Immunostimulatory and Antibacterial Effects of Cannabis sativa L. Leaves on Broilers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Keeping and Study Design
2.2. Industrial Hemp (Cannabis sativa L.) Preparation
2.2.1. Determination of Nutrient Concentration
2.2.2. Determination of Cannabinoid Concentration
2.3. Broiler Vaccination
2.4. Newcastle Disease Antibody Titre Assessment
2.5. Flow Cytometry for T Lymphocyte CD4+ and CD8+ Subpopulations in Peripheral Blood
2.6. Bacteriology
2.7. Statistical Analysis
3. Results
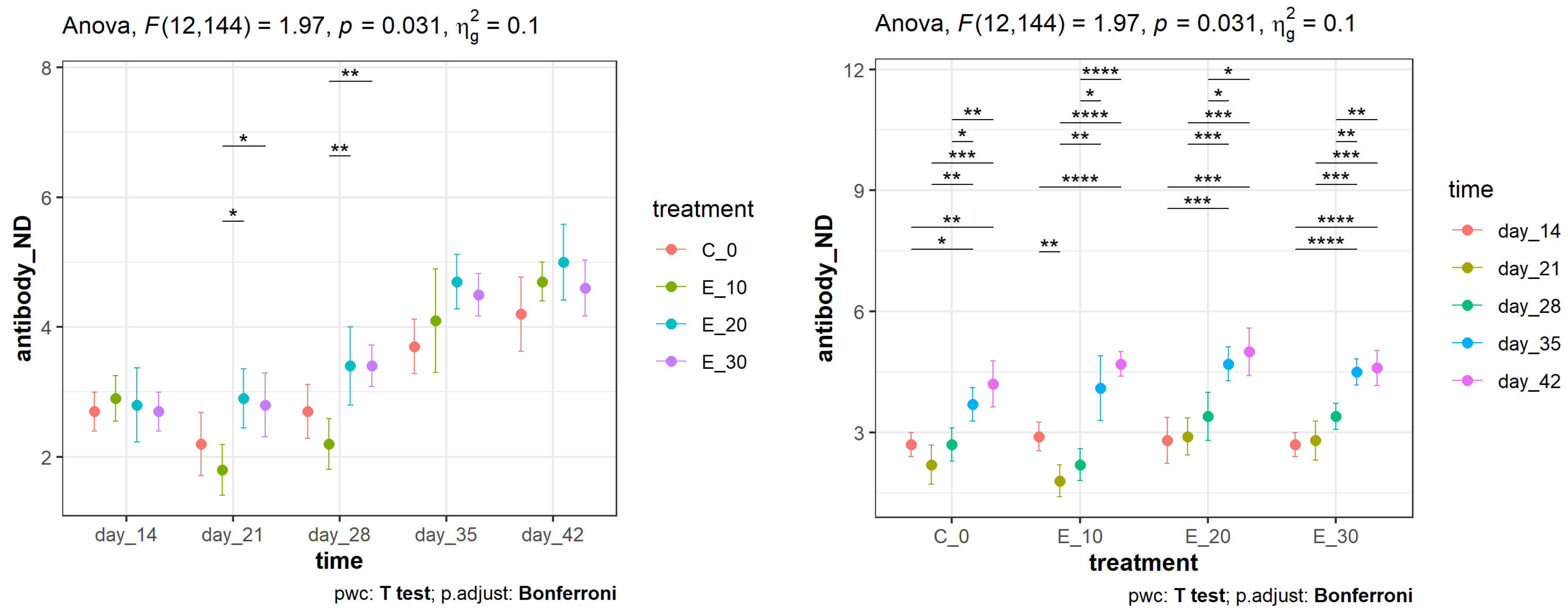
3.1. Effect of the Addition of C. sativa Leaves on NDV Antibody Titres
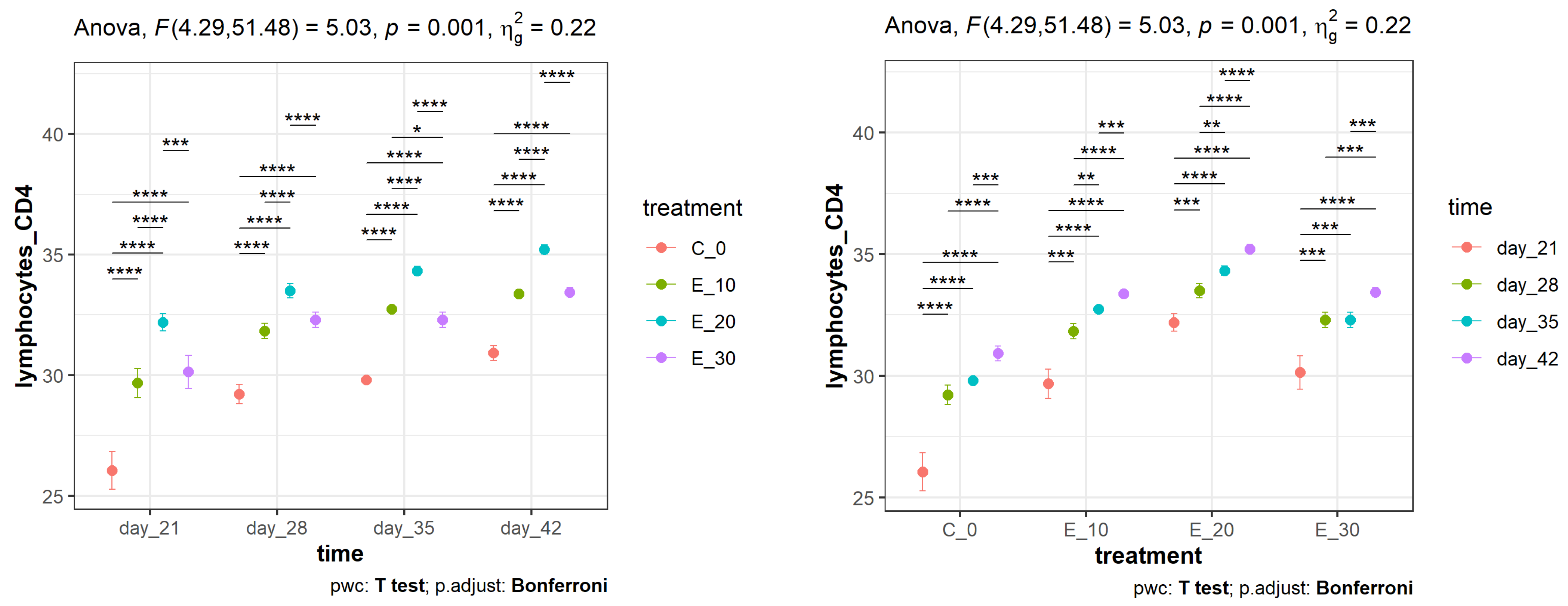
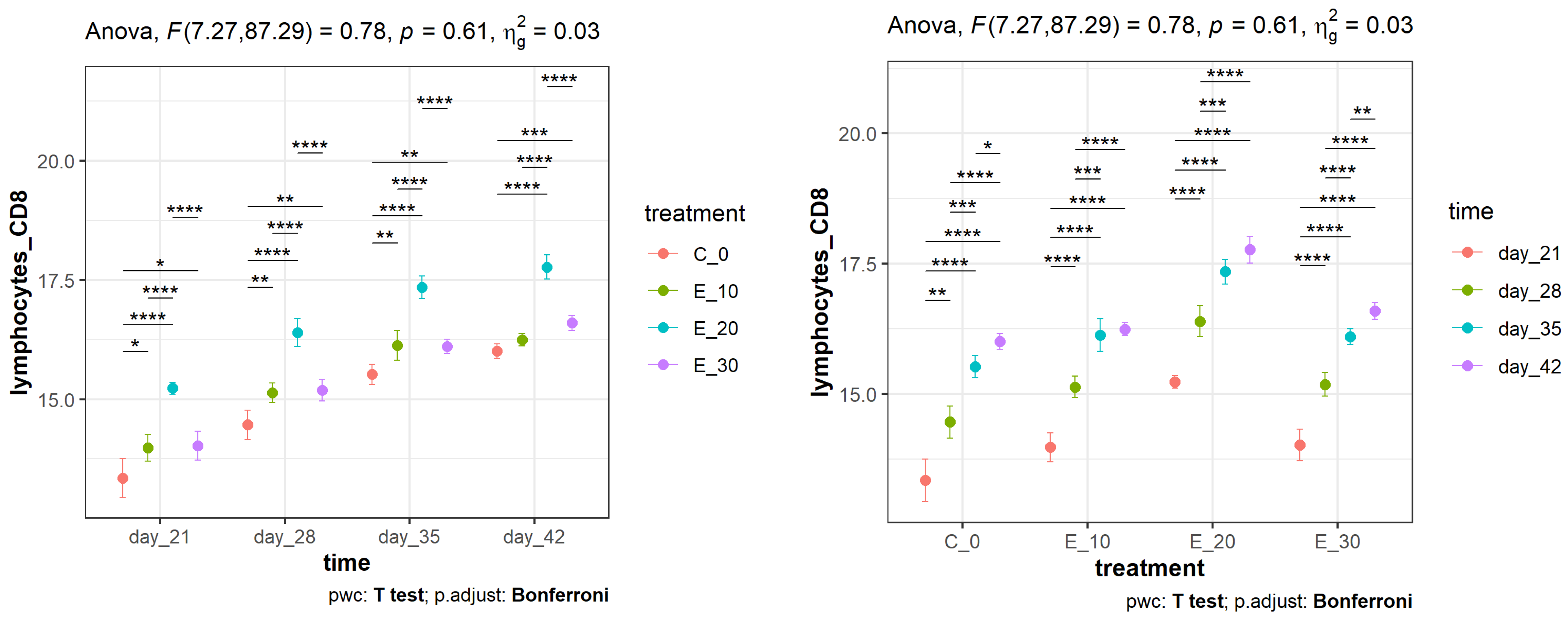
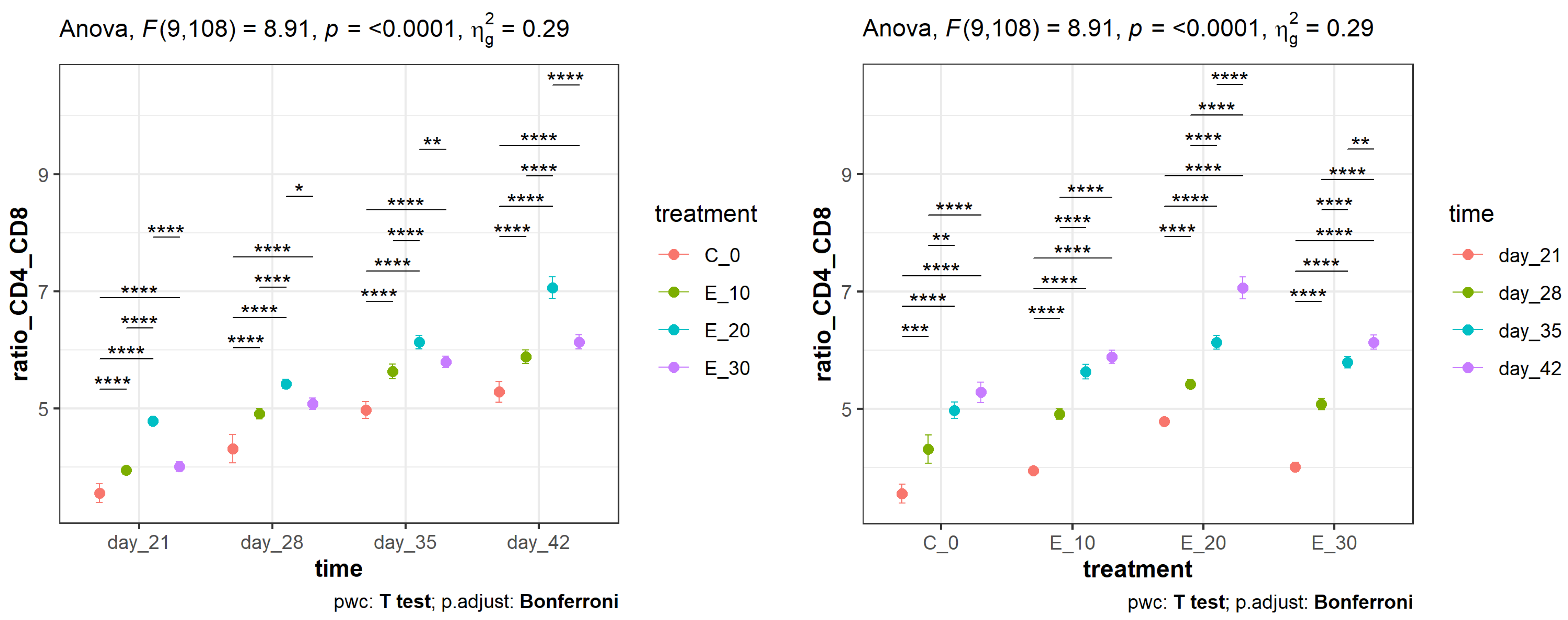
3.2. Effect of Adding C. sativa Leaves on Peripheral Blood CD4+ and CD8+ Lymphocyte Proliferation and CD4+:CD8+ Ratios
3.3. Effect of Adding C. sativa Leaves on E. coli Count in Faeces
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Windisch, W.; Schedle, K.; Plitzner, C.; Kroismayr, A. Use of phytogenic products as feed additives for swine and poultry. J. Anim. Sci. 2008, 86, E140–E148. [Google Scholar] [CrossRef] [PubMed]
- Lillehoj, H.S.; Lee, K.W. Immune modulation of innate immunity as alternatives-to-antibiotics strategies to mitigate the use of drugs in poultry production. Poult Sci. 2012, 91, 1286–1291. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Saad, A.M.; Salem, H.M.; Ashry, N.M.; Abo Ghanima, M.M.; Shukry, M.; Swelum, A.A.; Taha, A.E.; El-Tahan, A.M.; et al. Essential oils and their nanoemulsions as green alternatives to antibiotics in poultry nutrition: A comprehensive review. Poult. Sci. 2022, 101, 101584. [Google Scholar] [CrossRef] [PubMed]
- European Parliament, Council of the European Union. Consolidated text: Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition (Text with EEA relevance). Off. J. Eur. Union 2021, 268, 29–44. [Google Scholar]
- Grashorn, M.A. Use of Phytobiotics in Broiler Nutrition–An Alternative to in Feed Antibiotics? J. Anim. Feed Sci. 2010, 19, 338–347. [Google Scholar] [CrossRef]
- Wati, T.; Ghosh, T.K.; Syed, B.; Haldar, S. Comparative efficacy of a phytogenic feed additive and an antibiotic growth promoter on production performance, caecal microbial population and humoral immune response of broiler chickens inoculated with enteric pathogens. Anim. Nutr. 2015, 1, 213–219. [Google Scholar] [CrossRef]
- Hafeez, A.; Männer, K.; Schieder, C.; Zentek, J. Effect of supplementation of phytogenic feed additives (powdered vs. encapsulated) on performance and nutrient digestibility in broiler chickens. Poult. Sci. 2016, 95, 622–629. [Google Scholar] [CrossRef]
- Abdelli, N.; Solà-Oriol, D.; Pérez, J.F. Phytogenic Feed Additives in Poultry: Achievements, Prospective and Challenges. Animals 2021, 11, 3471. [Google Scholar] [CrossRef]
- Ahsan, U.; Adabi, S.G.; Sayın Özdemir, Ö.; Sevim, Ö.; Tatlı, O.; Kuter, E.; Cengiz, Ö. Growth performance, carcass yield and characteristics, meat quality, serum biochemistry, jejunal histomorphometry, oxidative stability of liver and breast muscle, and immune response of broiler chickens fed natural antioxidant alone or in combination with Bacillus licheniformis. Arch. Anim. Breed. 2022, 65, 183–197. [Google Scholar] [CrossRef]
- Ayalew, H.; Zhang, H.; Wang, J.; Wu, S.; Qiu, K.; Qi, G.; Tekeste, A.; Wassie, T.; Chanie, D. Potential Feed Additives as Antibiotic Alternatives in Broiler Production. Front. Vet. Sci. 2022, 9, 916473. [Google Scholar] [CrossRef]
- Shehata, A.A.; Yalçın, S.; Latorre, J.D.; Basiouni, S.; Attia, Y.A.; Abd El-Wahab, A.; Visscher, C.; El-Seedi, H.R.; Huber, C.; Hafez, H.M.; et al. Probiotics, Prebiotics, and Phytogenic Substances for Optimizing Gut Health in Poultry. Microorganisms 2022, 10, 395. [Google Scholar] [CrossRef] [PubMed]
- Gadde, U.; Kim, W.H.; Oh, S.T.; Lillehoj, H.S. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: A review. Anim. Health Res. Rev. 2017, 18, 26–45. [Google Scholar] [CrossRef] [PubMed]
- Alagawany, M.; Elnesr, S.S.; Farag, M.R.; Abd El-Hack, M.E.; Barkat, R.A.; Gabr, A.A.; Foda, M.A.; Noreldin, A.E.; Khafaga, A.F.; El-Sabrout, K.; et al. Potential role of important nutraceuticals in poultry performance and health–A comprehensive review. Res. Vet. Sci. 2021, 137, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Salem, H.M.; El-Tahan, A.M.; Soliman, M.M.; Youssef, G.B.A.; Taha, A.E.; Soliman, S.M.; Ahmed, A.E.; El-Kott, A.F.; et al. Alternatives to antibiotics for organic poultry production: Types, modes of action and impacts on bird’s health and production. Poult. Sci. 2022, 101, 101696. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Ghany, W.A. Phytobiotics in Poultry Industry as Growth Promoters, Antimicrobials and Immunomodulators–A Review. Worlds Poult. Sci. J. 2020, 10, 571–579. [Google Scholar] [CrossRef]
- Rafeeq, M.; Bilal, R.M.; Batool, F.; Yameen, K.; Farag, M.R.; Madkour, M.; Elnesr, S.S.; El-Shall, N.A.; Dhama, K.; Alagawany, M. Application of herbs and their derivatives in broiler chickens: A review. Worlds Poult. Sci. J. 2023, 79, 95–117. [Google Scholar] [CrossRef]
- Small, E.; Cronquist, A. A practical and natural taxonomy for Cannabis. Taxon 1976, 25, 405–435. [Google Scholar] [CrossRef]
- Radwan, M.M.; Chandra, S.; Gul, S.; ElSohly, M.A. Cannabinoids, Phenolics, Terpenes and Alkaloids of Cannabis. Molecules 2021, 26, 2774. [Google Scholar] [CrossRef] [PubMed]
- Kleinhenz, M.D.; Magnin, G.; Ensley, S.M.; Griffin, J.J.; Goeser, J.; Lynch, E.; Coetzee, J.F. Nutrient concentrations, digestibility, and cannabinoid concentrations of industrial hemp plant components. Appl. Anim. Sci. 2020, 36, 489–494. [Google Scholar] [CrossRef]
- Adams, T.K.; Masondo, N.A.; Malatsi, P.; Makunga, N.P. Cannabis sativa: From Therapeutic Uses to Micropropagation and Beyond. Plants 2021, 10, 2078. [Google Scholar] [CrossRef]
- Cerino, P.; Buonerba, C.; Cannazza, G.; D’Auria, J.; Ottoni, E.; Fulgione, A.; Di Stasio, A.; Pierri, B.; Gallo, A. A Review of Hemp as Food and Nutritional Supplement. Cannabis Cannabinoid Res. 2021, 6, 19–27. [Google Scholar] [CrossRef]
- Salami, S.A.; Martinelli, F.; Giovino, A.; Bachari, A.; Arad, N.; Mantri, N. It Is Our Turn to Get Cannabis High: Put Cannabinoids in Food and Health Baskets. Molecules 2020, 25, 4036. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Dai, K.; Xie, Z.; Chen, J. Secondary Metabolites Profiled in Cannabis Inflorescences, Leaves, Stem Barks, and Roots for Medicinal Purposes. Sci. Rep. 2020, 10, 3309. [Google Scholar] [CrossRef] [PubMed]
- Flores-Sanchez, I.J.; Verpoorte, R. Secondary metabolism in cannabis. Phytochem. Rev. 2008, 7, 615–639. [Google Scholar] [CrossRef]
- Tan, Z.; Halter, B.; Liu, D.; Gilbert, E.R.; Cline, M.A. Dietary Flavonoids as Modulators of Lipid Metabolism in Poultry. Front. Physiol. 2022, 13, 863860. [Google Scholar] [CrossRef] [PubMed]
- Basiouni, S.; Tellez-Isaias, G.; Latorre, J.D.; Graham, B.D.; Petrone-Garcia, V.M.; El-Seedi, H.R.; Yalçın, S.; El-Wahab, A.A.; Visscher, C.; May-Simera, H.L.; et al. Anti-Inflammatory and Antioxidative Phytogenic Substances against Secret Killers in Poultry: Current Status and Prospects. Vet. Sci. 2023, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- Della Rocca, G.; Di Salvo, A. Hemp in Veterinary Medicine: From Feed to Drug. Front. Vet. Sci. 2020, 7, 387. [Google Scholar] [CrossRef] [PubMed]
- Stastnik, O.; Pavlata, L.; Mrkvicova, E. The Milk Thistle Seed Cakes and Hempseed Cakes are Potential Feed for Poultry. Animals 2020, 10, 1384. [Google Scholar] [CrossRef]
- Fallahi, S.; Bobak, Ł.; Opaliński, S. Hemp in Animal Diets–Cannabidiol. Animals 2022, 12, 2541. [Google Scholar] [CrossRef]
- McPartland, J.M.; Russo, E.B. Cannabis and Cannabis Extracts: Greater Than the Sum of Their Parts? J. Cannabis Ther. 2001, 1, 103–132. [Google Scholar] [CrossRef]
- Aviagen Inc. Broiler Nutrition Specifications. 2022. Available online: https://aviagen.com/assets/Tech_Center/Ross_Broiler/Ross-BroilerNutritionSpecifications2022-EN.pdf (accessed on 8 January 2024).
- ISO 6865:2000; Animal Feeding Stuffs—Determination of Crude Fibre Content—Method with Intermediate Filtration. ISO International Organization for Standardization: Geneva, Switzerland, 2000. Available online: https://www.iso.org (accessed on 11 April 2023).
- ISO 6492:1999; Animal Feeding Stuffs—Determination of Fat Content. ISO International Organization for Standardization: Geneva, Switzerland, 1999. Available online: https://www.iso.org (accessed on 11 April 2023).
- ISO 6496:1999; Animal Feeding Stuffs—Determination of Moisture and Other Volatile Matt. ISO International Organization for Standardization: Geneva, Switzerland, 1999. Available online: https://www.iso.org (accessed on 11 April 2023).
- ISO 5983-1:2005; Animal Feeding Stuffs—Determination of Nitrogen Content and Calculation of Crude Protein Content—Part 1: Kjeldahl Method. ISO International Organization for Standardization: Geneva, Switzerland, 2005. Available online: https://www.iso.org (accessed on 11 April 2023).
- ISO 5983-2:2009; Animal Feeding Stuffs—Determination of Nitrogen Content and Calculation of Crude Protein Content—Part 2: Block Digestion and Steam Distillation Method. ISO International Organization for Standardization: Geneva, Switzerland, 2009. Available online: https://www.iso.org (accessed on 11 April 2023).
- ISO 6493:2000; Animal Feeding Stuffs—Determination of Starch Content—Polarimetric Method. ISO International Organization for Standardization: Geneva, Switzerland, 2000. Available online: https://www.iso.org (accessed on 11 April 2023).
- ISO 5984:2022; Animal Feeding Stuffs—Determination of Crude Ash. ISO International Organization for Standardization: Geneva, Switzerland, 2022. Available online: https://www.iso.org (accessed on 11 April 2023).
- ISO 6491:1998; Animal Feeding Stuffs—Determination of Phosphorus Content—Spectrometric Method. ISO International Organization for Standardization: Geneva, Switzerland, 1998. Available online: https://www.iso.org (accessed on 11 April 2023).
- ISO 6869:2000; Animal Feeding Stuffs—Determination of the Contents of Calcium, Copper, Iron, Magnesium, Manganese, Potassium, Sodium and Zinc—Method Using Atomic Absorption Spectrometry. ISO International Organization for Standardization: Geneva, Switzerland, 2000. Available online: https://www.iso.org (accessed on 11 April 2023).
- WPSA, WORLD’S POULTRY SCIENCE ASSOCIATION. Nutrition of the European Federation of Branches Subcommittee Energy of the Working Group (Beekbergen Netherlands). In European Table of Energy Values for Poultry Feedstuffs, 3rd ed.; WPSA: Beekbergen, The Netherlands, 1989. [Google Scholar]
- Nelson, N. A photometric adaptation of the Somogyi method for the determination of glucose. J. Biol. Chem. 1944, 153, 375–385. [Google Scholar] [CrossRef]
- Somogy, M. A reagent for the copper-iodometric determination of very small amounts of sugar. J. Biol. Chem. 1937, 117, 771–776. [Google Scholar] [CrossRef]
- Saingam, W.; Sakunpak, A. Development and validation of reverse phase high performance liquid chromatography method for the determination of delta-9-tetrahydrocannabinol and cannabidiol in oromucosal spray from cannabis extract. Rev. Bras. Farmacogn. 2018, 28, 669–672. [Google Scholar] [CrossRef]
- WOAH, The World Organisation for Animal Health. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. Newcastle Disease (Infection with Newcastle Disease Virus). 2022. Chapter 3.3.14. Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/A_summry.htm (accessed on 8 January 2024).
- ISO 21528-2:2017; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Enterobacteriaceae—Part 2: Colony-Count Technique (ISO 21528-2:2017, Corrected version 2018-06-01; EN ISO 21528-2:2017). ISO International Organization for Standardization: Geneva, Switzerland, 2017. Available online: https://www.iso.org (accessed on 14 March 2023).
- ISO 6887-6:2013; Microbiology of Food and Animal Feed—Preparation of Test Samples, Initial Suspension and Decimal Dilutions for Microbiological Examination—Part 6: Specific Rules for the Preparation of Samples Taken at the Primary Production Stage. ISO International Organization for Standardization: Geneva, Switzerland, 2013. Available online: https://www.iso.org (accessed on 14 March 2023).
- ISO 6887-1:2017; Microbiology of the Food Chain—Preparation of Test Samples, Initial Suspension and Decimal Dilutions for Microbiological Examination—Part 1: General Rules for the Preparation of the Initial Suspension and Decimal Dilutions. ISO International Organization for Standardization: Geneva, Switzerland, 2017. Available online: https://www.iso.org (accessed on 14 March 2023).
- ISO 6579-1:2017/AMD 1:2020; Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella spp.—Amendment 1: Broader Range of Incubation Temperatures, Amendment to the Status of Anex D, Correction of the Composition of MSRV and SC. ISO International Organization for Standardization: Geneva, Switzerland, 2020. Available online: https://www.iso.org (accessed on 14 March 2023).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org (accessed on 8 January 2024).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; D’Agostino McGowan, L.; François, R.; Grolemund, G.; Hayer, A.; Henry, L.; Hester, J.; et al. Welcome to the tidyverse. J. Open Source Softw. 2019, 43, 1686. [Google Scholar] [CrossRef]
- Hebbali, A.; descriptr: Generate Descriptive Statistics. R Package Version 0.5.2. 2021. Available online: https://CRAN.R-project.org/package=descriptr (accessed on 8 January 2024).
- Kassambara, A.; ggpubr: “ggplot2” Based Publication Ready Plots. R Package Version 0.4.0. 2020. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 8 January 2024).
- Kassambara, A.; rstatix: Pipe-Friendly Framework for Basic Statistical Tests. R Package Version 0.7.0. 2021. Available online: https://CRAN.R-project.org/package=rstatix (accessed on 8 January 2024).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: Cham, Switzerland; New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Kikusato, M. Phytobiotics to improve health and production of broiler chickens: Functions beyond the antioxidant activity. Anim. Biosci. 2021, 34, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Farhoomand, P.; Nourmohammadi, R. Effects of Different Levels of Hemp Seed (Cannabis Sativa L.) and Dextran Oligosaccharide on Growth Performance and Antibody Titer Response of Broiler Chickens. Ital. J. Anim. Sci. 2015, 14, 1. [Google Scholar] [CrossRef]
- Talebi, A.; Maham, M.; Asri-Rezaei, S.; Pournaghi, P.; Khorrami, M.-S.; Derakhshan, A. Effects of Nigella sativa on Performance, Blood Profiles, and Antibody Titer against Newcastle Disease in Broilers. J. Evid. Based. Complement. Altern. Med. 2021, 2021, 2070375. [Google Scholar] [CrossRef] [PubMed]
- Stüve, O.; Marra, C.M.; Bar-Or, A.; Niino, M.; Cravens, P.D.; Cepok, S.; Frohman, E.M.; Phillips, J.T.; Arendt, G.; Jerome, K.R.; et al. Altered CD4+/CD8+ T-cell ratios in cerebrospinal fluid of natalizumab-treated patients with multiple sclerosis. Arch. Neurol. 2006, 63, 1383–1387. [Google Scholar] [CrossRef] [PubMed]
- Spangler, J.B.; Tomala, J.; Luca, V.C.; Jude, K.M.; Dong, S.; Ring, A.M.; Votavova, P.; Pepper, M.; Kovar, M.; Garcia, K.C. Antibodies to Interleukin-2 Elicit Selective T Cell Subset Potentiation through Distinct Conformational Mechanisms. Immunity 2015, 42, 815–825. [Google Scholar] [CrossRef]
- Lee, S.H.; Lillehoj, H.S.; Hong, Y.H.; Jang, S.I.; Lillehoj, E.P.; Ionescu, C.; Mazuranok, L.; Bravo, D. In vitro effects of plant and mushroom extracts on immunological function of chicken lymphocytes and macrophages. Br. Poult. Sci. 2010, 51, 213–221. [Google Scholar] [CrossRef]
- Pourhossein, Z.; Qotbi, A.A.; Seidavi, A.; Laudadio, V.; Centoducati, G.; Tufarelli, V. Effect of different levels of dietary sweet orange (Citrus sinensis) peel extract on humoral immune system responses in broiler chickens. Anim. Sci. J. 2015, 86, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Bolukbasi, S.C.; Erhan, M.K. Effect of dietary thyme (Thyme vulgaris) on laying hens’ performance and Escherichia coli (E. coli) concentration in feces. Int. J. Nat. Engin. Sci. 2007, 1, 55–58. [Google Scholar]
- Balenović, M.; Savić, V.; Janječić, Z.; Popović, M.; Šimpraga, B.; Carović- Stanko, K.; Bedeković, D.; Amšel Zelenika, T. Immunomodulatory and antimicrobial effects of selected herbs on laying hens. Vet. Arhiv. 2018, 88, 673–686. [Google Scholar] [CrossRef]
| Item | Starter (Day 1–21) | Finisher (Day 22–42) |
|---|---|---|
| Ingredient (%) | ||
| Maize | 45.20 | 55.00 |
| Soybean meal (46%) | 17.80 | 9.50 |
| Soybean cake | 14.00 | 14.00 |
| Wheat | 11.00 | 9.50 |
| Sunflower meal (35%) | 7.00 | 7.00 |
| Premix 1,2 | 5.00 | 5.00 |
| Total | 100.00 | 100.00 |
| Nutrient content analysed | ||
| Crude protein (%) | 20.37 | 17.72 |
| Crude fibre (%) | 4.49 | 4.29 |
| Ash (%) | 5.45 | 3.84 |
| Total fat (%) | 5.26 | 4.31 |
| Water (%) | 11.15 | 12.91 |
| Starch (%) | 42.33 | 46.33 |
| Sugar content (%) | 3.96 | 2.99 |
| Ca (%) | 0.98 | 0.54 |
| P (%) | 0.73 | 0.45 |
| Na (%) | 0.196 | 0.158 |
| Mg (%) | 0.20 | 0.16 |
| K (%) | 0.10 | 0.683 |
| Cu (mg/kg) | 13.00 | 26.00 |
| Mn (mg/kg) | 110.00 | 130.00 |
| Zn (mg/kg) | 144.00 | 146.00 |
| Fe (mg/kg) | 362.00 | 244.00 |
| ME * (MJ/kg) | 12.54 | 12.35 |
| Unit | Industrial Hemp Leaves (C. sativa L.) | |
|---|---|---|
| Crude protein | % | 18.92 |
| Crude fibre | % | 15.75 |
| Ash | % | 14.34 |
| Total fat | % | 9.69 |
| Water | % | 5.26 |
| Minerals | ||
| Ca | % | 4.12 |
| P | % | 0.43 |
| Na | % | 0.017 |
| Mg | % | 0.58 |
| K | % | 1.61 |
| Cu | mg/kg | 15.10 |
| Mn | mg/kg | 35.53 |
| Zn | mg/kg | 47.96 |
| Fe | mg/kg | 186.53 |
| Cannabinoids in hemp leaves | ||
| CBD | µg/kg | 242.51 |
| CBG | µg/kg | 7.55 |
| CBN | µg/kg | 4.55 |
| Day of Experiment | E. coli Count (log CFU g−1) × 106x ± SEM | |||
|---|---|---|---|---|
| C_0 | E_10 | E_20 | E_30 | |
| 7 | 4.65 ± 0.06 | 3.58 ± 0.05 * | 2.78 ± 0.05 * | 3.78 ± 0.05 * |
| 14 | 5.15 ± 0.06 | 5.08 ± 0.01 | 5.33 ± 0.02 | 4.48 ± 0.05 |
| 21 | 5.75 ± 0.06 | 5.02 ± 0.01 | 5.04 ± 0.02 ** | 4.16 ± 0.02 ** |
| 28 | 5.58 ± 0.05 | 4.08 ± 0.05 ** | 3.78 ± 0.05 *,** | 3.58 ± 0.05 *,** |
| 35 | 5.50 ± 0.04 | 3.93 ± 0.05 * | 3.18 ± 0.05 *,** | 3.18 ± 0.05 *,** |
| 42 | 5.53 ± 0.03 | 3.80 ± 0.05 * | 3.20 ± 0.07 * | 3.13 ± 0.08 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balenović, M.; Janječić, Z.; Savić, V.; Kasap, A.; Popović, M.; Šimpraga, B.; Sokolović, M.; Bedeković, D.; Kiš, G.; Zglavnik, T.; et al. Immunostimulatory and Antibacterial Effects of Cannabis sativa L. Leaves on Broilers. Animals 2024, 14, 1159. https://doi.org/10.3390/ani14081159
Balenović M, Janječić Z, Savić V, Kasap A, Popović M, Šimpraga B, Sokolović M, Bedeković D, Kiš G, Zglavnik T, et al. Immunostimulatory and Antibacterial Effects of Cannabis sativa L. Leaves on Broilers. Animals. 2024; 14(8):1159. https://doi.org/10.3390/ani14081159
Chicago/Turabian StyleBalenović, Mirta, Zlatko Janječić, Vladimir Savić, Ante Kasap, Maja Popović, Borka Šimpraga, Marijana Sokolović, Dalibor Bedeković, Goran Kiš, Tihomir Zglavnik, and et al. 2024. "Immunostimulatory and Antibacterial Effects of Cannabis sativa L. Leaves on Broilers" Animals 14, no. 8: 1159. https://doi.org/10.3390/ani14081159






