Gene Expression in Porcine Bulbourethral Glands
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatments
2.2. Tissue Collection and Analyses
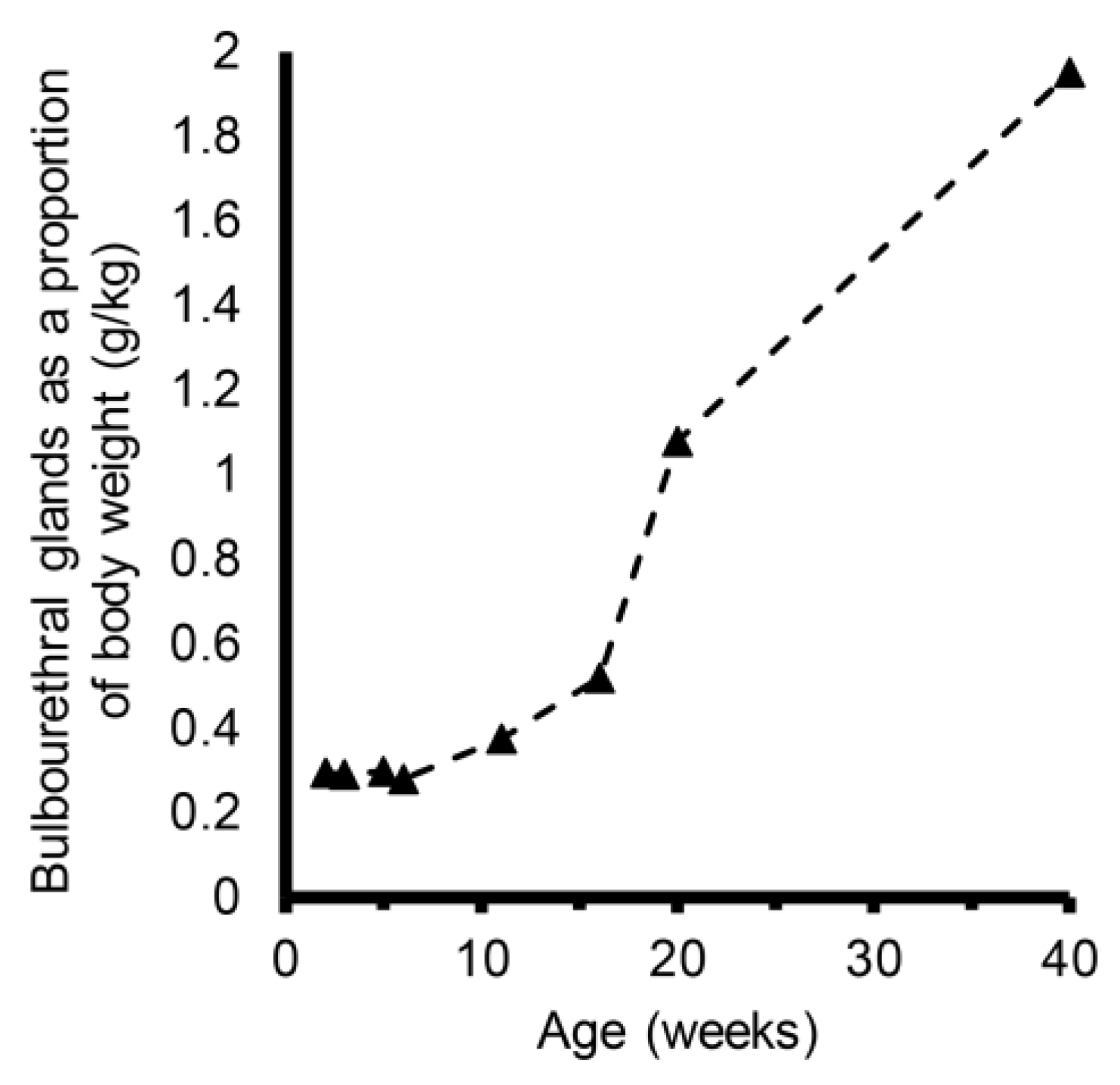
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Badia, E.; Briz, M.D.; Pinart, E.; Sancho, S.; Garcia, N.; Bassols, J.; Pruneda, A.; Bussalleu, E.; Yeste, M.; Casas, I.; et al. Structural and ultrastructural features of boar bulbourethral glands. Tissue Cell 2006, 38, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, E.H.; Sorensen, V.W.; Bjorkman, N. On the fine structure and mucosubstances in the bulbourethral gland in the domestic boar. Anat. Histol. Embryol. 1977, 6, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Boursnell, J.C.; Butler, E.J. Studies on properties of the seminal gel of the boar using natural gel and gel formed in vitro. J. Reprod. Fertil. 1973, 34, 457–465. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Berger, T.; McCarthy, M.; Pearl, C.A.; At-Taras, E.; Roser, J.F.; Conley, A. Reducing endogenous estrogens during the neonatal and juvenile periods affects reproductive tract development and sperm production in postpuberal boars. Anim. Reprod. Sci. 2008, 109, 218–235. [Google Scholar] [CrossRef] [PubMed]
- Parrott, R.F.; Booth, W.D. Behavioural and morphological effects of 5 alpha-dihydrotestosterone and oestradiol-17 beta in the prepubertally castrated boar. J. Reprod. Fertil. 1984, 71, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Prins, G.S.; Birch, L.; Habermann, H.; Chang, W.Y.; Tebeau, C.; Putz, O.; Bieberich, C. Influence of neonatal estrogens on rat prostate development. Reprod. Fertil. Dev. 2001, 13, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Risbridger, G.; Taylor, R.A. Physiology of the male accessory sex structures: The prostate gland, seminal vesicles, and bulbourethral glands. In Knobil and Neill’s Physiology of Reproduction, 3rd ed.; Neill, J.D., Ed.; Academic Press: St. Louis, MO, USA, 2006; Volume 1, pp. 1149–1172. [Google Scholar]
- Berger, T.; Guerrero, V.; Boeldt, R.; Legacki, E.; Roberts, M.; Conley, A.J. Development of Porcine Accessory Sex Glands. Animals 2024, 14, 462. [Google Scholar] [CrossRef] [PubMed]
- Berger, T.; Conley, A.J. Reducing endogenous estrogen during prepuberal life does not affect boar libido or sperm fertilizing potential. Theriogenology 2014, 82, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Berger, T.; Conley, A.J.; Van Klompenberg, M.; Roser, J.F.; Hovey, R.C. Increased testicular Sertoli cell population induced by an estrogen receptor antagonist. Mol. Cell. Endocrinol. 2013, 366, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Berger, T.; Tang, S.; Tu, L.; Soto, D.A.; Conley, A.J.; Nitta-Oda, B. Changes in testicular gene expression following reduced estradiol synthesis: A complex pathway to increased porcine Sertoli cell proliferation. Mol. Cell. Endocrinol. 2021, 523, 111099. [Google Scholar] [CrossRef] [PubMed]
- Kucera, H.; Puschner, B.; Conley, A.; Berger, T. Tissue steroid levels in response to reduced testicular estrogen synthesis in the male pig, Sus scrofa. PLoS ONE 2019, 14, e0215390. [Google Scholar] [CrossRef] [PubMed]
- Katleba, K.; Legacki, E.; Berger, T. Expression of CSF1, AR, and SRD5A2 during Postnatal Development of the Boar Reproductive Tract. Animals 2022, 12, 2167. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.R.; Berger, T. Regulation of apical blebbing in the porcine epididymis. J. Anat. 2018, 232, 515–522. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest package: Tests in linear mixed effects models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Aitken, R.N. A histochemical study of the accessory genital glands of the boar. J. Anat. 1960, 94, 130–142. [Google Scholar] [PubMed]
- At-Taras, E.E.; Conley, A.J.; Berger, T.; Roser, J.F. Reducing estrogen synthesis does not affect gonadotropin secretion in the developing boar. Biol. Reprod. 2006, 74, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Little, J.S., Jr.; Goode, R.L.; Neubauer, B.L. Prenatal in vivo bulbourethral gland development is not affected by prostaglandin E2 inhibition. J. Androl. 1995, 16, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Boronikhina, T.; Piavchenko, G.; Venediktov, A.; Kustavinova, E.; Mukhamedova, S.; Kartashkina, N.; Kuznetsov, S.; Meglinski, I.; Yatskovskiy, A. The number of the intraepithelial T cells correlate with the proliferation index in human bulbourethral gland epithelium. Heliyon 2022, 8, e11658. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noto, V.; Nitta-Oda, B.J.; Berger, T. Gene Expression in Porcine Bulbourethral Glands. Animals 2024, 14, 1115. https://doi.org/10.3390/ani14071115
Noto V, Nitta-Oda BJ, Berger T. Gene Expression in Porcine Bulbourethral Glands. Animals. 2024; 14(7):1115. https://doi.org/10.3390/ani14071115
Chicago/Turabian StyleNoto, Victoria, Barbara Jean Nitta-Oda, and Trish Berger. 2024. "Gene Expression in Porcine Bulbourethral Glands" Animals 14, no. 7: 1115. https://doi.org/10.3390/ani14071115
APA StyleNoto, V., Nitta-Oda, B. J., & Berger, T. (2024). Gene Expression in Porcine Bulbourethral Glands. Animals, 14(7), 1115. https://doi.org/10.3390/ani14071115




