Effects of Mixed Pasture Legume Phytoestrogens on Superovulatory Response and Embryo Quality in Angus Cows
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Information
2.2. Study Design, Feeding and Animals
2.3. Synchronization of Estrus and Superovulation
2.4. Blood Sampling
2.5. Embryo Recovery and Assessment of Quality
2.6. Pasture Sampling
2.7. Analytical Method
3. Results
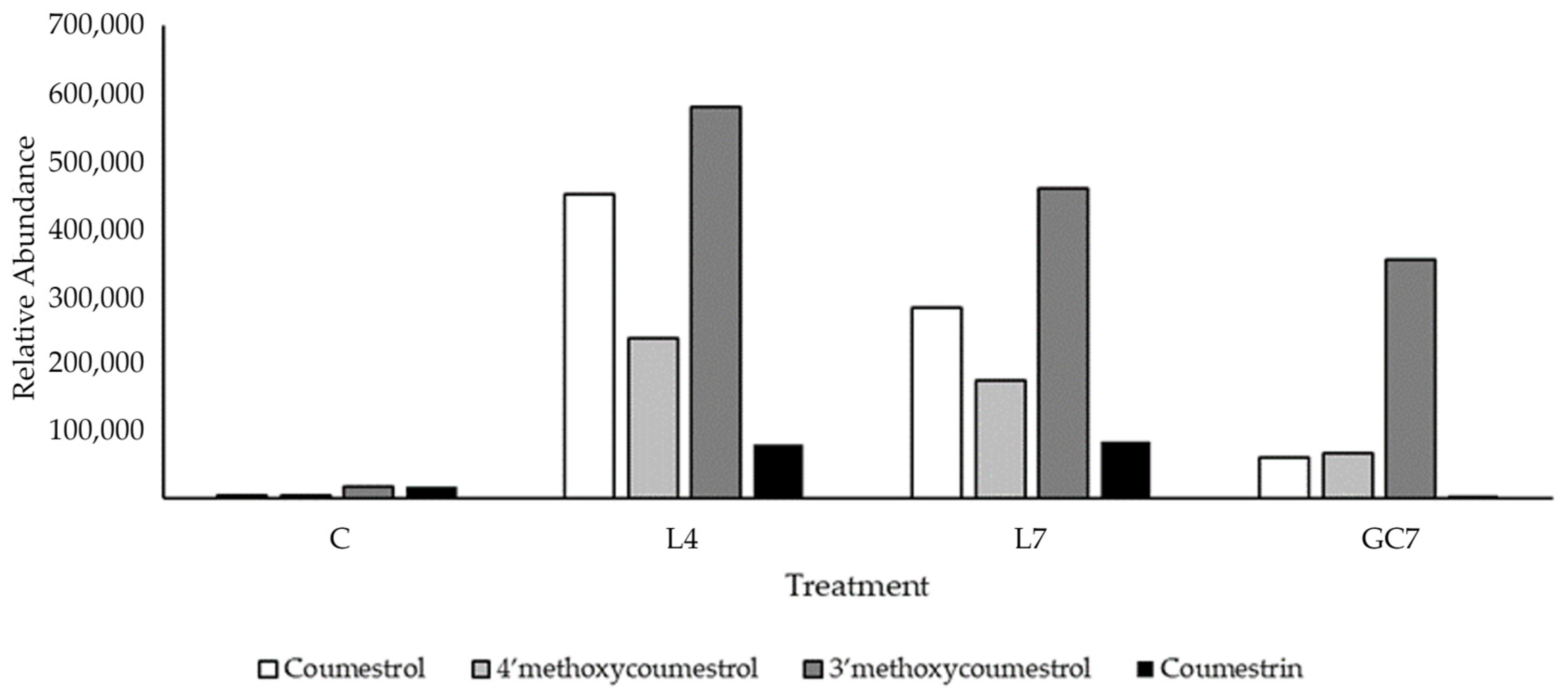
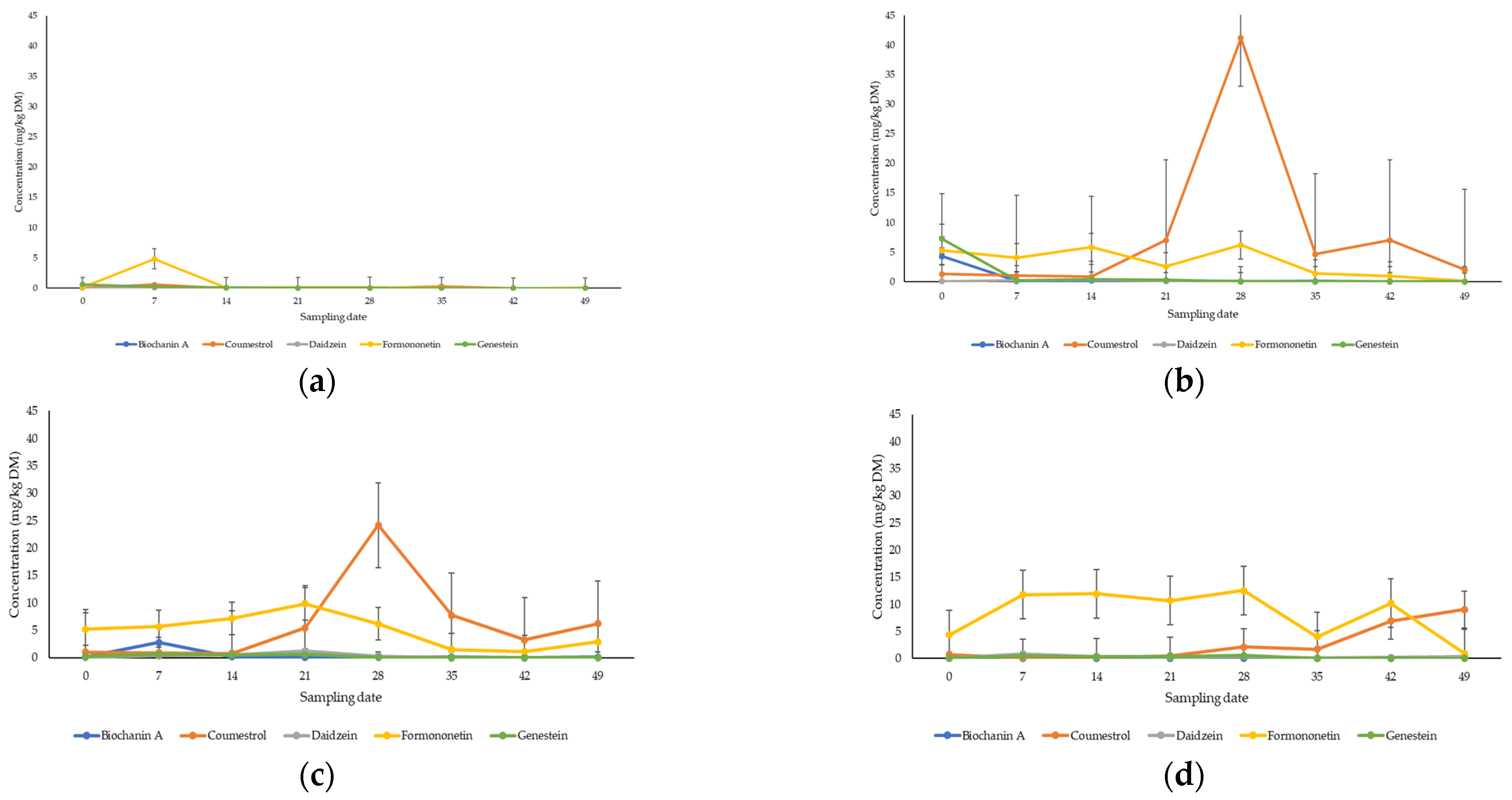
3.1. Quantification of Phytoestrogens in Pasture
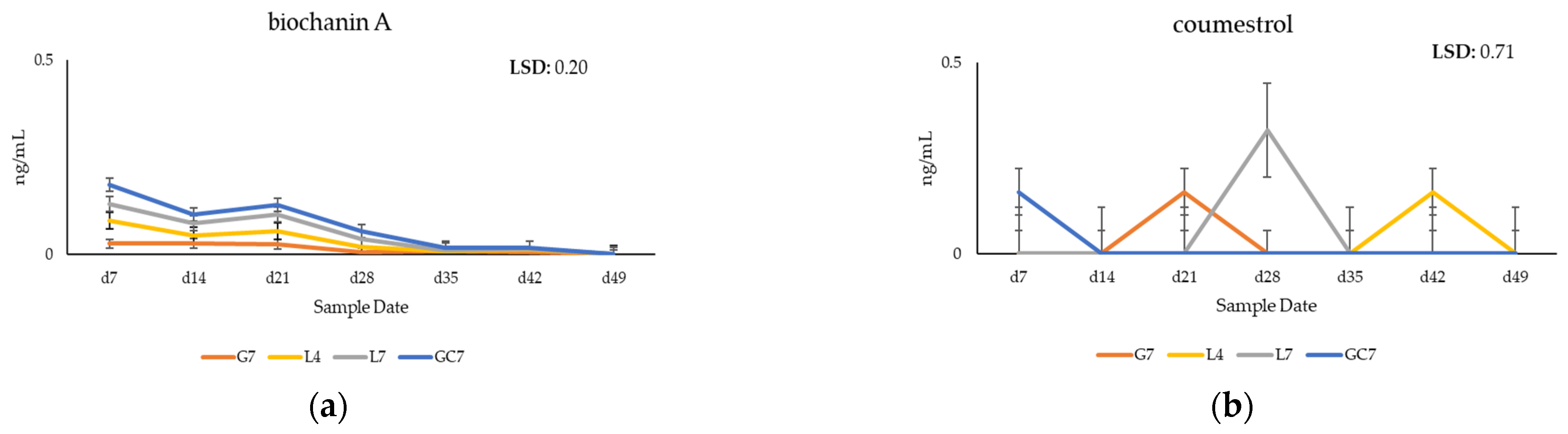
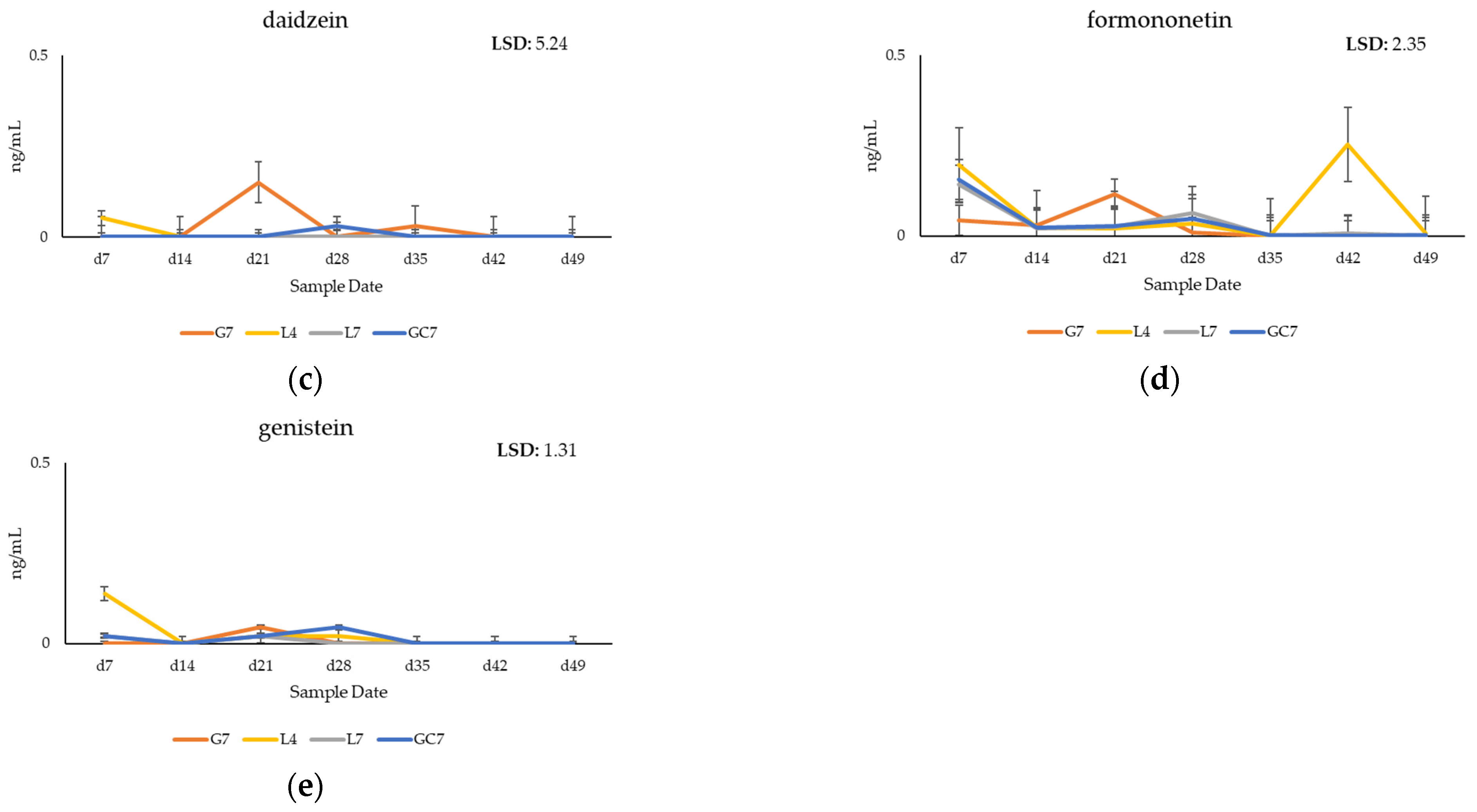
3.2. Quantification of Phytoestrogens in Plasma of Grazing Cows
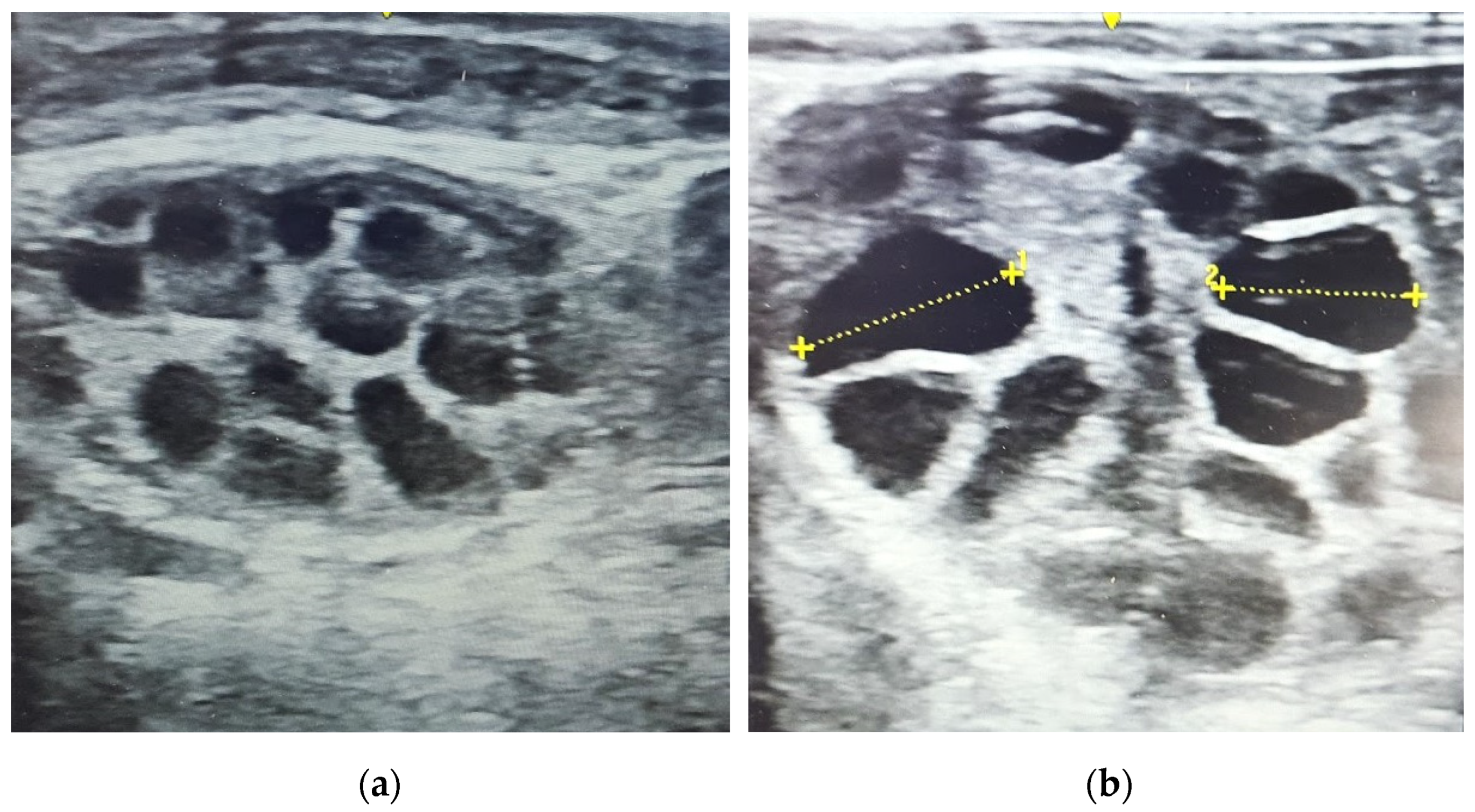
3.3. Follicular Development
3.4. Embryo Quantity and Quality
4. Discussion
4.1. Metabolomic Profiling of Key Phytoestrogens in Legume Pastures and Bovine Plasma
4.2. Effects on the Hypothalamo–Pituitary–Gonadal Axis
4.3. Factors Affecting Embryo Quality, Embryo Stage and Development
4.4. Superovulation/Factors Affecting the Reproductive Cycle
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Biochanin A | Coumestrol | Daidzein | Formononetin | Genistein | |
|---|---|---|---|---|---|
| Arrowleaf clover | 2.6 | 698.1 | 0.0 | 233.4 | 6.7 |
| Cluster clover | 0.1 | 1189.3 | 866.2 | 151.2 | 8.0 |
| Haresfoot clover | 6.3 | 0.0 | 658.0 | 172.8 | 52.4 |

References
- Nichols, P.G.H.; Revell, C.K.; Humphries, A.W.; Howie, J.H.; Hall, E.J.; Sandral, G.A.; Ghamkhar, K.; Harris, C.A. Temperate pasture legumes in Australia—Their history, current use, and future prospects. Crop Pasture Sci. 2012, 63, 691–725. [Google Scholar] [CrossRef]
- Doyle, P.; Grimm, M.; Thompson, A. Grazing for pasture and sheep management in the annual pasture zone. In Pasture Management Technology for the 21st Century; Kemp, D.R., Michalk, D.L., Eds.; CSIRO: Melbourne, VIC, Australia, 1994; pp. 71–90. [Google Scholar]
- Thomas, D.T.; Milton, J.T.B.; Revell, C.K.; Ewing, M.A.; Dynes, R.A.; Murray, K.; Lindsay, D.R. Preference of sheep among annual legumes is more closely related to plant nutritive characteristics as plants mature. Anim. Prod. Sci. 2010, 50, 114–123. [Google Scholar] [CrossRef]
- Wyse, J.; Latif, S.; Gurusinghe, S.; McCormick, J.; Weston, L.A.; Stephen, C.P. Phytoestrogens: A Review of Their Impacts on Reproductive Physiology and Other Effects upon Grazing Livestock. Animals 2022, 12, 2709. [Google Scholar] [CrossRef] [PubMed]
- Melchior, E.A.; Myer, P.R. Fescue toxicosis and its influence on the rumen microbiome: Mitigation of production losses through clover isoflavones. J. Appl. Anim. Res. 2018, 46, 1280–1288. [Google Scholar] [CrossRef]
- Wyse, J.M.; Latif, S.; Gurusinghe, S.; Berntsen, E.D.; Weston, L.A.; Stephen, C.P. Characterization of Phytoestrogens in Medicago sativa L. and Grazing Beef Cattle. Metabolites 2021, 11, 550. [Google Scholar] [CrossRef]
- Puschner, B. Anti-nutritional factors in alfalfa hay. In Proceedings of the National Alfalfa Symposium, Las Vegas, NV, USA, 10–12 December 2000; The California Alfalfa Workgroup and the Alfalfa Council: Las Vegas, NV, USA, 2000; pp. 157–162. [Google Scholar]
- Horrocks, R.D.; Valentine, J.F. Harvested Forages; Academic Press: San Diego, CA, USA, 1999; pp. 68–70. [Google Scholar]
- Norman, H.C.; Hughes, S.J.; Hulm, E.L.; Humphries, K.; Oldach, K.; Revell, D.K.; Durmic, Z.; Vadhanabhuti, J.; Vercoe, P.E. Improving the feeding value of dryland lucerne in Australia. In Proceedings of the International Grasslands Congress, Covington, KY, USA, 14–19 May 2023; Internation Grassland Congress: Covington, KY, USA, 2023. [Google Scholar]
- Allworth, M.; McQuillan, M.; McGrath, S.; Wilson, C.; Hernandez-Jover, M. A survey on bloat in southern Australian beef production systems. Aust. Vet.J. 2023, 101, 121–126. [Google Scholar] [CrossRef]
- Venkatanagappa, S. Identifying lucerne growers’ usage and needs through a survey for breeding new generation lucerne varieties. In Capturing Opportunities and Overcoming Obstacles in Australian Agronomy, Proceedings of the 16th Australian Agronomy Conference 2012, Armidale, NSW, Australia, 14–18 October 2012; Australian Society of Agronomy Inc.: Armidale, NSW, Australia, 2012; pp. 14–18. [Google Scholar]
- Moot, D.; Bennett, S.; Mills, A.; Smith, M. Optimal grazing management to achieve high yields and utilisation of dryland lucerne. J. N. Z. Grassl. 2016, 78, 27–34. [Google Scholar] [CrossRef]
- Karki, K.B.; Mishra, A.K.; Choi, S.J.; Baek, K.H. Effect of Ultraviolet C Irradiation on Isoflavone Concentrations in Different Cultivars of Soybean (Glycine max). Plants 2020, 9, 1043. [Google Scholar] [CrossRef]
- Reed, K. Fertility of herbivores consuming phytoestrogen-containing Medicago and Trifolium species. Agriculture 2016, 6, 35. [Google Scholar] [CrossRef]
- Mlynarczuk, J.; Wrobel, M.; Kotwica, J. The adverse effect of phytoestrogens on the synthesis and secretion of ovarian oxytocin in cattle. Reprod. Domest. Anim. 2011, 46, 21–28. [Google Scholar] [CrossRef]
- Hughes, C.L., Jr. Phytochemical mimicry of reproductive hormones and modulation of herbivore fertility by phytoestrogens. Environ. Health Perspect. 1988, 78, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Zdunczyk, S.; Piskula, M.; Janowski, T.; Baranski, W.; Ras, M. Concentrations of isoflavones in blood plasma of dairy cows with different incidence of silent heat. Bull. Vet. Inst. Pulawy 2005, 49, 189–191. [Google Scholar]
- Borzym, E.; Bobowiec, R.; Kosior-Korzecka, U.; Martelli, F.; Burmaczuk, A. Disturbances of cow oocyte maturation by phytoestrogens. Med. Weter 2008, 64, 1107–1111. [Google Scholar]
- Mostrom, M.; Evans, T.J. Phytoestrogens. Reprod. Develop. Toxicol. 2011, 707–722. [Google Scholar] [CrossRef]
- Jefferson, W.N.; Patisaul, H.B.; Williams, C.J. Reproductive consequences of developmental phytoestrogen exposure. Reproduction 2012, 143, 247. [Google Scholar] [CrossRef] [PubMed]
- Shore, L.S.; Rios, C.; Marcus, S.; Bernstein, M.; Shemesh, M. Relationship between peripheral estrogen concentrations at insemination and subsequent fetal loss in cattle. Theriogenology 1998, 50, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Dias, G.; Botelho, M.; Zagrajczuk, A.; Rebordão, M.R.; Galvão, A.M.; Pinto Bravo, P.; Piotrowska-Tomala, K.; Szóstek, A.Z.; Wiczkowski, W.; Piskula, M.; et al. Coumestrol and its metabolite in mares’ plasma after ingestion of phytoestrogen-rich plants: Potent endocrine disruptors inducing infertility. Theriogenology 2013, 80, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Williamson, L.N.; Bartlett, M.G. Quantitative liquid chromatography/time-of-flight mass spectrometry. Biomed. Chromatogr. 2007, 21, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Mapletoft, R.J.; Bó, G.A. Superovulation in cattle. In Bovine Reproduction, 2nd ed.; Hopper, R.M., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2021; pp. 696–702. [Google Scholar]
- Noga, N.; Looney, C. Embryo Transfer and Embryo Collection. In Bovine Reproduction, 2nd ed.; Hopper, R.M., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2021; pp. 1041–1060. [Google Scholar]
- Stringfellow, D.A.; Givens, M.D. Manual of the International Embryo Transfer Society (IETS): A Procedural Guide and General Information for the Use of Embryo Transfer Technology Emphasizing Sanitary Procedures, 4th ed.; The International Embryo Transfer Society: Champaign, IL, USA, 2010. [Google Scholar]
- McIntyre, G. A method for unbiased selective sampling, using ranked sets. Aus. J. Agric. Res. 1952, 3, 385–390. [Google Scholar] [CrossRef]
- Rayburn, E.; Lozier, J.; Sanderson, M. Estimating Pasture Forage Mass from Pasture Height; West Virginia University: Morgantown, WV, USA, 2003. [Google Scholar]
- Graham, P. Pasture Assessment. In PROGRAZE Profitable, Sustainable Grazing, 9th ed.; New South Wales Department of Primary Industries: Yass, Australia, 2017; p. 180. [Google Scholar]
- Adams, G.P.; Singh, J. Ovarian Follicular and Luteal Dynamics in Cattle. In Bovine Reproduction, 2nd ed.; Hopper, R.M., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2021; pp. 292–323. [Google Scholar]
- Njåstad, K.M.; Adler, S.A.; Hansen-Møller, J.; Thuen, E.; Gustavsson, A.M.; Steinshamn, H. Gastrointestinal metabolism of phytoestrogens in lactating dairy cows fed silages with different botanical composition. J. Dairy Sci. 2014, 97, 7735–7750. [Google Scholar] [CrossRef]
- Madej, A.; Lundh, T. Risk of adverse effects of phytoestrogens in animal feed. In Bioactive Compounds in Plants–Benefits and Risks for Man and Animals; Bernhoft, A., Ed.; The Norwegian Academy of Science and Letters: Oslo, Norway, 2008; pp. 94–103. [Google Scholar]
- Bó, G.A.; Mapletoft, R.J. Historical perspectives and recent research on superovulation in cattle. Theriogenology 2014, 81, 38–48. [Google Scholar] [CrossRef]
- Romero, R.C.M.; Castellanos, M.d.R.T.; Mendoza, R.M.; Reyes, R.A.; García, A.R. Oestrogenic syndrome in dairy cows by alfalfa comsuption with large amount of coumestrol. Vet. Méx. 1997, 28, 25–30. [Google Scholar]
- Scales, G.H.; Moss, R.A. Mating ewes on lucerne. N. Z. J. Agr. 1976, 132, 21–22. [Google Scholar]
- Adams, N. Phytoestrogens. In Toxicants of Plant Origin. Volume IV Phenolics; Cheeke, P.R., Ed.; CRC Press: Boca Raton, FL, USA, 1989; pp. 23–51. [Google Scholar]
- Wocławek-Potocka, I.; Okuda, K.; Acosta, T.; Korzekwa, A.; Pilawski, W.; Skarzynski, D. Phytoestrogen metabolites are much more active than phytoestrogens themselves in increasing prostaglandin F2α synthesis via prostaglanin F2α synthase-like 2 stimulation in bovine endometrium. Prostag. Oth. Lipid M. 2005, 78, 202–217. [Google Scholar] [CrossRef]
- Cox, R.; Braden, A. The metabolism and physiological effects of phyto-oestrogens in livestock. In Proceedings of the Australian Society of Animal Production, Perth, WA, Australia, July 1974; pp. 122–129. [Google Scholar]
- Wada, H.; Yuhara, M.; Ikemoto, T.; Senovo, M.; Okushima, S.; Fukushima, S.; Morita, M. Intake of forage by Jersey heifers grazing on a ladino clover dominant pasture, and its influence on fertility and udder of the cattle. In Proceedings of the Faculty of Agriculture, Tokai University, Kumamoto, Japan, 1988; pp. 37–45. [Google Scholar]
- Kallela, K.; Heinonen, K.; Saloniemi, H. Plant oestrogens; the cause of decreased fertility in cows. A case report. Nord. Vet. Med. 1984, 36, 124–129. [Google Scholar]
- Hashem, N.; El-Azrak, K.; El-Din, A.N.; Sallam, S.; Taha, T.; Salem, M. Effects of Trifolium alexandrinum phytoestrogens on oestrous behaviour, ovarian activity and reproductive performance of ewes during the non-breeding season. Anim. Reprod. Sci. 2018, 196, 1–8. [Google Scholar] [CrossRef]
- Lindner, H. Study of the fate of phyto-oestrogens in the sheep by determination of isoflavones and coumestrol in the plasma and adipose tissue. Aust. J. Agric. Res. 1967, 18, 305–333. [Google Scholar] [CrossRef]
- Mathieson, R.; Kitts, W. Binding of phyto-oestrogen and oestradiol-17β by cytoplasmic receptors in the pituitary gland and hypothalamus of the ewe. J. Endocrinol. 1980, 85, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Wocławek-Potocka, I.; Mannelli, C.; Boruszewska, D.; Kowalczyk-Zieba, I.; Waśniewski, T.; Skarżyński, D.J. Diverse effects of phytoestrogens on the reproductive performance: Cow as a model. Int. J. Endocrinol. 2013, 2013, 650984. [Google Scholar] [CrossRef]
- Robinson, J.; Ashworth, C.; Rooke, J.; Mitchell, L.; McEvoy, T. Nutrition and fertility in ruminant livestock. Anim. Feed Sci. Technol. 2006, 126, 259–276. [Google Scholar] [CrossRef]
- Brito, A.F.; Zang, Y. A review of lignan metabolism, milk enterolactone concentration, and antioxidant status of dairy cows fed flaxseed. Molecules 2018, 24, 41. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, H.F.; Neuendorff, D.A.; Pemberton, I.J.; Randel, R.D. Soybean Meal Phytoestrogens Do Not Affect Superovulatory Response or Gross Reporductive System Morphology in Beef Heifers; No. 94-1; Texas A&M University: Overton, TX, USA, 1994. [Google Scholar]

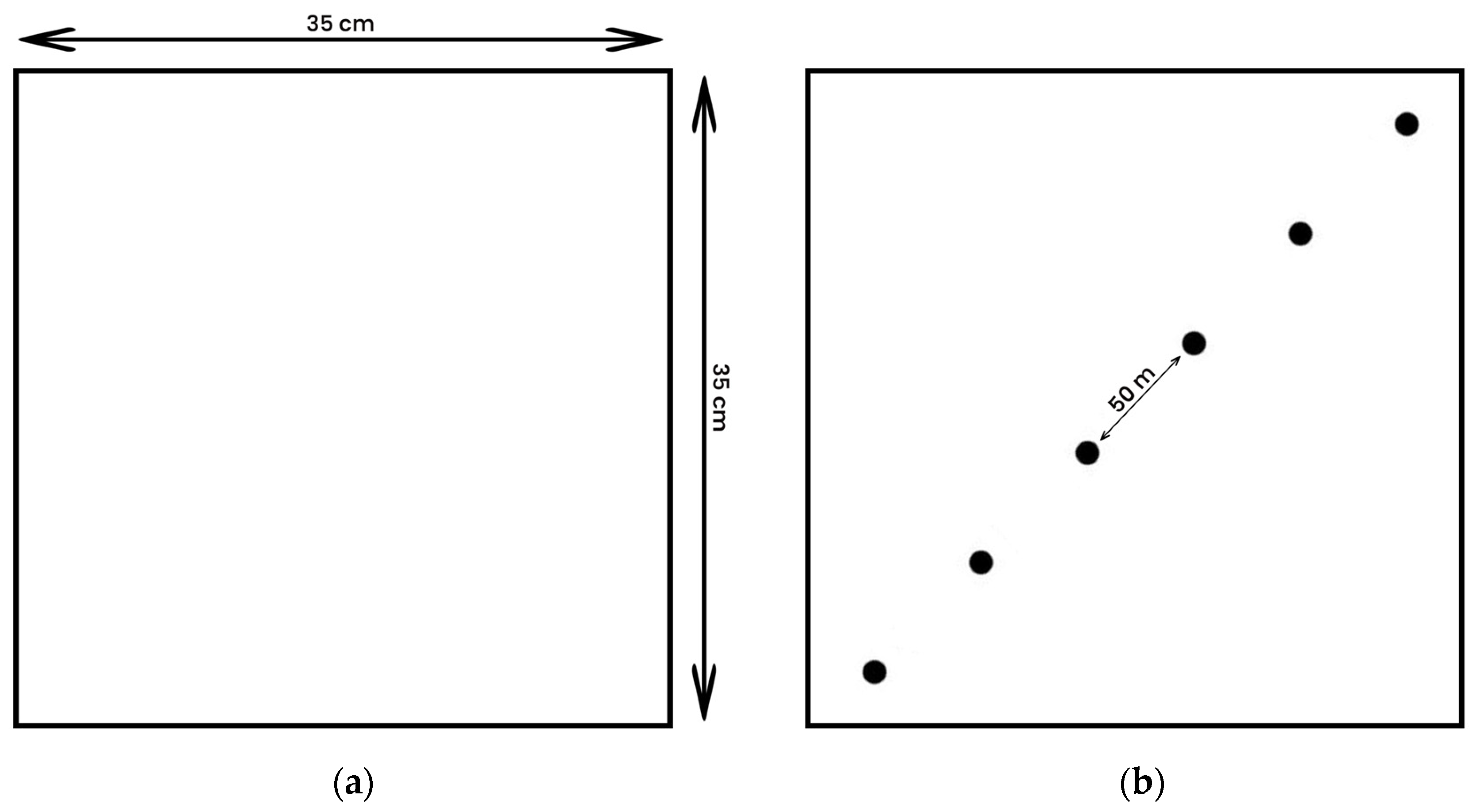

| Area | Lucerne % | Other Legumes a % | Desirable Grasses % | Broadleaf Weeds % | |
|---|---|---|---|---|---|
| G7 | 5.07 ha | 0 | 2 | 81 | 2 |
| L7 | 4.73 ha | 76 | 5 | 15 | 4 |
| L4 | 4.73 ha | 71 | 5 | 21 | 3 |
| GC7 | 5.86 ha | 0 | 19 | 73 | 2 |
| G7 | L7 | L4 | GC7 | |
|---|---|---|---|---|
| biochanin A | 1.13 | 0.68 | 1.04 | 0.23 |
| coumestrol | 2.66 | 2.62 | 0.62 | 0.00 |
| daidzein | 14.02 | 20.81 | 1.02 | 0.00 |
| formononetin | 11.50 | 4.72 | 3.28 | 0.86 |
| genistein | 6.12 | 3.69 | 1.68 | 0.28 |
| Compound | Formula | Exact Mass g/mol | Basis for Identification 1 | RT |
|---|---|---|---|---|
| 3′methoxycoumestrol | C16H10O6 | 298.047738 | AM | 11.96 |
| 4′methoxycoumestrol | C15H8O6 | 282.052823 | AM | 11.4 |
| apigenin | C15H10O5 | 270.052823 | AM | 9.45 |
| apigetrin (apigenin 7-O-glucoside) | C21H20O10 | 432.105647 | AM | 6.7 |
| azelaic acid | C9H16O4 | 188.104859 | AM | 7.45 |
| biochanin A | C16H12O5 | 284.068474 | STD | 11.26 |
| coumestrin (coumestrol 3-O-glucoside) | C21H18O10 | 430.089997 | AM | 7.6 |
| coumestrol | C15H8O5 | 268.037173 | STD | 9.53 |
| daidzein | C15H10O4 | 254.057909 | STD | 8.3 |
| ferulic acid | C10H10O4 | 194.057909 | AM | 6.7 |
| formononetin | C16H12O4 | 268.073559 | STD | 10.0 |
| genistein | C15H10O5 | 270.052824 | STD | 9.37 |
| hyperoside (quercetin 3-galactoside) | C21H20O12 | 464.095476 | AM | 6.8 |
| isorhamnetin 3-O-glucoside | C22H22O12 | 478.111126 | AM | 7.2 |
| luteoloside (luteolin 7-glucoside) | C21H20O11 | 448.100561 | AM | 7.3 |
| p-coumaric acid | C9H8O3 | 164.047344 | AM | 6.3 |
| pratensein | C16H12O6 | 300.063388 | AM | 10.6 |
| quercetin | C15H8O6 | 302.042653 | AM | 8.7 |
| rutin | C27H30O16 | 610.153385 | AM | 6.6 |
| Day 35 | Day 39 | |||||
|---|---|---|---|---|---|---|
| >1 cm | <1 cm | AF | >1 cm | <1 cm | AF | |
| G7 | 5 | 1 | 41 | 14 | 2 | 75 |
| L4 | 4 | 0 | 40 | 14 | 2 | 76 |
| L7 | 5 | 0 | 67 | 17 | 2 | 66 |
| GC7 | 4 | 2 | 45 | 13 | 5 | 75 |
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|
| G7 | 13 | 3 | 7 | 0 |
| L4 | 9 | 0 | 0 | 0 |
| L7 | 10 | 10 | 4 | 0 |
| GC7 | 0 | 0 | 0 | 0 |
| TOTAL | 32 | 13 | 11 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wyse, J.M.; Nevard, R.P.; Loy, J.; Weston, P.A.; Gurusinghe, S.; McCormick, J.; Weston, L.A.; Stephen, C.P. Effects of Mixed Pasture Legume Phytoestrogens on Superovulatory Response and Embryo Quality in Angus Cows. Animals 2024, 14, 1125. https://doi.org/10.3390/ani14071125
Wyse JM, Nevard RP, Loy J, Weston PA, Gurusinghe S, McCormick J, Weston LA, Stephen CP. Effects of Mixed Pasture Legume Phytoestrogens on Superovulatory Response and Embryo Quality in Angus Cows. Animals. 2024; 14(7):1125. https://doi.org/10.3390/ani14071125
Chicago/Turabian StyleWyse, Jessica M., Rory P. Nevard, Jaymie Loy, Paul A. Weston, Saliya Gurusinghe, Jeffrey McCormick, Leslie A. Weston, and Cyril P. Stephen. 2024. "Effects of Mixed Pasture Legume Phytoestrogens on Superovulatory Response and Embryo Quality in Angus Cows" Animals 14, no. 7: 1125. https://doi.org/10.3390/ani14071125






