H.M. Effect of chronic heat stress and long-term feed festriction on broiler performance. Int. J. Poult. Sci. 2006, 5, 185–190. [Google Scholar]
- Elnagar, S.A.; Scheideler, S.E.; Beck, M.M. Reproductive hormones, hepatic deiodinase messenger ribonucleic acid, and vasoactive intestinal polypeptide-immunoreactive cells in hypothalamus in the heat stress-induced or chemically induced hypothyroid laying hen. Poult. Sci. 2010, 89, 2001–2009. [Google Scholar] [CrossRef] [PubMed]
- Razuki, W.M.; Farhan, S.H.; Jasim, F.H.; AlKhilani, F.M.; Jameel, F.R. Effects of genetic strain and protein concentrate removal from finisher ration on performance and carcass parameters of broilers reared under hot climate. Int. J. Poult. Sci. 2015, 14, 92. [Google Scholar] [CrossRef]
- Nathan, D.B.; Heller, E.D.; Perek, M. The effect of short heat stress upon leucocyte count, plasma corticosterone level, plasma and leukocyte ascorbic acid content. Br. Poult. Sci. 1976, 17, 481–485. [Google Scholar] [CrossRef]
- Halliwell, B.; Whiteman, M. Measuring reactive species and oxidative damage in vivo and in cell culture: How should you do it and what do the results mean. Br. J. Pharmacol. 2004, 142, 231–255. [Google Scholar] [CrossRef] [PubMed]
- Akbarian, A.; Michiels, J.; Degroote, J.; Majdeddin, M.; Golian, A.; De Smet, S. Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. J. Anim. Sci. Biotechnol. 2016, 7, 37. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Halliwell, B.; Lunt, G.G.; Davies, K.J. Oxidative stress: The paradox of aerobic life. Biochem. Soc. Symp. 1995, 61, 1–31. [Google Scholar] [CrossRef]
- Zulovich, J.M.; DeShazer, J.A. Estimating Egg Production Declines at High Environmental Temperatures and Humidities; Paper; American Society of Agricultural Engineers: St. Joseph, MI, USA, 1990; No. 90-4021. [Google Scholar]
- Ataallahi, M.; Ghassemi Nejad, J.; Song, J.I.; Kim, J.S.; Park, K.H. Effects of feather processing methods on quantity of extracted corticoste-rone in broiler chickens. J. Anim. Sci. Technol. Short Commun. 2020, 62, 884–892. [Google Scholar] [CrossRef]
- Ma, X.; Lin, Y.; Zhang, H.; Chen, W.; Wang, S.; Ruan, D.; Jiang, Z. Heat stress impairs the nutritional metabolism and reduces the productivity of egg-laying ducks. Anim. Reprod. Sci. 2014, 145, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Reece, F.N.; Deaton, J.W. Use of Evaporative Cooling for Broiler Chicken Production in Areas of High Relative Humidity. Poult. Sci. 1970, 50, 100–104. [Google Scholar] [CrossRef]
- Winn, P.N.; Godfrey, E.F. The effect of humidity on growth and feed conversion of broiler chickens. Int. J. Biometeorol. 1967, 11, 39–50. [Google Scholar] [CrossRef]
- Spiers, D.E. Physiological basics of temperature regulation in domestic animals. In Environmental Physiology of Livestock; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 17–34. [Google Scholar]
- He, S.P.; Arowolo, M.A.; Medrano, R.F.; Li, S.; Yu, Q.F.; Chen, J.Y.; He, J.H. Impact of heat stress and nutritional interventions on poultry production. World’s Poult. Sci. J. 2019, 74, 647–664. [Google Scholar] [CrossRef]
- Liu, J.B.; Yan, H.L.; Liao, Y.P.; Xie, Z.J.; Yin, Y.L. Effects of feed intake level on the additivity of apparent and standardized ileal digestibility of amino acids in diets for growing pigs. Anim. Feed. Sci. Technol. 2020, 266, 114525. [Google Scholar] [CrossRef]
- Lin, H.; Jiao, H.C.; Buyse, J.; Decuypere, E. Strategies for preventing heat stress in poultry. World’s Poult. Sci. J. 2006, 62, 71–86. [Google Scholar] [CrossRef]
- Yahav, S.; Shinder, D.; Razpakovski, V.; Rusal, M.; Bar, A. Lack of response of laying hens to relative humidity at high ambient temperature. Br. Poult. Sci. 2000, 41, 660–663. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Lu, Y.; Lan, R. Chitosan oligosaccharide as an effective feed additive to maintain growth performance, meat quality, muscle glycolytic metabolism, and oxidative status in yellow-feather broilers under heat stress. Poult. Sci. 2020, 99, 4824–4831. [Google Scholar] [CrossRef]
- Rostagno, M.H.; North, P.; Llc, A. Effects of heat stress on the gut health of poultry. J. Anim. Sci. 2020, 98, skaa090. [Google Scholar] [CrossRef]
- El Hadi, H.; Sykes, A.H. Thermal panting and respiratory alkalosis in the laying hen. Br. Poult. Sci. 1982, 23, 49–57. [Google Scholar] [CrossRef]
- Vandana, G.D.; Sejian, V. Heat stress and poultry production: Impact and amelioration. Int. J. Biometeorol. 2021, 65, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Khone, H.J.; Jones, J.G. Changes in plasma electrolytes acid-base balance and other physiological parameters of adult female turkeys under conditions of acute hyperthermia. Poult. Sci. J. 1975, 54, 2034–2038. [Google Scholar] [CrossRef] [PubMed]
- Bortolotti, G.R.; Marchant, T.A.; Blas, J.; German, T. Corticosterone in feathers is a long-term, integrated measure of avian stress physiology. Funct. Ecol. 2008, 22, 494–500. [Google Scholar] [CrossRef]
- Freeman, N.E.; Newman, A.E. Quantifying corticosterone in feathers: Validations for an emerging technique. Conserv. Physiol. 2018, 6, coy051. [Google Scholar] [CrossRef] [PubMed]
- Carbajal, A.; Tallo-Parra, O.; Sabes-Alsina, M.; Mular, I.; Lopez-Bejar, M. Feather corticosterone evaluated by ELISA in broilers: A potential tool to evaluate broiler welfare. Poult. Sci. 2014, 93, 2884–2886. [Google Scholar] [CrossRef] [PubMed]
- Weimer, S.L.; Wideman, R.F.; Scanes, C.G.; Mauromoustakos, A.; Christensen, K.D.; Vizzier-Thaxton, Y. An evaluation of methods for measuring stress in broiler chickens. Poult. Sci. 2018, 97, 3381–3389. [Google Scholar] [CrossRef] [PubMed]
- Häffelin, K.E.; Lindenwald, R.; Kaufmann, F.; Döhring, S.; Spindler, B.; Preisinger, R.; Rautenschlein, S.; Kemper, N.; Andersson, R. Corticosterone in feathers of laying hens: An assay validation for evidence-based assessment of animal welfare. Poult. Sci. 2020, 99, 4685–4694. [Google Scholar] [CrossRef] [PubMed]
- Adamkova, M.; Bilkova, Z.; Tomasek, O.; Simek, Z.; Albrecht, T. Feather steroid hormone concentrations in relation to age, sex, and molting time in a long-distance migratory passerine. Ecol. Evol. 2019, 9, 9018–9026. [Google Scholar] [CrossRef]
| Item | Treatment 1 | SEM 2 | p-Value | ||||
|---|---|---|---|---|---|---|---|
| THI 68 | THI 72 | THI 78 | THI 81 | THI 85 | |||
| Egg production (%) | |||||||
| Day 7 | 99.38 | 97.4 | 97.51 | 96.89 | 94.4 | 1.48 | 0.222 |
| Day 14 | 97.51 a | 98.76 a | 98.81 a | 96.89 a | 92.85 b | 1.28 | 0.010 |
| Day 21 | 96.27 a | 98.05 a | 98.81 a | 95.65 a | 85.70 b | 1.56 | <0.001 |
| Day 28 | 98.70 a | 96.89 a | 95.83 a | 95.03 a | 88.30 b | 1.79 | 0.002 |
| Feed intake (g/day/bird) | |||||||
| Day 7 | 129.5 a | 125.2 a | 120.3 ab | 110.6 b | 88.3 c | 4.26 | <0.001 |
| Day 14 | 127.5 a | 123.2 a | 119.0 a | 101.7 b | 91.2 c | 2.86 | <0.001 |
| Day 21 | 127.2 a | 118.2 ab | 115.4 b | 108.4 b | 81.4 c | 3.45 | <0.001 |
| Day 28 | 121.2 a | 122.0 a | 118.3 a | 114.2 a | 95.1 b | 3.12 | <0.001 |
| Egg weight (g) | |||||||
| Day 7 | 58.0 a | 55.7 b | 55.5 b | 56.1 ab | 52.0 c | 0.68 | <0.001 |
| Day 14 | 57.8 a | 57.8 a | 57.2 a | 55.4 a | 48.9 b | 0.89 | <0.001 |
| Day 21 | 59.1 a | 56.7 b | 57.3 ab | 56.5 b | 50.1 c | 0.78 | <0.001 |
| Day 28 | 59.7 a | 57.3 ab | 55.8 bc | 53.6 c | 48.1 c | 0.87 | <0.001 |
| Feed conversion ratio (g/g) | |||||||
| Day 7 | 2.40 a | 2.33 a | 2.08 b | 2.08 b | 1.78 c | 0.069 | <0.001 |
| Day 14 | 2.31 a | 2.23 ab | 2.06 bc | 1.82 d | 1.88 cd | 0.069 | <0.001 |
| Day 21 | 2.26 | 2.12 | 2.06 | 2.00 | 1.64 | 0.116 | 0.337 |
| Day 28 | 2.19 | 2.23 | 2.18 | 2.07 | 2.12 | 0.061 | 0.353 |
| Body weight gain (g/day/bird) | |||||||
| Day 0 to 7 | 2.57 ab | 4.14 a | 2.43 ab | 3.94 b | 22.56 c | 2.48 | <0.001 |
| Day 7 to 14 | 7.07 a | 0.99 b | 0.27 b | 0.53 a | 8.68 c | 1.91 | 0.001 |
| Day 14 to 21 | 4.32 a | 1.95 ab | 3.27 b | 0.44 ab | 12.57 c | 2.27 | 0.003 |
| Day 21 to 28 | 2.05 | 4.39 | −0.37 | 0.76 | 2.40 | 1.75 | 0.097 |
| Day 0 to 28 | 15.62 a | 8.49 ab | 0.94 bc | 5.67 c | 46.21 d | 4.05 | <0.001 |
| Items | Treatment 1 | SEM 2 | p-Value | ||||
|---|---|---|---|---|---|---|---|
| THI 68 | THI 72 | THI 78 | THI 81 | THI 85 | |||
| Water consumption (mL/day/bird) | |||||||
| d7 | 202.1 b | 210.7 b | 308.8 a | 270.0 ab | 365.0 a | 30.84 | 0.005 |
| d14 | 207.1 b | 164.3 b | 353.8 a | 270.6 ab | 325.4 a | 36.26 | 0.006 |
| d21 | 202.1 c | 232.9 bc | 327.1 a | 293.8 ab | 283.1 ab | 27.07 | 0.027 |
| d28 | 192.9 b | 190.0 b | 263.8 ab | 205.0 ab | 285.0 a | 26.35 | 0.049 |
| Respiration rate (breaths/min) | |||||||
| d0 | 46.44 | 45.11 | 43.78 | 45.80 | 46.44 | 2.312 | 0.918 |
| d7 | 48.44 | 46.40 | 48.40 | 47.40 | 48.29 | 3.060 | 0.988 |
| d14 | 44.22 | 51.60 | 55.20 | 43.20 | 51.50 | 3.385 | 0.077 |
| d21 | 50.89 | 49.40 | 64.80 | 53.80 | 58.44 | 4.977 | 0.205 |
| d28 | 48.67 | 45.00 | 53.40 | 51.60 | 61.75 | 5.776 | 0.405 |
| Rectal temperature (°C) | |||||||
| d0 | 41.50 | 41.55 | 41.56 | 41.52 | 41.49 | 0.094 | 0.351 |
| d7 | 41.61 | 41.54 | 41.85 | 41.79 | 41.80 | 0.083 | 0.050 |
| d14 | 41.52 b | 41.61 b | 41.80 ab | 41.6 b | 41.99 a | 0.105 | 0.031 |
| d21 | 41.56 | 41.59 | 41.75 | 41.72 | 41.68 | 0.076 | 0.340 |
| d28 | 41.49 b | 41.60 b | 41.58 b | 41.68 b | 41.90 a | 0.065 | 0.002 |
| Item | Treatment 1 | SEM 2 | p-Value | ||||
|---|---|---|---|---|---|---|---|
| THI 68 | THI 72 | THI 78 | THI 81 | THI 85 | |||
| Haugh unit | |||||||
| d7 | 99.29 a | 98.76 a | 97.13 a | 99.01 a | 92.09 b | 1.001 | 0.002 |
| d14 | 100.2 a | 101.6 a | 100.14 a | 99.27 a | 93.59 b | 1.345 | 0.003 |
| d21 | 99.69 a | 100.5 a | 97.92 ab | 101.1 a | 95.78 b | 1.109 | 0.015 |
| d28 | 94.09 | 92.16 | 92.99 | 94.09 | 93.11 | 1.518 | 0.889 |
| Egg yolk color | |||||||
| d7 | 2.693 ab | 2.725 a | 2.817 a | 2.503 bc | 2.478 c | 0.068 | 0.006 |
| d14 | 3.503 a | 3.197 b | 3.160 b | 3.047 b | 2.593 c | 0.104 | <0.001 |
| d21 | 3.868 a | 3.495 ab | 3.477 ab | 3.222 b | 2.660 c | 0.176 | 0.001 |
| d28 | 3.460 a | 3.003 b | 3.208 ab | 2.918 b | 2.587 c | 0.110 | <0.001 |
| Eggshell thickness (mm) | |||||||
| d7 | 0.388 a | 0.388 a | 0.370 a | 0.347 b | 0.327 c | 0.007 | <0.001 |
| d14 | 0.386 a | 0.378 a | 0.375 a | 0.352 b | 0.317 c | 0.008 | <0.001 |
| d21 | 0.402 a | 0.393 a | 0.368 a | 0.355 bc | 0.333 c | 0.008 | <0.001 |
| d28 | 0.390 a | 0.388 a | 0.365 b | 0.343 c | 0.322 d | 0.007 | <0.001 |
| Eggshell strength (kgf/cm2) | |||||||
| d7 | 39.18 a | 40.49 a | 38.18 a | 32.80 b | 32.23 b | 1.130 | <0.001 |
| d14 | 41.53 a | 41.13 a | 39.22 a | 37.51 a | 30.41 b | 1.623 | 0.001 |
| d21 | 43.29 ab | 44.01 a | 41.26 ab | 38.64 b | 35.33 c | 1.522 | 0.003 |
| d28 | 41.98 a | 42.21 a | 41.15 a | 38.19 ab | 34.18 b | 1.435 | 0.003 |
| Items | Treatment 1 | SEM 2 | p-Value | ||||
|---|---|---|---|---|---|---|---|
| THI 68 | THI 72 | THI 78 | THI 81 | THI 85 | |||
| Na (mmol/L) | |||||||
| d14 | 159.5 | 166.5 | 162.5 | 171.0 | 163.3 | 4.166 | 0.426 |
| d28 | 192.9 b | 190.0 b | 263.8 ab | 205.0 ab | 285.0 a | 26.35 | 0.049 |
| K (mmol/L) | |||||||
| d14 | 4.500 b | 4.733 b | 4.925 ab | 5.438 a | 4.943 ab | 0.177 | 0.021 |
| d28 | 4.163 c | 4.400 bc | 4.600 b | 4.638 b | 5.038 a | 0.130 | <0.001 |
| Cl (mmol/L) | |||||||
| d14 | 123.8 | 126.3 | 124.8 | 130.5 | 126.1 | 3.099 | 0.639 |
| d28 | 115.1 | 115.6 | 114.4 | 115.5 | 114.0 | 0.829 | 0.575 |
| Items | Treatment 1 | SEM 3 | p-Value | ||||
|---|---|---|---|---|---|---|---|
| THI 68 | THI 72 | THI 78 | THI 81 | THI 85 | |||
| Feather length(mm) | 126.5 | 139.4 | 133.6 | 137.4 | 139.5 | 3.132 | 0.2109 |
| FCC 2 (pg/mm) | 2.626 | 2.776 | 2.502 | 2.576 | 2.485 | 0.336 | 0.9187 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-R.; Ryu, C.; Lee, S.-D.; Cho, J.-H.; Kang, H. Effects of Heat Stress on the Laying Performance, Egg Quality, and Physiological Response of Laying Hens. Animals 2024, 14, 1076. https://doi.org/10.3390/ani14071076
Kim H-R, Ryu C, Lee S-D, Cho J-H, Kang H. Effects of Heat Stress on the Laying Performance, Egg Quality, and Physiological Response of Laying Hens. Animals. 2024; 14(7):1076. https://doi.org/10.3390/ani14071076
Chicago/Turabian StyleKim, Hye-Ran, Chaehwa Ryu, Sung-Dae Lee, Jin-Ho Cho, and Hwanku Kang. 2024. "Effects of Heat Stress on the Laying Performance, Egg Quality, and Physiological Response of Laying Hens" Animals 14, no. 7: 1076. https://doi.org/10.3390/ani14071076
APA StyleKim, H.-R., Ryu, C., Lee, S.-D., Cho, J.-H., & Kang, H. (2024). Effects of Heat Stress on the Laying Performance, Egg Quality, and Physiological Response of Laying Hens. Animals, 14(7), 1076. https://doi.org/10.3390/ani14071076





