Two Separate Brain Networks for Predicting Trainability and Tracking Training-Related Plasticity in Working Dogs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Dog Training and Preparation
2.2. Longitudinal Experimental Design
2.3. Working Dog Assessments
2.4. Data Acquisition
2.5. Image Preprocessing
2.6. Characterization of Resting-State Brain Networks
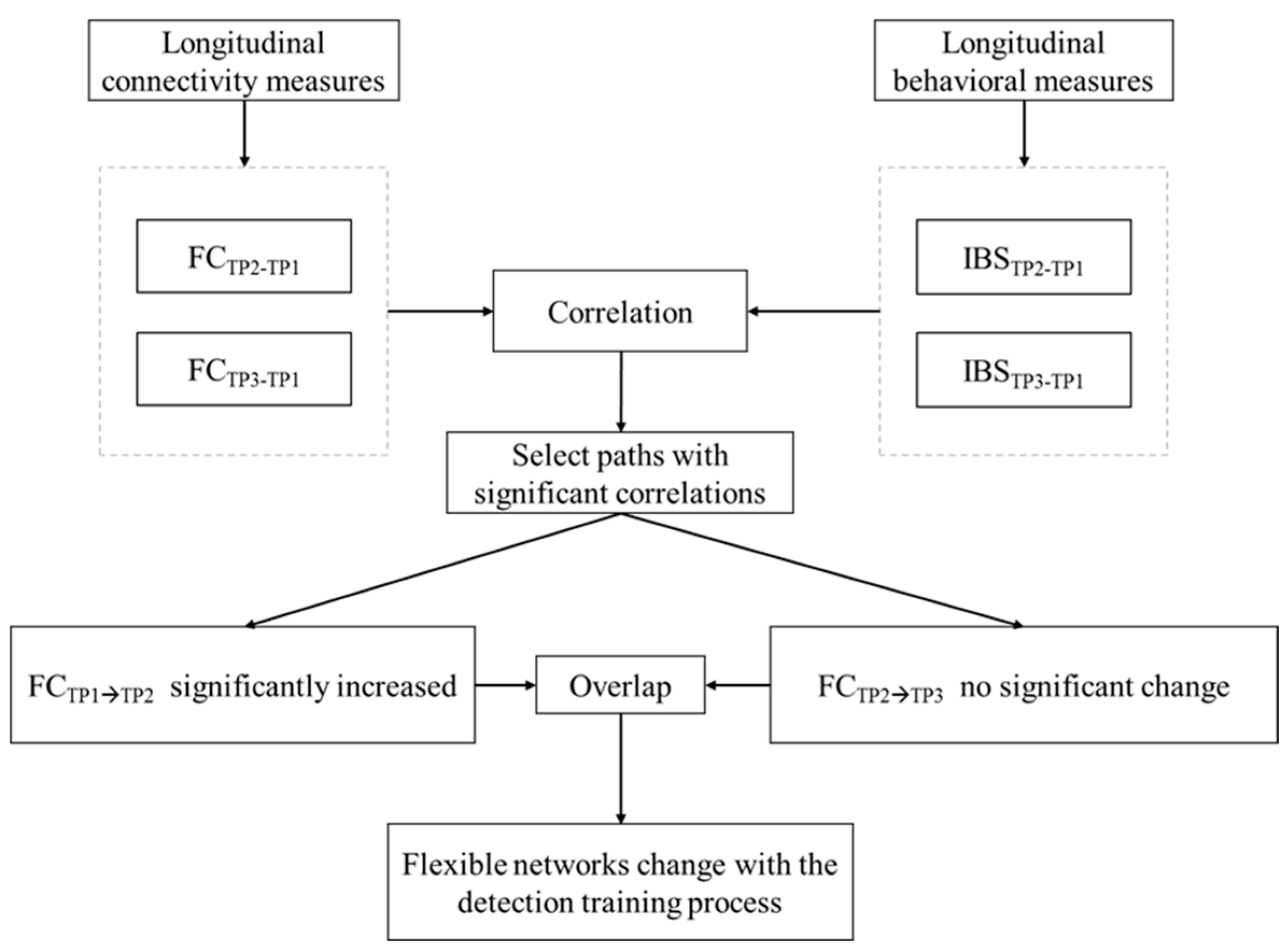
2.7. Connectivity–Behavior Correlations across Timepoints
2.8. Brain Networks Predictive of Dogs’ Suitability for Detection Work
3. Results
3.1. Successful vs. Non-Successful Working Dogs
3.2. Classification Analyses
4. Discussion
4.1. Flexible Periphery Network Underlying Detection Training
4.2. Stable Core Network for Predicting Training Outcomes
4.3. Insights from Human Homology
5. Limitations and Future Research
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miyadera, K.; Acland, G.M.; Aguirre, G.D. Genetic and phenotypic variations of inherited retinal diseases in dogs: The power of within- and across-breed studies. Mamm. Genome 2012, 23, 40–61. [Google Scholar] [CrossRef] [PubMed]
- Sachs-Ericsson, N.; Hansen, N.K.; Fitzgerald, S.G. Benefits of assistance dogs: A review. Rehabil. Psychol. 2002, 47, 251–277. [Google Scholar] [CrossRef]
- Gee, N.R.; Harris, S.L.; Johnson, K.L. The role of therapy dogs in speed and accuracy to complete motor skills tasks for preschool children. Anthrozoos 2007, 20, 375–386. [Google Scholar] [CrossRef]
- Yount, R.A.; Olmert, M.D.; Lee, M.R. Service dog training program for treatment of posttraumatic stress in service members. U.S. Army Med. Dep. J. 2012, 63–69. Available online: http://www.ncbi.nlm.nih.gov/pubmed/22388685 (accessed on 1 April 2012).
- Cobb, M.; Branson, N.; McGreevy, P.; Lill, A.; Bennett, P. The advent of canine performance science: Offering a sustainable future for working dogs. Behav. Process. 2015, 110, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Slabbert, J.M.; Odendaal, J.S.J. Early prediction of adult police dog efficiency-A longitudinal study. Appl. Anim. Behav. Sci. 1999, 64, 269–288. [Google Scholar] [CrossRef]
- Berns, G.S.; Cook, P.F. Why Did the Dog Walk Into the MRI? Curr. Dir. Psychol. Sci. 2016, 25, 363–369. [Google Scholar] [CrossRef]
- Thompkins, A.M.; Deshpande, G.; Waggoner, P.; Katz, J.S. Functional Magnetic Resonance Imaging of the Domestic Dog: Research, Methodology, and Conceptual Issues. Comp. Cogn. Behav. Rev. 2016, 11, 63–82. [Google Scholar] [CrossRef][Green Version]
- Biswal, B.; Yetkin, F.Z.; Haughton, V.M.; Hyde, J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar. Magn. Reson. Med. 1995, 34, 537–541. [Google Scholar] [CrossRef]
- Buckner, R.L.; Andrews-Hanna, J.R.; Schacter, D.L. The brain’s default network: Anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef]
- Gilbert, D.T.; Wilson, T.D. Prospection: Experiencing the future. Science 2007, 317, 1351–1354. [Google Scholar] [CrossRef] [PubMed]
- Hampson, M.; Driesen, N.R.; Skudlarski, P.; Gore, J.C.; Constable, R.T. Brain connectivity related to working memory performance. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 13338–13343. [Google Scholar] [CrossRef] [PubMed]
- Leech, R.; Kamourieh, S.; Beckmann, C.F.; Sharp, D.J. Fractionating the default mode network: Distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 3217–3224. [Google Scholar] [CrossRef] [PubMed]
- Seeley, W.W.; Menon, V.; Schatzberg, A.F.; Keller, J.; Glover, G.H.; Kenna, H.; Reiss, A.L.; Greicius, M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Szabó, D.; Czeibert, K.; Kettinger, Á.; Gácsi, M.; Andics, A.; Miklósi, Á.; Kubinyi, E. Resting-state fMRI data of awake dogs (Canis familiaris) via group-level independent component analysis reveal multiple, spatially distributed resting-state networks. Sci. Rep. 2019, 9, 15270. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, K.M.; Wang-Leandro, A.; Dennler, M.; Carrera, I.; Richter, H.; Bektas, R.N.; Steiner, A.; Haller, S. Resting state networks of the canine brain under sevoflurane anaesthesia. PLoS ONE 2020, 15, e0231955. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, F.; Dillenburger, B.C.; Friedman, R.M.; Chen, L.M.; Gore, J.C.; Avison, M.J.; Roe, A.W. Functional magnetic resonance imaging of awake monkeys: Some approaches for improving imaging quality. Magn. Reson. Imaging 2012, 30, 36–47. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kulkarni, P.; Stolberg, T.; Sullivan, J.M.; Ferris, C.F. Imaging evolutionarily conserved neural networks: Preferential activation of the olfactory system by food-related odor. Behav. Brain Res. 2012, 230, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zuo, Y.; Gu, H.; Waltz, J.A.; Zhan, W.; Scholl, C.A.; Rea, W.; Yang, Y.; Stein, E.A. Synchronized delta oscillations correlate with the resting-state functional MRI signal. Proc. Natl. Acad. Sci. USA 2007, 104, 18265–18269. [Google Scholar] [CrossRef]
- Wang, K.; Van Meer, M.P.A.; Van Der Marel, K.; Van Der Toorn, A.; Xu, L.; Liu, Y.; Viergever, M.A.; Jiang, T.; Dijkhuizen, R.M. Temporal scaling properties and spatial synchronization of spontaneous blood oxygenation level-dependent (BOLD) signal fluctuations in rat sensorimotor network at different levels of isoflurane anesthesia. NMR Biomed. 2011, 24, 61–67. [Google Scholar] [CrossRef]
- Jia, H.; Pustovyy, O.M.; Waggoner, P.; Beyers, R.J.; Schumacher, J.; Wildey, C.; Barrett, J.; Morrison, E.; Salibi, N.; Denney, T.S.; et al. Functional MRI of the olfactory system in conscious dogs. PLoS ONE 2014, 9, e86362. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Pustovyy, O.M.; Wang, Y.; Waggoner, P.; Beyers, R.J.; Schumacher, J.; Wildey, C.; Morrison, E.; Salibi, N.; Denney, T.S.; et al. Enhancement of odor-induced activity in the canine brain by zinc nanoparticles: A functional MRI study in fully unrestrained conscious dogs. Chem. Sens. 2016, 41, 53–67. [Google Scholar] [CrossRef]
- Kyathanahally, S.P.; Jia, H.; Pustovyy, O.M.; Waggoner, P.; Beyers, R.; Schumacher, J.; Barrett, J.; Morrison, E.E.; Salibi, N.; Denney, T.S.; et al. Anterior-posterior dissociation of the default mode network in dogs. Brain Struct. Funct. 2015, 220, 1063–1076. [Google Scholar] [CrossRef] [PubMed]
- Andics, G.A.; Gácsi, M.; Faragó, T.; Szabó, D.; Miklósi, Á.; Nieto-Castanon, A. Neural mechanisms for lexical processing in dogs. Science 2016, 353, 1030–1032. [Google Scholar] [CrossRef] [PubMed]
- Andics, G.M.; Faragó, T.; Kis, A.; Miklósi, Á. Voice-sensitive regions in the dog and human brain are revealed by comparative fMRI. Curr. Biol. 2014, 24, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Berns, G.S.; Brooks, A.M.; Spivak, M. Functional MRI in awake unrestrained dogs. PLoS ONE 2012, 7, e38027. [Google Scholar] [CrossRef] [PubMed]
- Berns, G.S.; Brooks, A.M.; Spivak, M. Scent of the familiar: An fMRI study of canine brain responses to familiar and unfamiliar human and dog odors. Behav. Process. 2015, 110, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Berns, G.S.; Brooks, A.M.; Spivak, M.; Levy, K. Functional Mri in Awake Dogs Predicts Suitability for Assistance Work. Sci. Rep. 2016, 90, 43704. [Google Scholar] [CrossRef] [PubMed]
- Bunford, N.; Andics, A.; Kis, A.; Miklósi, Á.; Gácsi, M. Canis familiaris as model for non-invasive comparative neuroscience. Trends Neurosci. 2017, 40, 438–452. [Google Scholar] [CrossRef]
- Cook, P.F.; Prichard, A.; Spivak, M.; Berns, G.S. Awake Canine fMRI Predicts Dogs’ Preference for Praise Versus Food. Soc. Cogn. Affect. Neurosci. 2016, 11, 1853–1862. [Google Scholar] [CrossRef]
- Cook, P.F.; Spivak, M.; Berns, G. Neurobehavioral evidence for individual differences in canine cognitive control: An awake fMRI study. Anim. Cogn. 2016, 19, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Cuaya, L.V.; Hernández-Pérez, R.; Concha, L. Our faces in the dog’s brain: Functional imaging reveals temporal cortex activation during perception of human faces. PLoS ONE 2016, 11, e0149431. [Google Scholar] [CrossRef] [PubMed]
- Dilks, D.D.; Cook, P.; Weiller, S.K.; Berns, H.P.; Spivak, M.; Berns, G.S. Awake fMRI reveals a specialized region in dog temporal cortex for face processing. PeerJ 2015, 3, e1115. [Google Scholar] [CrossRef] [PubMed]
- Huber, L.; Lamm, C. Understanding dog cognition by functional magnetic resonance imaging. Learn. Behav. 2017, 45, 101–102. [Google Scholar] [CrossRef] [PubMed]
- Ramaihgari, B.; Pustovyy, O.M.; Waggoner, P.; Beyers, R.J.; Wildey, C.; Morrison, E.; Salibi, N.; Katz, J.S.; Denney, T.S.; Vodyanoy, V.J.; et al. Zinc nanoparticles enhance brain connectivity in the canine olfactory network: Evidence from an fMRI study in unretsrained awake dogs. Front. Vet. Med. 2018, 5, 127. [Google Scholar] [CrossRef] [PubMed]
- Thompson, T.W.; Waskom, M.L.; Gabrieli, J.D. Intensive Working Memory Training Produces Functional Changes in Large-scale Frontoparietal Networks. J. Cogn. Neurosci. 2016, 28, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Thompkins, A.M.; Lazarowski, L.; Ramaiahgari, B.; Gotoor, S.S.R.; Waggoner, P.; Denney, T.S.; Deshpande, G.; Katz, J.S. Dog–human social relationship: Representation of human face familiarity and emotions in the dog brain. Anim. Cogn. 2021, 24, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Rocznik, D.; Sinn, D.L.; Thomas, S.; Gosling, S.D. Criterion analysis and content validity for standardized behavioral tests in a detector-dog breeding program. J. Forensic Sci. 2015, 60, S213–S221. [Google Scholar] [CrossRef]
- Sinn, D.L.; Gosling, S.D.; Hilliard, S. Personality and performance in military working dogs: Reliability and predictive validity of behavioral tests. Appl. Anim. Behav. Sci. 2010, 127, 51–65. [Google Scholar] [CrossRef]
- Power, J.D.; Barnes, K.A.; Snyder, A.Z.; Schlaggar, B.L.; Petersen, S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 2012, 59, 2142–2154. [Google Scholar] [CrossRef]
- Chao-Gan, Y.; Yu-Feng, Z. DPARSF: AMATLABToolbox for “Pipeline” Data Analysis of Resting-State fMRI. Front. Syst. Neurosci. 2010, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.J.; Holmes, A.P.; Worsley, K.J.; Poline, J.P.; Frith, C.D.; Frackowiak, R.S. Statistical parametric mapping in functional imaging: A general linear approach. Hum. Brain Mapp. 1995, 2, 189–210. [Google Scholar] [CrossRef]
- Bluhm, R.; Williamson, P.; Lanius, R.; Théberge, J.; Densmore, M.; Bartha, R.; Osuch, E. Resting state default-mode network connectivity in early depression using a seed region-of-interest analysis: Decreased connectivity with caudate nucleus. Psychiatry Clin. Neurosci. 2009, 63, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Fair, D.A.; Cohen, A.L.; Dosenbach, N.U.F.; Church, J.A.; Miezin, F.M.; Barch, D.M.; Raichle, M.E.; Petersen, S.E.; Schlaggar, B.L. The maturing architecture of the brain’s default network. Proc. Natl. Acad. Sci. USA 2008, 105, 4028–4032. [Google Scholar] [CrossRef] [PubMed]
- Sheline, Y.I.; Raichle, M.E.; Snyder, A.Z.; Morris, J.C.; Head, D.; Wang, S.; Mintun, M.A. Amyloid Plaques Disrupt Resting State Default Mode Network Connectivity in Cognitively Normal Elderly. Biol. Psychiatry 2010, 67, 584–587. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.D.; Raichle, M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007, 8, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Raichle, M.E.; MacLeod, A.M.; Snyder, A.Z.; Powers, W.J.; Gusnard, D.A.; Shulman, G.L. A default mode of brain function. Proc. Natl. Acad. Sci. USA 2001, 98, 676–682. [Google Scholar] [CrossRef] [PubMed]
- van den Heuvel, M.P.; Stam, C.J.; Boersma, M.; Hulshoff Pol, H.E. Small-world and scale-free organization of voxel-based resting-state functional connectivity in the human brain. NeuroImage 2008, 43, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.R. The regression analysis of binary sequences (with discussion). J. R. Stat. Soc. Ser. B 1958, 20, 215–242. [Google Scholar]
- Patronek, G.J.; Waters, D.J.; Glickman, L.T. Comparative longevity of pet dpgs and humans: Implication for gerontology research. J. Gerontol. Biol. Sci. 1997, 52A, B171–B178. [Google Scholar] [CrossRef]
- Smith, S.M.; Andersson, J.; Auerbach, E.J.; Beckmann, C.F.; Bijsterbosch, J.; Douaud, G.; Duff, E.; Feinberg, D.A.; Griffanti, L.; Harms, M.P.; et al. Resting-state fMRI in the Human Connectome Project. NeuroImage 2013, 80, 144–168. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, A. Domestic Dog Cognition and Behavior: The Scientific Study of Canis familiaris; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Xia, M.; Wang, J.; He, Y. BrainNet Viewer: A Network Visualization Tool for Human Brain Connectomics. PLoS ONE 2013, 8, e68910. [Google Scholar] [CrossRef] [PubMed]
- Bassett, W.N.F.; Porter, M.A.; Mucha, P.J.; Carlson, J.M.; Grafton, S.T. Dynamic reconfiguration of human brain networks during learning. Proc. Natl. Acad. Sci. USA 2011, 108, 7641–7646. [Google Scholar] [CrossRef] [PubMed]
- Bassett, D.S.; Wymbs, N.F.; Rombach, M.P.; Porter, M.A.; Mucha, P.J.; Grafton, S.T. Task-Based Core-Periphery Organization of Human Brain Dynamics. PLoS Comput. Biol. 2013, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Braun, U.; Schäfer, A.; Walter, H.; Erk, S.; Romanczuk-Seiferth, N.; Haddad, L.; Bassett, D.S. Dynamic reconfiguration of frontal brain networks during executive cognition in humans. Proc. Natl. Acad. Sci. USA 2015, 112, 11678–11683. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.R.; Mattar, M.G.; Blank, I.A.; Fedorenko, E.; Bassett, D.S. Functional Network Dynamics of the Language System. Cereb. Cortex 2016, 26, 4148–4159. [Google Scholar] [CrossRef] [PubMed]
- Ekman, M.; Derrfuss, J.; Tittgemeyer, M.; Fiebach, C.J. Predicting errors from reconfiguration patterns in human brain networks. Proc. Natl. Acad. Sci. USA 2012, 109, 16714–16719. [Google Scholar] [CrossRef] [PubMed]
- Gollo, L.L.; Roberts, J.A.; Cocchi, L. Mapping how local perturbations influence systems-level brain dynamics. NeuroImage 2017, 160, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Poellinger, A.; Thomas, R.; Lio, P.; Lee, A.; Makris, N.; Rosen, B.R.; Kwong, K.K. Activation and habituation in olfaction—An fMRI study. NeuroImage 2001, 13, 547–560. [Google Scholar] [CrossRef]
- Savic, I.; Gulyas, B.; Larsson, M.; Roland, P. Olfactory functions are mediated by parallel and hierarchical processing. Neuron 2000, 26, 735–745. [Google Scholar] [CrossRef]
- Vedaei, F.; Oghabian, M.A.; Firouznia, K.; Harirchian, M.H.; Lotfi, Y.; Fakhri, M. The Human Olfactory System: Cortical Brain Mapping Using fMRI. Iran. J. Radiol. 2016, in press. [Google Scholar] [CrossRef]
- Kollndorfer, K.; Fischmeister, F.P.S.; Kowalczyk, K.; Hoche, E.; Mueller, C.A.; Trattnig, S.; Schöpf, V. Olfactory training induces changes in regional functional connectivity in patients with long-term smell loss. NeuroImage Clin. 2015, 9, 401–410. [Google Scholar] [CrossRef]
- Huerta, C.I.; Sarkar, P.R.; Duong, T.Q.; Laird, A.R.; Fox, P.T. Neural bases of food perception: Coordinate-based meta-analyses of neuroimaging studies in multiple modalities. Obesity 2014, 22, 1439–1446. [Google Scholar] [CrossRef]
- Dosenbach, N.U.F.; Fair, D.A.; Cohen, A.L.; Schlaggar, B.L.; Petersen, S.E. A dual-networks architecture of top-down control. Trends Cogn. Sci. 2008, 12, 99–105. [Google Scholar] [CrossRef]
- Markett, S.; Reuter, M.; Montag, C.; Voigt, G.; Lachmann, B.; Rudorf, S.; Elger, C.E.; Weber, B. Assessing the function of the fronto-parietal attention network: Insights from resting-state fMRI and the attentional network test. Hum. Brain Mapp. 2014, 35, 1700–1709. [Google Scholar] [CrossRef]
- Olsson, A.; Ochsner, K.N. The role of social cognition in emotion. Trends Cogn. Sci. 2008, 12, 65–71. [Google Scholar] [CrossRef]
- Ghahremani, M.; Hutchison, R.M.; Menon, R.S.; Everling, S. Frontoparietal Functional Connectivity in the Common Marmoset. Cereb. Cortex 2016, 27, 3890–3905. [Google Scholar] [CrossRef]
- Hearne, L.J.; Mattingley, J.B.; Cocchi, L. Functional brain networks related to individual differences in human intelligence at rest. Sci. Rep. 2016, 6, 32328. [Google Scholar] [CrossRef]
- Song, M.; Zhou, Y.; Li, J.; Liu, Y.; Tian, L.; Yu, C.; Jiang, T. Brain spontaneous functional connectivity and intelligence. NeuroImage 2008, 41, 1168–1176. [Google Scholar] [CrossRef]
- Montaldi, D.; Spencer, T.J.; Roberts, N.; Mayes, A.R. The neural system that mediates familiarity memory. Hippocampus 2006, 16, 504–520. [Google Scholar] [CrossRef]
- Vilberg, K.L.; Rugg, M.D. Dissociation of the neural correlates of recognition memory according to familiarity, recollection, and amount of recollected information. Neuropsychologia 2007, 45, 2216–2225. [Google Scholar] [CrossRef]
- Woodruff, C.C.; Johnson, J.D.; Uncapher, M.R.; Rugg, M.D. Content-specificity of the neural correlates of recollection. Neuropsychologia 2005, 43, 1022–1032. [Google Scholar] [CrossRef]
- Vilberg, K.L.; Rugg, M.D. Memory retrieval and the parietal cortex: A review of evidence from a dual-process perspective. Neuropsychologia 2008, 46, 1787–1799. [Google Scholar] [CrossRef]
- Herregodts, P.; Ebinger, G.; Michotte, Y. Distribution of monoamines in human brain: Evidence for neurochemical heterogeneity in subcortical as well as in cortical areas. Brain Res. 1991, 542, 300–306. [Google Scholar] [CrossRef]
- Sara, S.J. The locus coeruleus and noradrenergic modulation of cognition. Nature Reviews. Neuroscience 2009, 10, 211–223. [Google Scholar] [CrossRef]
- Sara, S.J.; Bouret, S. Orienting and Reorienting: The Locus Coeruleus Mediates Cognition through Arousal. Neuron 2012, 76, 130–141. [Google Scholar] [CrossRef]
- Harley, C.W. Norepinephrine and dopamine as learning signals. Neural Plast. 2004, 11, 191–204. [Google Scholar] [CrossRef]
- Murchison, C.F.; Zhang, X.-Y.; Zhang, W.-P.; Ouyang, M.; Lee, A.; Thomas, S.A. A distinct role for norephinephrine in memory retrieval. Cell 2004, 117, 131–142. [Google Scholar] [CrossRef]
- Guedj, C.; Monfardini, E.; Reynaud, A.J.; Farnè, A.; Meunier, M.; Hadj-Bouziane, F. Boosting Norepinephrine Transmission Triggers Flexible Reconfiguration of Brain Networks at Rest. Cereb. Cortex 2016, 27, 4691–4700. [Google Scholar] [CrossRef]
- Pinsk, M.A.; Arcaro, M.; Weiner, K.S.; Kalkus, J.F.; Inati, S.J.; Gross, C.G.; Kastner, S. Neural representations of faces and body parts in macaque and human cortex: A comparative FMRI study. J. Neurophysiol. 2009, 101, 2581–2600. [Google Scholar] [CrossRef]
- Langers, D.R.; van Dijk, P.; Backes, W.H. Lateralization, connectivity and plasticity in the human central auditory system. Neuroimage 2005, 28, 490–499. [Google Scholar] [CrossRef]
- Siniscalchi, M.; d’Ingeo, S.; Fornelli, S.; Quaranta, A. Lateralized behavior and cardiac activity of dogs in response to human emotional vocalizations. Sci. Rep. 2018, 8, 77. [Google Scholar] [CrossRef]
- Overall, K.L. That Dog Is Smarter Than You Know: Advances in Understanding Canine Learning, Memory, and Cognition. Top. Companion Anim. Med. 2011, 26, 2–9. [Google Scholar] [CrossRef]
- ChaeYoung, L.; ChangHoon, K.; SooAn, S.; DaeSung, S.; JooHyun, K.; ChanKyu, P. The dopamine D4 receptor polymorphism affects the canine fearfulness. Anim. Cells Syst. 2008, 12, 77–83. [Google Scholar] [CrossRef][Green Version]
- Wan, M.; Hejjas, K.; Ronai, Z.; Elek, Z.; Sasvari-Szekely, M.; Champagne, F.A.; Miklósi, Á.; Kubinyi, E. DRD4 and TH gene polymorphisms are associated with activity, impulsivity and inattention in Siberian Husky dogs. Anim. Genet. 2013, 44, 717–727. [Google Scholar] [CrossRef]
- Primus, R.J.; Thurkauf, A.; Xu, J.; Yevich, E.; Mcinerney, S.; Shaw, K.; Tallman, J.F.; Gallager, D.W., II. Localization and Characterization of Dopamine D4 Binding Sites in Rat and Human Brain by Use of the Novel, D4 Receptor-Selective Ligand [3H] NGD 94-1. J. Pharmacol. Exp. Ther. 1997, 282, 1020–1027. [Google Scholar]
- Haruno, M. A Neural Correlate of Reward-Based Behavioral Learning in Caudate Nucleus: A Functional Magnetic Resonance Imaging Study of a Stochastic Decision Task. J. Neurosci. 2004, 24, 1660–1665. [Google Scholar] [CrossRef]
- Haruno, M.; Kawato, M. Heterarchical reinforcement-learning model for integration of multiple cortico-striatal loops: fMRI examination in stimulus-action-reward association learning. Neural Netw. 2006, 19, 1242–1254. [Google Scholar] [CrossRef]
- Cook, P.F.; Spivak, M.; Berns, G.S. One pair of hands is not like another: Caudate BOLD response in dogs depends on signal source and canine temperament. PeerJ 2014, 2, e596. [Google Scholar] [CrossRef]
- Van Essen, D.C.; Dierker, D.L. Surface-based and probabilistic atlases of primate cerebral cortex. Neuron 2007, 56, 209–225. [Google Scholar] [CrossRef]
- Orban, G.A.; Claeys, K.; Nelissen, K.; Smans, R.; Sunaert, S.; Todd, J.T.; Wardak, C.; Durand, J.B.; Vanduffel, W. Mapping the parietal cortex of human and non-human primates. Neuropsychologia 2006, 44, 2647–2667. [Google Scholar] [CrossRef] [PubMed]
- Striedter, G.F. Brain homology and function: An uneasy alliance. Brain Res. Bull. 2002, 57, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Mars, R.B.; Verhagen, L.; Gladwin, T.E.; Neubert, F.X.; Sallet, J.; Rushworth, M.F.S. Comparing brains by matching connectivity profiles. Neurosci. Biobehav. Rev. 2016, 60, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Passingham, R.E.; Stephan, K.E.; Kotter, R. The anatomical basis of functional localization in the cortex. Nat. Rev. Neurosci. 2002, 3, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, R.M.; Gallivan, J.P. Functional coupling between frontoparietal and occipitotemporal pathways during action and perception. Cortex 2016, 98, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Sallet, J.; Mars, R.B.; Noonan, M.P.; Neubert, F.-X.; Jbabdi, S.; O’Reilly, J.X.; Filippini, N.; Thomas, A.G.; Rushworth, M.F. The organization of dorsal frontal cortex in humans and macaques. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 12255–12274. [Google Scholar] [CrossRef]
- Reicher, V.; Bunford, N.; Kis, A.; Carreiro, C.; Csibra, B.; Kratz, L.; Gácsi, M. Developmental features of sleep electrophysiology in family dogs. Sci. Rep. 2021, 11, 22760. [Google Scholar] [CrossRef]



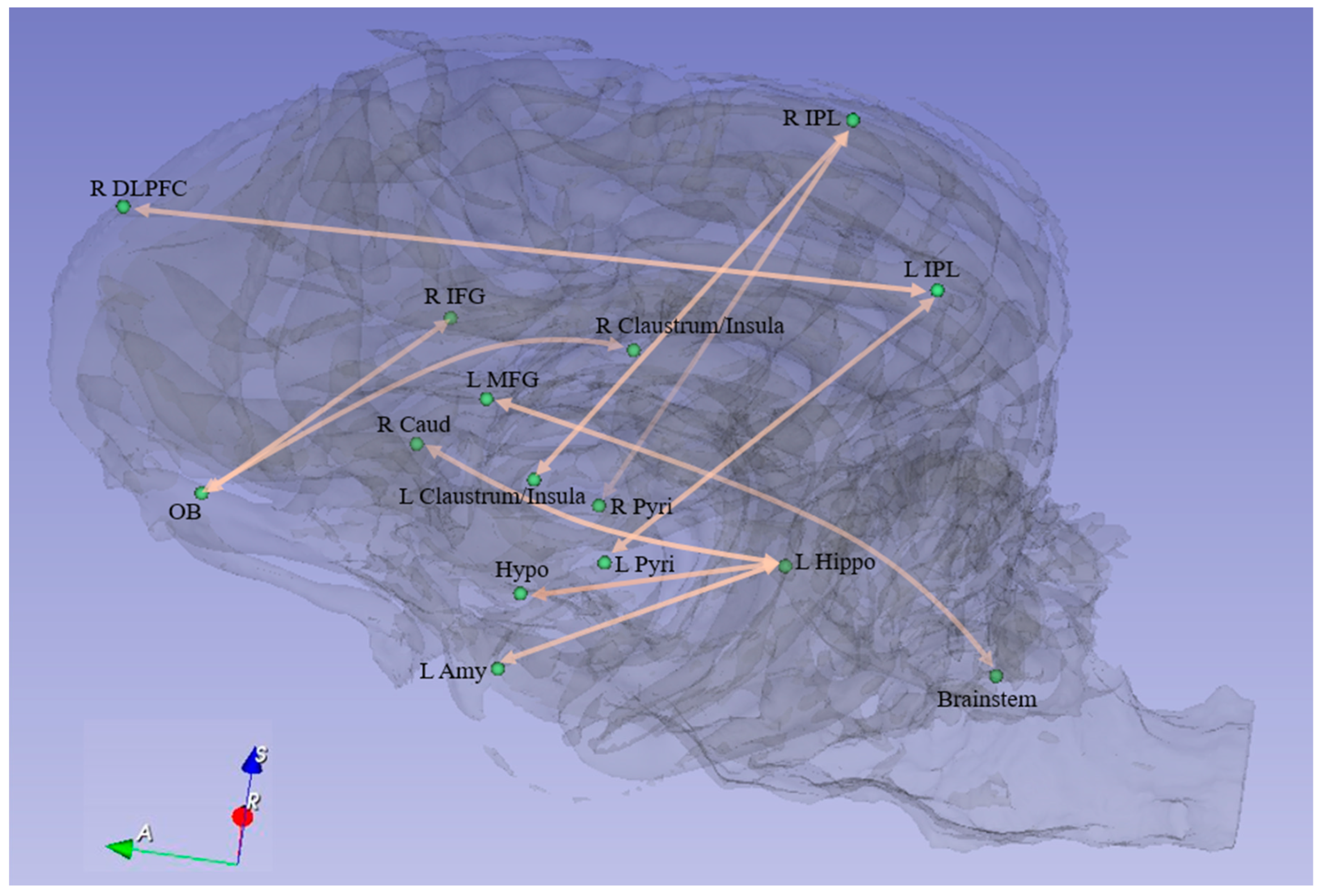
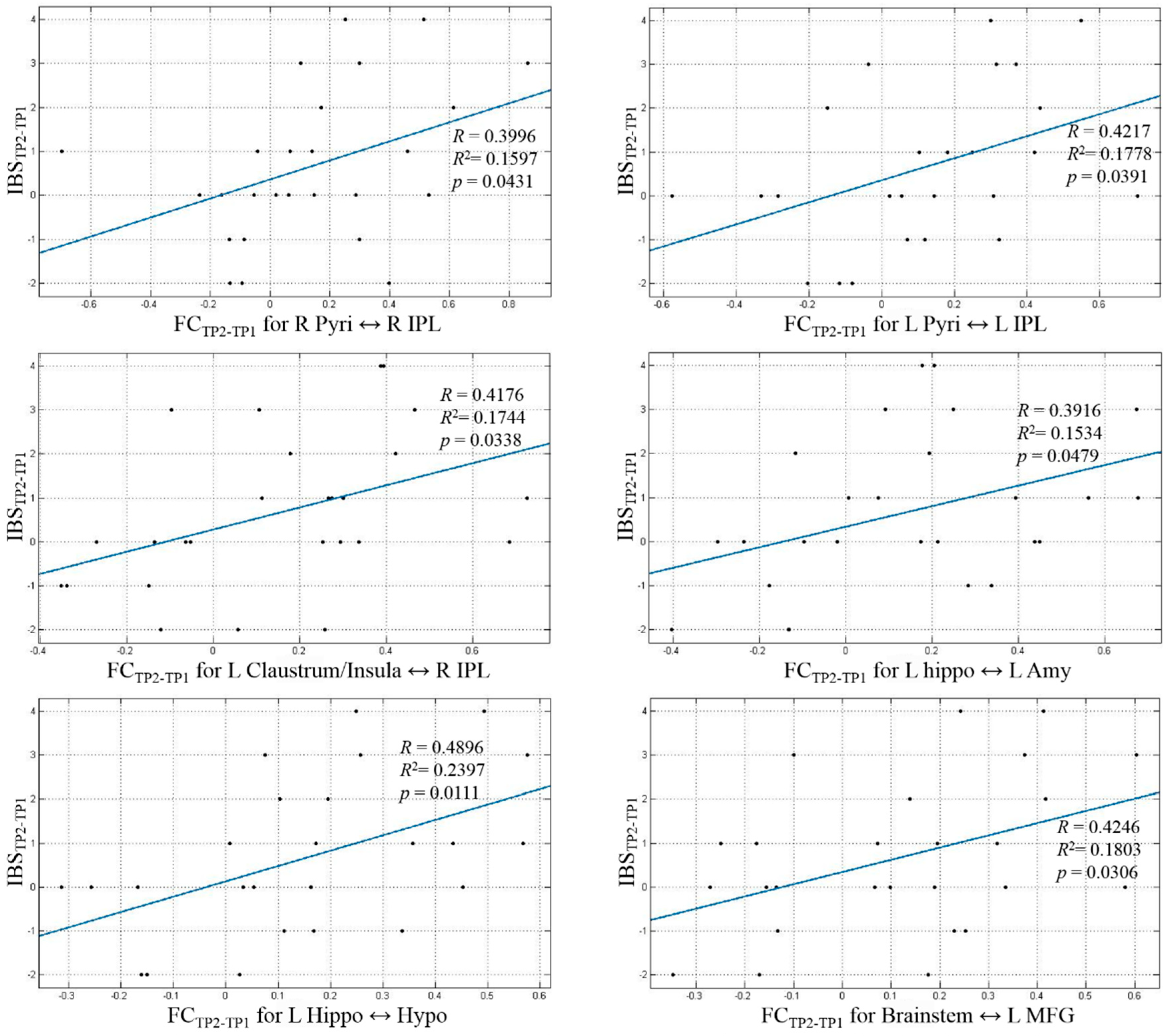

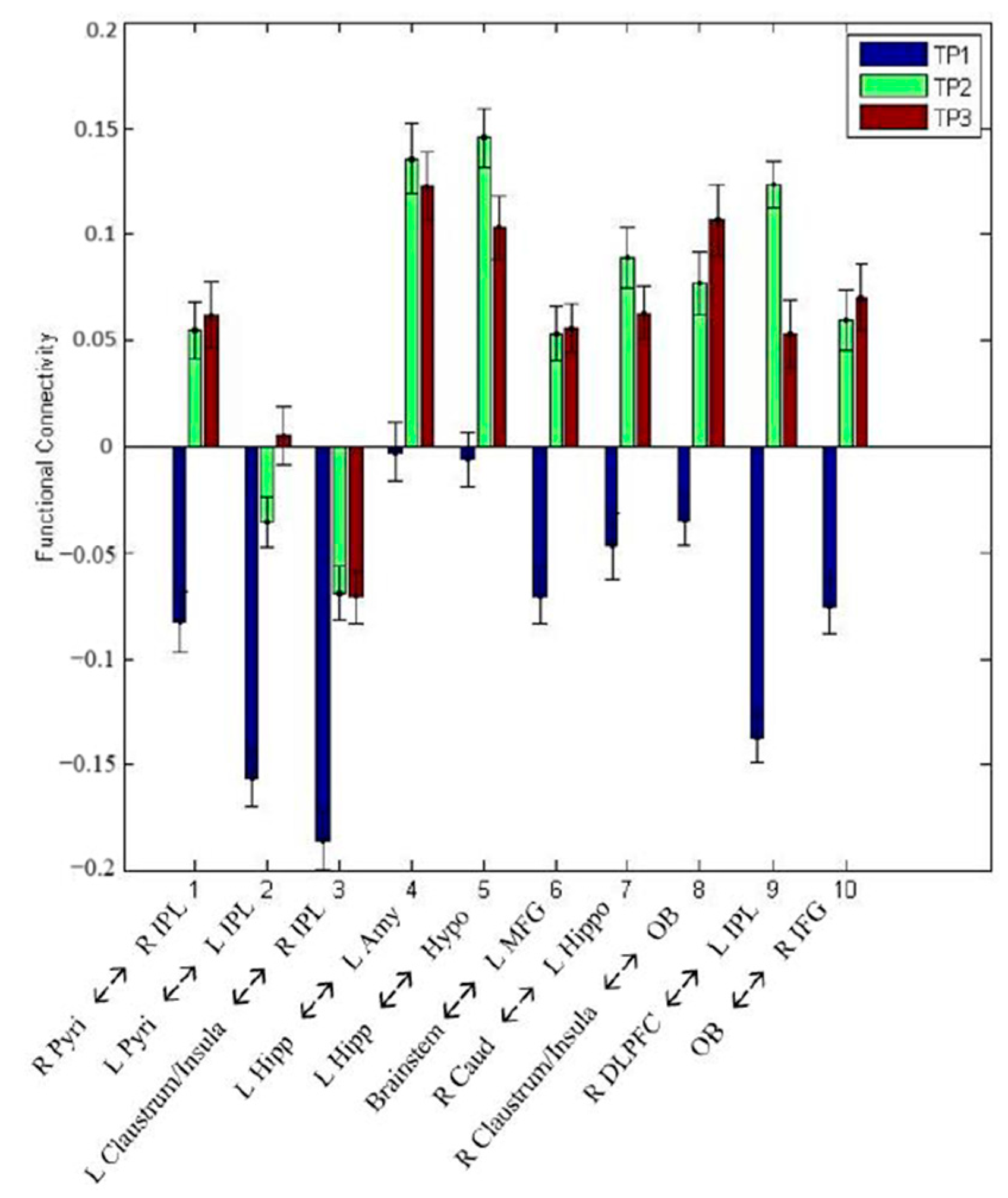

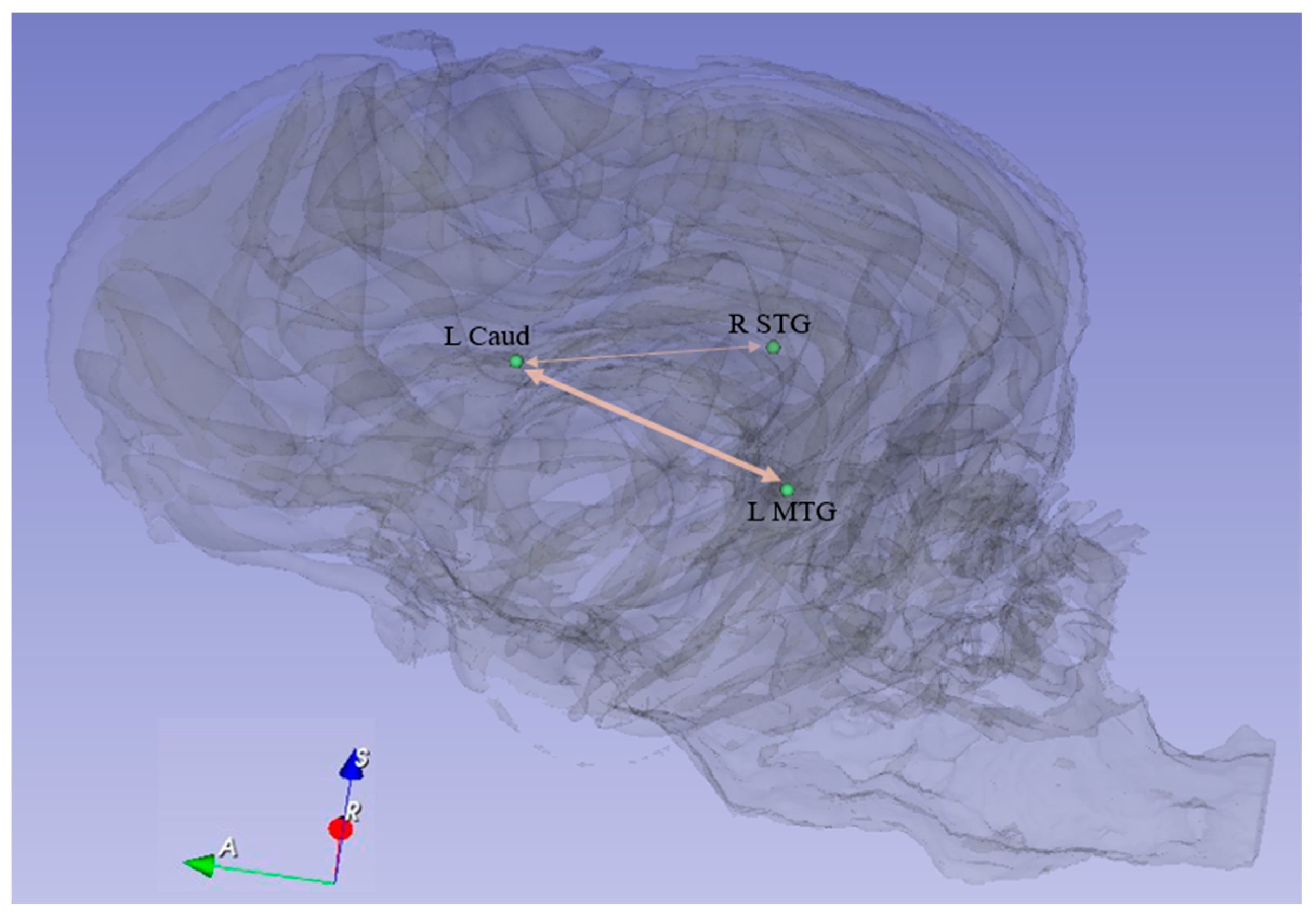

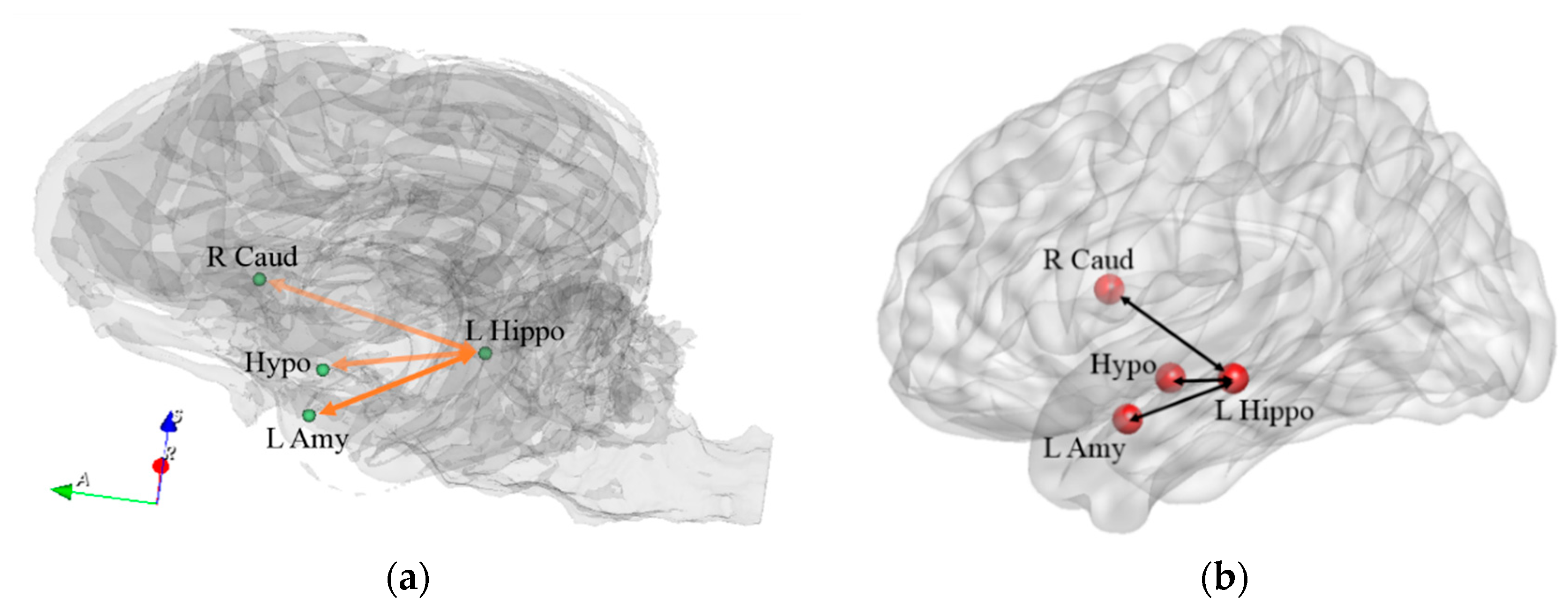
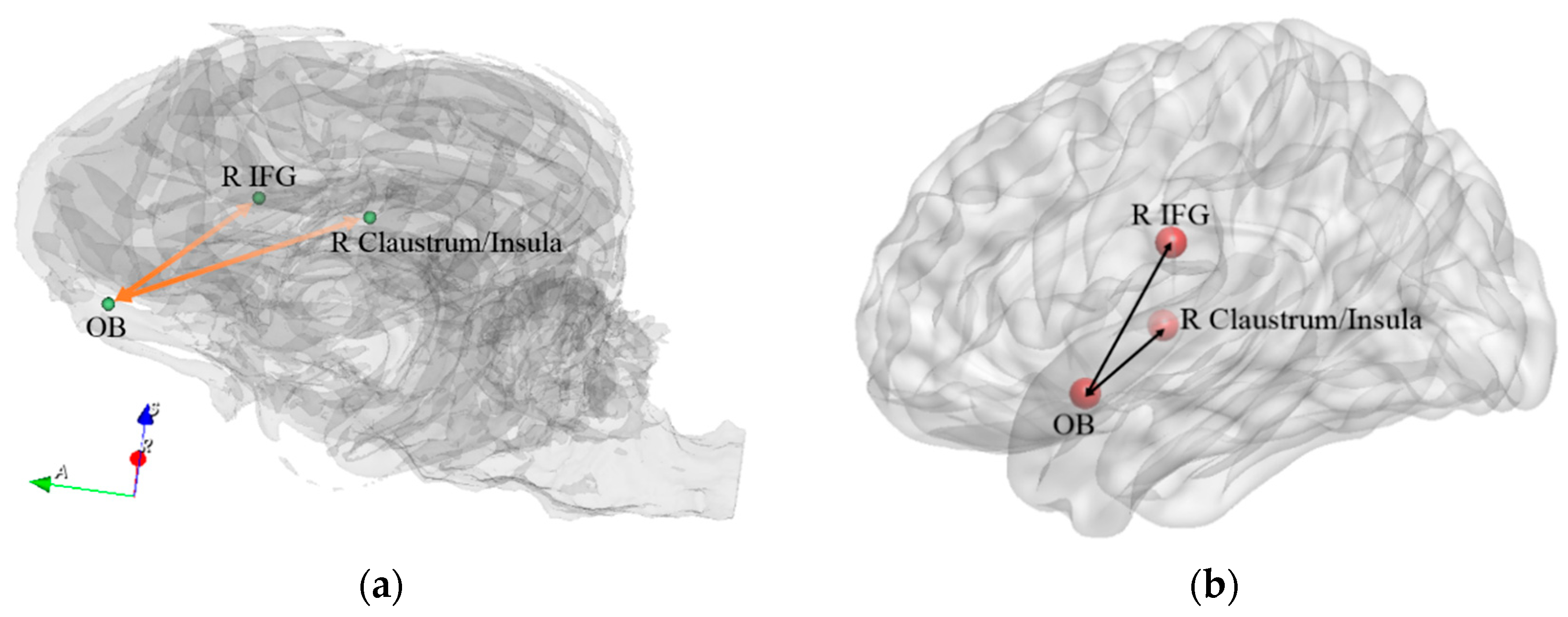
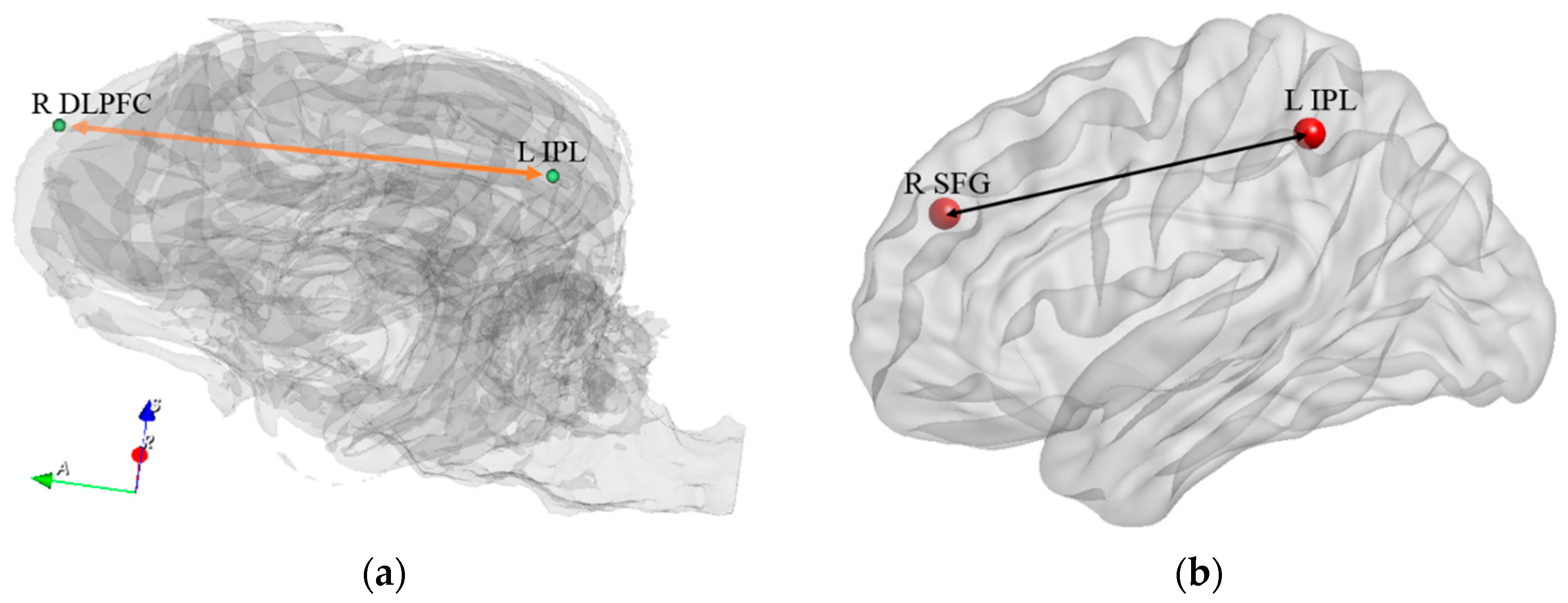
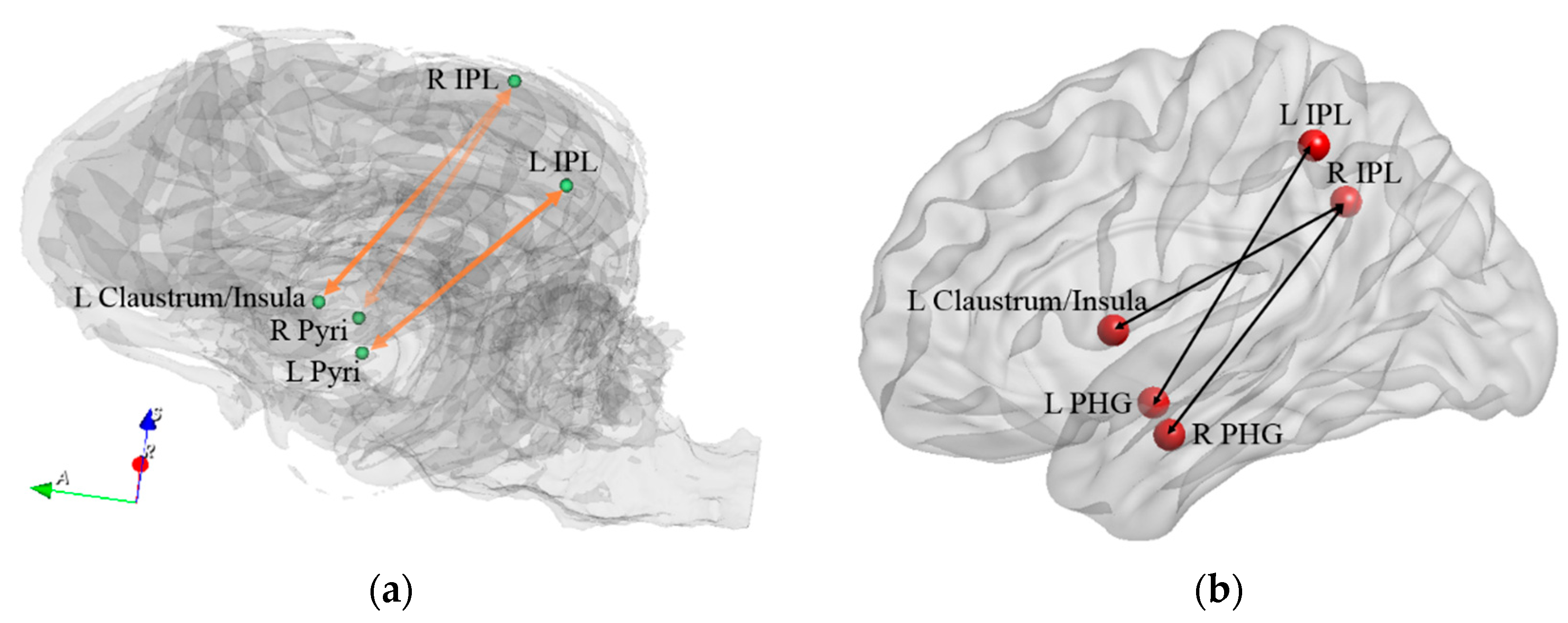
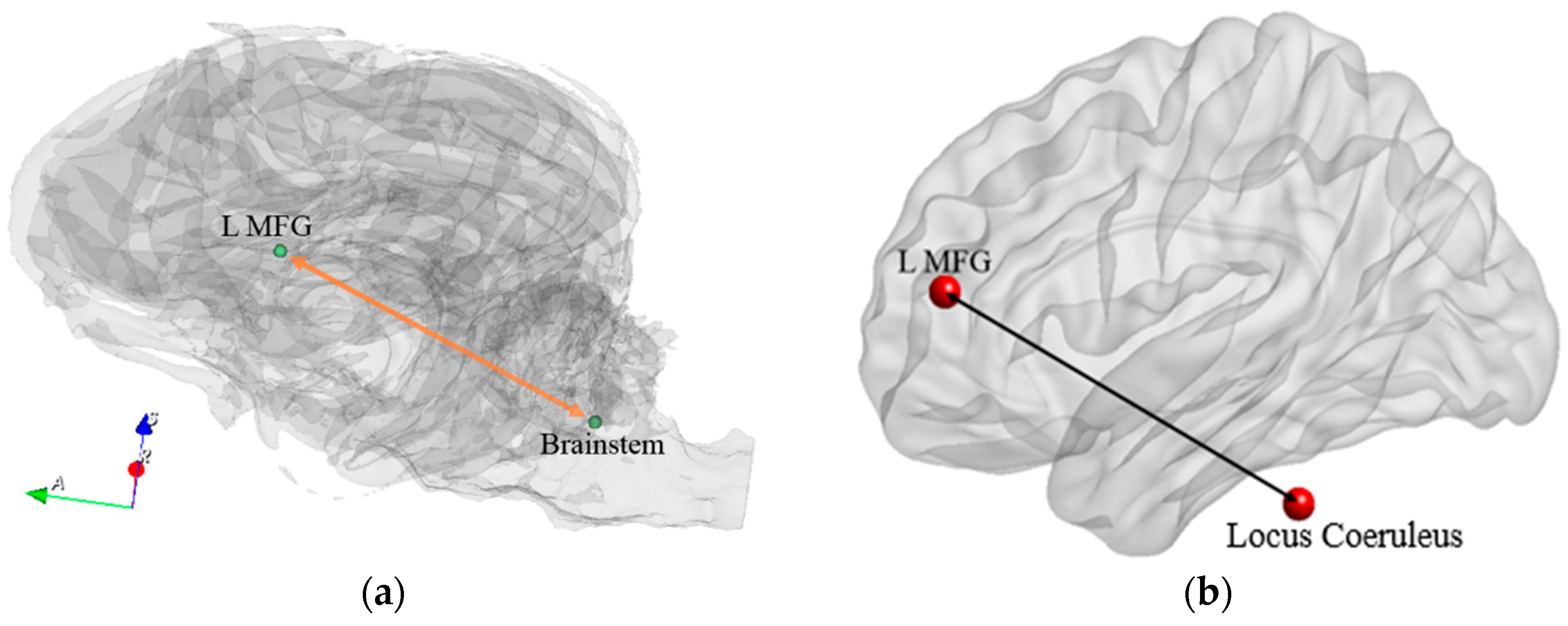
| Path No. | Path |
|---|---|
| 1 | R Pyri ↔ R IPL |
| 2 | L Pyri ↔ L IPL |
| 3 | L Claustrum/Insula ↔ R IPL |
| 4 | L Hippo ↔ L Amy |
| 5 | L Hippo ↔ Hypo |
| 6 | Brainstem ↔ L MFG |
| 7 | R Caud ↔ L Hippo |
| 8 | R Claustrum/Insula ↔ OB |
| 9 | R DLPFC ↔ L IPL |
| 10 | OB ↔ R IFG |
| TP2 > TP1 | TP3 > TP1 | TP2 ≠ TP3 | |
|---|---|---|---|
| R Pyri ↔ R IPL | 1.49 × 10−2 | 1.76 × 10−2 | 9.12 × 10−1 |
| L Pyri ↔ L IPL | 1.83 × 10−2 | 5.52 × 10−3 | 4.58 × 10−1 |
| L Claustrum/Insula ↔ R IPL | 2.66 × 10−2 | 3.06 × 10−2 | 9.75 × 10−1 |
| L Hippo ↔ L Amy | 2.23 × 10−2 | 3.53 × 10−2 | 8.54 × 10−1 |
| L Hippo ↔ Hypo | 7.71 × 10−3 | 4.39 × 10−2 | 5.18 × 10−1 |
| Brainstem ↔ L MFG | 1.69 × 10−2 | 1.42 × 10−2 | 9.65 × 10−1 |
| R Caud ↔ L Hippo | 2.29 × 10−2 | 4.61 × 10−2 | 6.69 × 10−1 |
| R Claustrum/Insula ↔ OB | 3.73 × 10−2 | 1.92 × 10−2 | 6.79 × 10−1 |
| R DLPFC ↔ L IPL | 2.75 × 10−6 | 2.17 × 10−3 | 2.62 × 10−1 |
| OB ↔ R IFG | 1.57 × 10−2 | 1.51 × 10−2 | 8.72 × 10−1 |
| p-Value of FCsuccessful > FCnon-successful | |||
|---|---|---|---|
| Path | TP1 | TP2 | TP3 |
| 1–6. Caudate ↔ L MTG | 3.38 × 10−4 | 2.9 × 10−3 | 1.9 × 10−3 |
| 7.97 × 10−4 | 2.1 × 10−3 | 1.6 × 10−3 | |
| 8.55 × 10−5 | 1.6 × 10−3 | 4.1 × 10−3 | |
| 9.91 × 10−5 | 9.4 × 10−4 | 1.7 × 10−3 | |
| 2.8 × 10−3 | 1.6 × 10−3 | 1.9 × 10−3 | |
| 3.7 × 10−3 | 4.2 × 10−3 | 1.8 × 10−3 | |
| 7. L Caud ↔ R STG | 3.8 × 10−6 | 1.9 × 10−3 | 4.8 × 10−3 |
| p-Value of Successful Group | p-Value of Non-Successful Group | |||||
|---|---|---|---|---|---|---|
| Path No. | TP1 ≠ TP2 | TP1 ≠ TP3 | TP2 ≠ TP3 | TP1 ≠ TP2 | TP1 ≠ TP3 | TP2 ≠ TP3 |
| 1 | 5.6 × 10−1 | 2.3 × 10−1 | 9.5 × 10−2 | 7.8 × 10−1 | 5.2 × 10−1 | 4.4 × 10−1 |
| 2 | 3.8 × 10−1 | 3.5 × 10−1 | 1.2 × 10−1 | 3.4 × 10−1 | 8.7 × 10−1 | 3.3 × 10−1 |
| 3 | 6.4 × 10−1 | 5.7 × 10−1 | 2.9 × 10−1 | 5.1 × 10−1 | 2.4 × 10−1 | 5.1 × 10−1 |
| 4 | 4.3 × 10−1 | 8.7 × 10−1 | 4.1 × 10−1 | 3.5 × 10−1 | 8.9 × 10−1 | 5.4 × 10−1 |
| 5 | 1.7 × 10−1 | 6.9 × 10−1 | 1.7 × 10−1 | 8.8 × 10−2 | 5.1 × 10−1 | 3.5 × 10−1 |
| 6 | 6.2 × 10−2 | 6.2 × 10−1 | 2.6 × 10−1 | 6.3 × 10−2 | 9.3 × 10−2 | 9.6 × 10−1 |
| 7 | 3.9 × 10−1 | 9.1 × 10−1 | 4.1 × 10−1 | 1.1 × 10−1 | 6.6 × 10−2 | 5.4 × 10−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deshpande, G.; Zhao, S.; Waggoner, P.; Beyers, R.; Morrison, E.; Huynh, N.; Vodyanoy, V.; Denney, T.S., Jr.; Katz, J.S. Two Separate Brain Networks for Predicting Trainability and Tracking Training-Related Plasticity in Working Dogs. Animals 2024, 14, 1082. https://doi.org/10.3390/ani14071082
Deshpande G, Zhao S, Waggoner P, Beyers R, Morrison E, Huynh N, Vodyanoy V, Denney TS Jr., Katz JS. Two Separate Brain Networks for Predicting Trainability and Tracking Training-Related Plasticity in Working Dogs. Animals. 2024; 14(7):1082. https://doi.org/10.3390/ani14071082
Chicago/Turabian StyleDeshpande, Gopikrishna, Sinan Zhao, Paul Waggoner, Ronald Beyers, Edward Morrison, Nguyen Huynh, Vitaly Vodyanoy, Thomas S. Denney, Jr., and Jeffrey S. Katz. 2024. "Two Separate Brain Networks for Predicting Trainability and Tracking Training-Related Plasticity in Working Dogs" Animals 14, no. 7: 1082. https://doi.org/10.3390/ani14071082
APA StyleDeshpande, G., Zhao, S., Waggoner, P., Beyers, R., Morrison, E., Huynh, N., Vodyanoy, V., Denney, T. S., Jr., & Katz, J. S. (2024). Two Separate Brain Networks for Predicting Trainability and Tracking Training-Related Plasticity in Working Dogs. Animals, 14(7), 1082. https://doi.org/10.3390/ani14071082







