Contaminants of Emerging Concern: Antibiotics Research in Mussels from the Coasts of the Tyrrhenian Sea (Sardinia, Italy)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
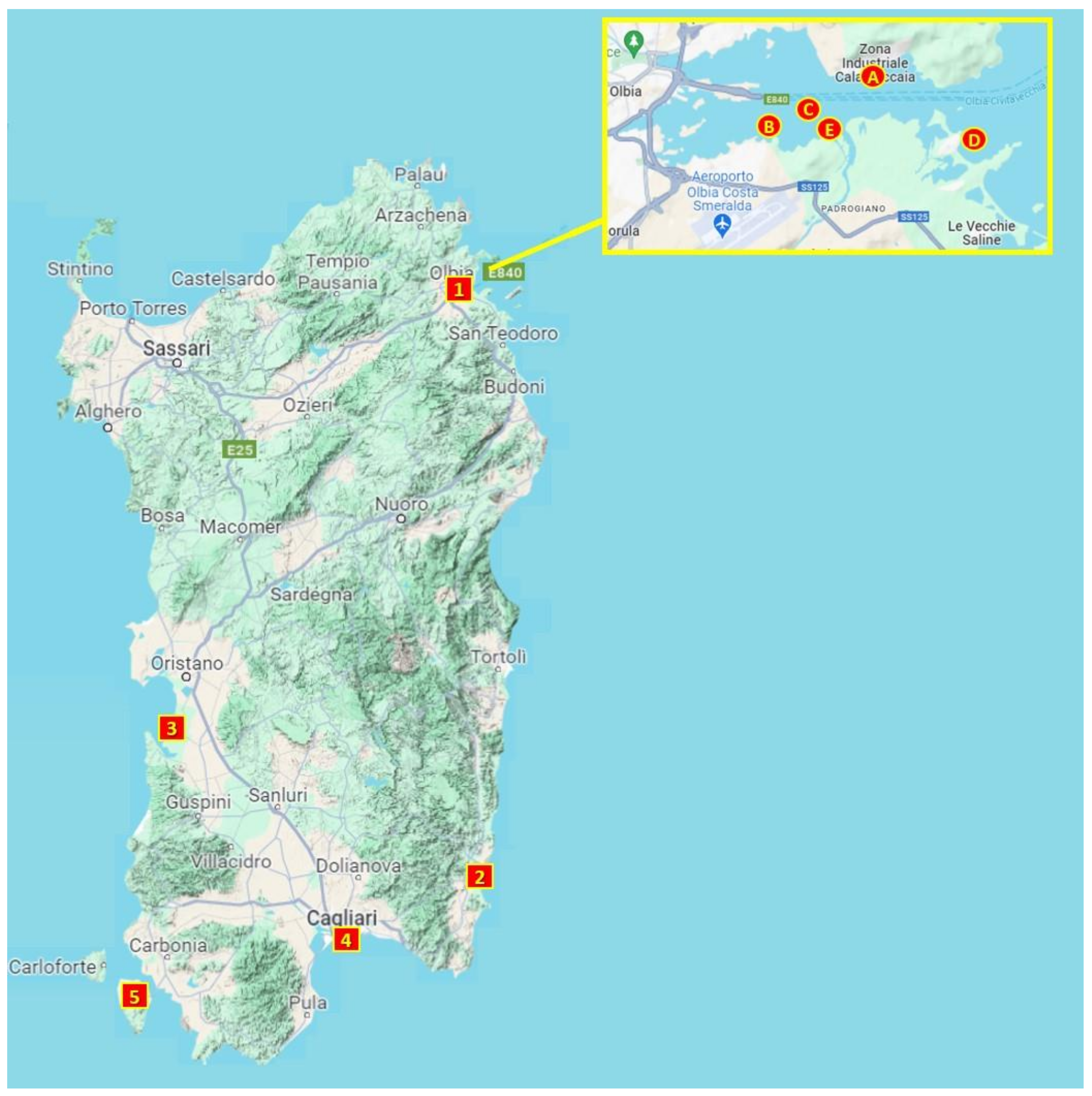
2.2. Sample Collection and Study Area
2.3. Sample Extraction
2.4. LC-MS/MS Analysis
2.5. Method Validation
2.6. Statistical Analysis
3. Results and Discussion
3.1. Method Validation
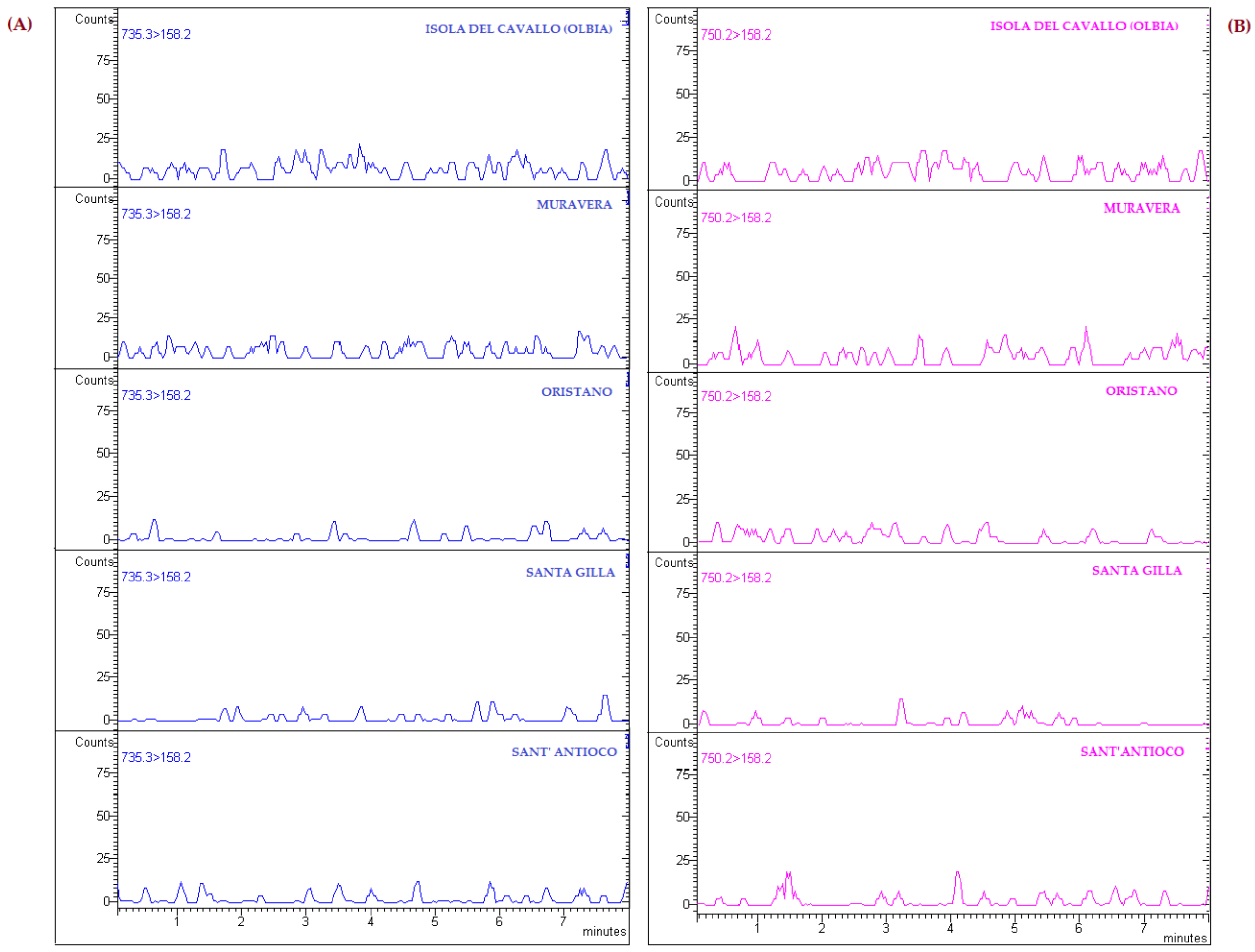
3.2. Application to Real Samples
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, M.; Rajput, D.; Kumar, V.; Jatain, I.; Aminabhavi, T.M.; Mohanakrishna, G.; Kumar, R.; Dubey, K.K. Photocatalytic degradation of four emerging antibiotic contaminants and toxicity assessment in wastewater: A comprehensive study. Environ. Res. 2023, 231, 116132. [Google Scholar] [CrossRef] [PubMed]
- Browne, A.J.; Chipeta, M.G.; Haines-Woodhouse, G.; Kumaran, E.P.A.; Hamadani, B.H.K.; Zaraa, S.; Henry, N.J.; Deshpande, A.; Reiner, R.C.; Day, N.P.J.; et al. Global antibiotic consumption and usage in humans, 2000–2018: A spatial modelling study. Lancet Planet. Health 2021, 5, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Oharisi, O.-o.L.; Ncube, S.; Nyoni, H.; Madikizela, M.L.; Olowoyo, O.J.; Maseko, B.R. Occurrence and prevalence of antibiotics in wastewater treatment plants and effluent receiving rivers in South Africa using UHPLC-MS determination. J. Environ. Manag. 2023, 345, 118621. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.W.K.; Millar, B.C.; Moore, J.E. Antimicrobial Resistance (AMR). Br. J. Biomed. Sci. 2023, 80, 11387. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, S.; Shrestha, P.; Adhikari, B. Antimicrobial use in food animals and human health: Time to implement ‘One Health’ approach. Antimicrob. Resist. Infect. Control 2020, 9, 181. [Google Scholar] [CrossRef] [PubMed]
- Burridge, L.; Weis, J.S.; Cabello, F.; Pizarro, J.; Bostick, K. Chemical use in salmon aquaculture: A review of current practices and possible environmental effects. Aquaculture 2010, 306, 7–23. [Google Scholar] [CrossRef]
- Okocha, R.C.; Olatoye, I.O.; Adedeji, O.B. Food safety impacts of antimicrobial use and their residues in aquaculture. Public Health Rev. 2018, 39, 21. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, A.F.; Santos, L.; Rosa, J.; Leston, S.; Barbosa, J.; Vila Pouca, A.S.; Freitas, A.; Ramos, F. Development and validation of a multi-residue and multi-class screening method of 44 antibiotics in salmon (Salmo salar) using ultra-high-performance liquid chromatography/time-of-flight mass spectrometry: Application to farmed salmon. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1118–1119, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Ya, H.; Zhang, T.; Xing, Y.; Lv, M.; Wang, X.; Jiang, B. Co-existence of polyethylene microplastics and tetracycline on soil microbial community and ARGs. Chemosphere 2023, 335, 139082. [Google Scholar] [CrossRef]
- Xiang, Y.; Wu, H.; Li, L.; Ren, M.; Qie, H.; Lin, A. A review of distribution and risk of pharmaceuticals and personal care products in the aquatic environment in China. Ecotoxicol. Environ. Saf. 2021, 213, 112044. [Google Scholar] [CrossRef]
- Charuaud, L.; Jarde, E.; Jaffrezic, A.; Thomas, M.F.; Le Bot, B. Veterinary pharmaceutical residues from natural water to tap water: Sales, occurrence and fate. J. Hazard. Mater. 2019, 321, 169–186. [Google Scholar] [CrossRef] [PubMed]
- Smaldone, G.; Marrone, R.; Cappiello, S.; Martin, G.A.; Oliva, G.; Cortesi, M.L.; Anastasio, A. Occurrence of antibiotic resistance in bacteria isolated from seawater organisms caught in Campania Region: Preliminary study. BMC Vet. Res. 2014, 10, 161. [Google Scholar] [CrossRef] [PubMed]
- Mello, F.V.; Cunha, S.C.; Fogaça, F.H.S.; Alonso, M.B.; Torres, J.P.M.; Fernandes, J.O. Occurrence of pharmaceuticals in seafood from two Brazilian coastal areas: Implication for human risk assessment. Sci. Total Environ. 2022, 803, 14974. [Google Scholar] [CrossRef] [PubMed]
- Baralla, E.; Demontis, M.P.; Dessì, F.; Varoni, M.V. An overview of antibiotics as emerging contaminants: Occurrence in bivalves as biomonitoring organisms. Animals 2021, 11, 3239. [Google Scholar] [CrossRef] [PubMed]
- Varol, M.; Sünbül, M.R. Organochlorine pesticide, antibiotic and heavy metal residues in mussel, crayfish and fish species from a reservoir on the Euphrates River, Turkey. Environ. Pollut. 2017, 230, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Capolupo, M.; Franzellitti, S.; Valbonesi, P.; Lanzas, C.S.; Fabbri, E. Uptake and transcriptional effects of polystyrene microplastics in larval stages of the Mediterranean mussel Mytilus galloprovincialis. Environ. Pollut. 2018, 241, 1038–1042. [Google Scholar] [CrossRef] [PubMed]
- Rocha, T.L.; Gomes, T.; Cardoso, C.; Letendre, J.; Pinheiro, J.P.; Sousa, V.S.; Teixeira, M.R.; Bebianno, M.J. Immunocytotoxicity, cytogenotoxicity and genotoxicity of cadmiumbased quantum dots in the marine mussel Mytilus galloprovincialis. Mar. Environ. Res. 2014, 101, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Alesci, A.; Di Paola, D.; Fumia, A.; Marino, S.; D’Iglio, C.; Famulari, S.; Albano, M.; Spanò, N.; Lauriano, E. Internal Defense System of Mytilus galloprovincialis (Lamarck, 1819): Ecological Role of Hemocytes as Biomarkers for Thiacloprid and Benzo[a]Pyrene Pollution. Toxins 2023, 11, 731. [Google Scholar] [CrossRef]
- Farrington, J.W.; Tripp, B.W.; Tanabe, S.; Subramanian, A.; Sericano, J.L.; Wade, T.L.; Knap, A.H.; Edward, D. Goldberg’s proposal of “the Mussel Watch”: Reflections after 40 years. Mar. Pollut. Bull. 2016, 110, 501–510. [Google Scholar] [CrossRef]
- Barreira, J.; Araújo, D.F.; Knoery, J.; Briant, N.; Machado, W.; Grouhel-Pellouin, A. The French Mussel Watch Program reveals the attenuation of coastal lead contamination over four decades. Mar. Pollut. Bull. 2024, 199, 115975. [Google Scholar] [CrossRef]
- European Commission Implementing Decision (EU) 2015/495. Off. J. Eur. Union 2015, L78/40, 40–42.
- Sarkar, S.; Okafor, C. Effect of veterinary feed directive rule changes on tetracycline-resistant and erythromycin-resistant bacteria (Salmonella, Escherichia, and Campylobacter) in retail meats in the United States. PLoS ONE 2023, 18, e0289208. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Laslop, N.; Mankin, A.S. How Macrolide Antibiotics Work. Trends Biochem. Sci. 2018, 43, 668–684. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.S.; Rupasinghe, T.W.; Simpson, J.A.; Vodstrcil, L.A.; Fairley, C.K.; McConville, M.J.; Hocking, J.S. Pharmacokinetics of a single 1g dose of azithromycin in rectal tissue in men. PLoS ONE 2017, 28, e0174372. [Google Scholar] [CrossRef]
- Parnham, M.J.; Haber, V.E.; Giamarellos-Bourboulis, E.J.; Perletti, G.; Verleden, G.M.; Vos, R. Azithromycin: Mechanisms of action and their relevance for clinical applications. Pharmacol. Ther. 2014, 143, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Tadi, D.A.; Fu, J.; Azizian, K.; Kouhsari, E. Global status of Azithromycin and Erythromycin Resistance Rates in Neisseria gonorrhoeae: A Systematic Review and Meta-analysis. Yale J. Biol. Med. 2022, 95, 465–478. [Google Scholar] [PubMed]
- World Health Organization. WHO Model List of Essential Medicines: 21st List; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Heidary, M.; Ebrahimi Samangani, A.; Kargari, A.; Kiani Nejad, A.; Yashmi, I.; Motahar, M.; Taki, E.; Khoshnood, S. Mechanism of action, resistance, synergism, and clinical implications of azithromycin. J. Clin. Lab. Anal. 2022, 36, e24427. [Google Scholar] [CrossRef]
- Gatimu, S.M.; Kimani, R.W. Does mass drug administration of azithromycin reduce child mortality? Lancet Glob. Health 2021, 9, 1485–1486. [Google Scholar] [CrossRef] [PubMed]
- Doan, T.; Worden, L.; Hinterwirth, A.; Arzika, A.M.; Maliki, R.; Abdou, A.; Zhong, L.; Chen, C.; Cook, C.; Lebas, E.; et al. Macrolide and Nonmacrolide Resistance with Mass Azithromycin Distribution. N. Engl. J. Med. 2020, 383, 1941–1950. [Google Scholar] [CrossRef]
- Monahan, C.; Morris, D.; Nag, R.; Cummins, E. Risk ranking of macrolide antibiotics—Release levels, resistance formation potential and ecological risk. Sci. Total Environ. 2023, 859, 160022. [Google Scholar] [CrossRef]
- Li, J.; Liu, L.; Zhang, H.; Guo, J.; Wei, X.; Xue, M.; Ma, X. Severe problem of macrolides resistance to common pathogens in China. Front. Cell. Infect. Microbiol. 2023, 13, 1181633. [Google Scholar] [CrossRef]
- Gautier-Bouchardon, A.V. Antimicrobial Resistance in Mycoplasma spp. Microbiol. Spectr. 2018, 6, 4. [Google Scholar] [CrossRef]
- Desmarchelier, A.; Bessaire, T.; Savoy, M.C.; Tarres, A.; Mujahid, C.; Beck, A.; Mottier, P.; Delatour, T. Screening of 154 Veterinary Drug Residues in Foods of Animal Origin Using LC-MS/MS: First Action 2020.04. J. AOAC Int. 2021, 104, 650–681. [Google Scholar] [CrossRef] [PubMed]
- Guidi, L.R.; Santos, F.A.; Ribeiro, A.C.S.R.; Fernandes, C.; Silva, L.H.M.; Gloria, M.B.A. Quinolones and tetracyclines in aquaculture fish by a simple and rapid LC-MS/MS method. Food Chem. 2018, 245, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Fabregat-Safont, D.; Gracia-Marín, E.; Ibáñez, M.; Pitarch, E.; Hernández, F. Analytical key issues and challenges in the LC-MS/MS determination of antibiotics in wastewater. Anal. Chim. Acta 2023, 1239, 340739. [Google Scholar] [CrossRef]
- Nunes, M.J.; Paz, V.; Cordas, C.M.; Noronha, J.P.; Branco, L.C. LC-MS/MS methodology development and validation for the screening and quantification of five antibiotics in water. Anal. Methods 2022, 14, 935–948. [Google Scholar] [CrossRef]
- Shen, K.; Zou, X.; Wang, J. Simultaneous determination of the four key fluoroquinolones and two antipsychotics in fish and shrimp by LC-MS/MS. Food Addit. Contam.—Part A Chem. Anal. Control Expo. Risk Assess. 2022, 39, 678–686. [Google Scholar] [CrossRef]
- Jadhav, M.R.; Pudale, A.; Raut, P.; Utture, S.; Ahammed Shabeer, T.P.; Banerjee, K. A unified approach for high-throughput quantitative analysis of the residues of multi-class veterinary drugs and pesticides in bovine milk using LC-MS/MS and GC–MS/MS. Food Chem. 2019, 272, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Alija, G.; Hajrulai-Musliu, Z.; Uzunov, R. Development and validation of confirmatory LC–MS/MS method for multi-residue analysis of antibiotic drugs in bovine milk. SN Appl. Sci. 2020, 2, 1563. [Google Scholar] [CrossRef]
- Magalhães, D.; Freitas, A.; Sofia Vila Pouca, A.; Barbosa, J.; Ramos, F. The use of ultra-high-pressure-liquid-chromatography tandem time-of-flight mass spectrometry as a confirmatory method in drug residue analysis: Application to the determination of antibiotics in piglet liver. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1153, 122264. [Google Scholar] [CrossRef]
- Viale, I.; Olla, G.; Salati, F. Acquacultura in Sardegna, Tradizioni, Innovazione, Sapori e Ambiente. Agenzia Laore Sardegna: Cagliari, Italy, 2016; 52p. Available online: https://www.sardegnaagricoltura.it/documenti/14_43_20160616142206.pdf (accessed on 20 February 2024).
- Mudadu, A.G.; Spanu, C.; Salza, S.; Piras, G.; Uda, M.T.; Giagnoni, L.; Fois, G.; Pereira, J.G.; Pantoja, J.C.F.; Virgilio, S.; et al. Association between rainfall and Escherichia coli in live bivalve molluscs harvested in Sardinia, Italy. Food Res. Int. 2023, 174, 113563. [Google Scholar] [CrossRef] [PubMed]
- Peters, F.T.; Drummer, O.H.; Musshoff, F. Validation of new methods. Forensic Sci. Int. 2007, 165, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Taverniers, I.; De Loose, M.; Van Bockstaele, E. Trends in quality in the analytical laboratory. II. Analytical method validation and quality assurance. TrAC Trends Anal. Chem. 2004, 23, 535–552. [Google Scholar] [CrossRef]
- Bacanlı, M.; Başaran, N. Importance of antibiotic residues in animal food. Food Chem. Toxicol. 2019, 125, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ying, G.G.; Deng, W.J. Antibiotic Residues in Food: Extraction, Analysis, and Human Health Concerns. J. Agric. Food Chem. 2019, 67, 7569–7586. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Huang, Z.; Ohore, O.E.; Yang, J.; Peng, K.; Li, S.; Li, X. Impact of antibiotics on microbial community in aquatic environment and biodegradation mechanism: A review and bibliometric analysis. Environ. Sci. Pollut. Res. 2023, 25, 66431–66444. [Google Scholar] [CrossRef] [PubMed]
- Santos-Santórum Suárez, C.; Sanders, P.; Perrin-Guyomard, A.; Hurtaud-Pessel, D.; Laurentie, M.; Viel, A.; Taillandier, J.F.; Lagree, M.P.; Gaugain, M. Validation of a LC-MS/MS method for the quantitative analysis of four antibiotics in pig tissues and plasma to assess the risk of transfer of residues to edible matrices after exposure to cross-contaminated feed. Food Addit. Contam. Part A 2022, 39, 1818–1827. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Analysis of macrolide antibiotics, using liquid chromatography-mass spectrometry, in food, biological and environmental matrices. Mass Spectrom. Rev. 2009, 28, 50–92. [Google Scholar] [CrossRef] [PubMed]
- Messens, W.; Fernandez-Escamez, P.S.; Lees, D.; Lindqvist, R.; O’Mahony, M.; Suffredini, E.; Cortiñas Abrahantes, J.; Chantzis, E.; Koutsoumanis, K. Thermal processing of live bivalve molluscs for controlling viruses: On the need for a risk-based design. Crit. Rev. Food Sci. Nutr. 2018, 58, 2854–2865. [Google Scholar] [CrossRef]
- O’Mahony, M. Eu regulatory risk management of marine biotoxins in the marine bivalve mollusc food-chain. Toxins 2018, 10, 118. [Google Scholar] [CrossRef]
- Walker, D.I.; Younger, A.; Stockley, L.; Baker-Austin, C. Escherichia coli testing and enumeration in live bivalve shellfish—Present methods and future directions. Food Microbiol. 2018, 73, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Arnich, N.; Abadie, E.; Amzil, Z.; Bottein, M.Y.D.; Comte, K.; Chaix, E.; Delcourt, N.; Hort, V.; Mattei, C.; Molgó, J.; et al. Guidance level for brevetoxins in french shellfish. Mar. Drugs 2021, 19, 520. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, P.; Salerno, B.; Bordin, P.; Peruzzo, A.; Orsini, M.; Arcangeli, G.; Barco, L.; Losasso, C. Tetrodotoxin in live bivalve mollusks from Europe: Is it to be considered an emerging concern for food safety? Compr. Rev. Food Sci. Food Saf. 2022, 21, 719–737. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M.; Tahir, A.; Williams, S.L.; Baxa, D.V.; Lam, R.; Miller, J.T.; Teh, F.C.; Werorilangi, S.; Teh, S.J. Anthropogenic debris in seafood: Plastic debris and fibers from textiles in fish and bivalves sold for human consumption. Sci. Rep. 2015, 5, 14340. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.; Jo, H.; Kim, S.H.; Kang, G.J. Exposure assessment to paralytic shellfish toxins through the shellfish consumption in Korea. Food Res. Int. 2018, 108, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Tedde, G.; Lorenzoni, G.; Meloni, D.; Salza, S.; Melillo, R.; Bazzardi, R.; Cau, S.; Tiziana, T.; Piras, G.; Uda, M.T.; et al. Trend of Pathogenic Vibrio parahaemolyticus Occurrences in Bivalve Molluscs Harvested in Sardinian Coastal Environments Between 2011 and 2018. J. Food Prot. 2023, 86, 100150. [Google Scholar] [CrossRef] [PubMed]
- Sedda, T.; Baralla, E.; Varoni, M.V.; Pasciu, V.; Lorenzoni, G.; Demontis, M.P. Determination of microcystin-LR in clams (Tapes decussatus) of two Sardinian coastal ponds (Italy). Mar. Pollut. Bull. 2016, 108, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Mudadu, A.G.; Lorenzoni, G.; Bazzoni, A.M.; Bazzardi, R.; Tedde, G.; Arras, I.; Sanna, G.; Santucciu, C.; Marongiu, E.; Virgilio, S. Yessotoxin detection in bivalve molluscs: A case study from coastal mussel farms (Sardinia, Italy). Ital. J. Food Saf. 2017, 6, 7015. [Google Scholar] [CrossRef] [PubMed]
- Lorenzoni, G.; Melillo, R.; Mudadu, A.G.; Piras, G.; Cau, S.; Usai, K.; Corda, L.; Salza, S.; Tedde, T.; Vodret, B.; et al. Identification and quantification of potential microplastics in shellfish harvested in Sardinia (Italy) by using transillumination stereomicroscopy. Ital. J. Food Saf. 2022, 11, 10738. [Google Scholar] [CrossRef]
- Esposito, G.; Mudadu, A.G.; Abete, M.C.; Pederiva, S.; Griglione, A.; Stella, C.; Ortu, S.; Bazzoni, A.M.; Meloni, D.; Squadrone, S. Seasonal accumulation of trace elements in native Mediterranean mussels (Mytilus galloprovincialis Lamarck, 1819) collected in the Calich Lagoon (Sardinia, Italy). Environ. Sci. Pollut. Res. 2021, 28, 25770–25781. [Google Scholar] [CrossRef]
- Baralla, E.; Pasciu, V.; Varoni, M.V.; Nieddu, M.; Demuro, R.; Demontis, M.P. Bisphenols’ occurrence in bivalves as sentinel of environmental contamination. Sci. Total Environ. 2021, 785, 147263. [Google Scholar] [CrossRef]
- Sferlazzo, G.; Meloni, D.; Lamon, S.; Marceddu, M.; Mureddu, A.; Consolati, S.G.; Pisanu, M.; Virgilio, S. Evaluation of short purification cycles in naturally contaminated Mediterranean mussels (Mytilus galloprovincialis) harvested in Sardinia (Italy). Food Microbiol. 2018, 74, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EU) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off. J. Eur. Union 2010, L15/1, 1–72.
- Alvarez-Muñoz, D.; Huerta, B.; Fernandez-Tejedor, M.; Rodríguez-Mozaz, S.; Barceló, D. Multi-residue method for the analysis of pharmaceuticals and some of their metabolites in bivalves. Talanta 2015, 136, 132–174. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, L.M.; Nobile, M.; Malandra, R.; Panseri, S.; Arioli, F. Occurrence of antibiotics in mussels and clams from various FAO areas. Food Chem. 2018, 240, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lu, Y.; Sun, B.; Zhang, M.; Wang, C.; Xiu, C.; Johnson, A.C.; Wang, P. Ecological and human health risks of antibiotics in marine species through mass transfer from sea to land in a coastal area: A case study in Qinzhou Bay, the South China sea. Environ. Pollut. 2023, 316, 120502. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, W.; Liu, K.; Guo, Y.; Ding, C.; Han, J.; Li, P. Global review of macrolide antibiotics in the aquatic environment: Sources, occurrence, fate, ecotoxicity, and risk assessment. J. Hazard. Mater. 2022, 439, 129628. [Google Scholar] [CrossRef]
- Tang, Y.; Lou, X.; Yang, G.; Tian, L.; Wang, Y.; Huang, X. Occurrence and human health risk assessment of antibiotics in cultured fish from 19 provinces in China. Front. Cell. Infect. Microbiol. 2022, 12, 964283. [Google Scholar] [CrossRef]
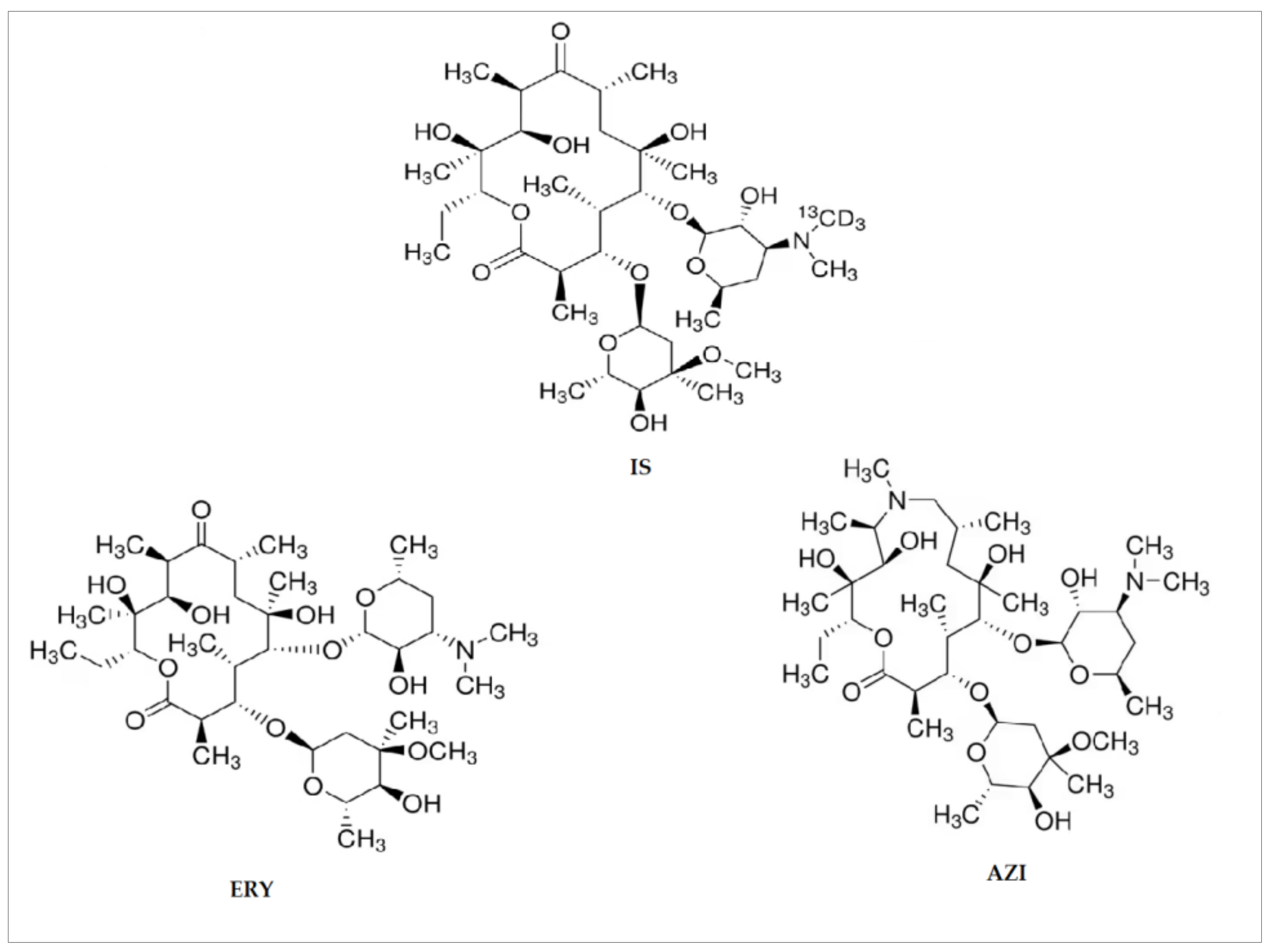

| Analyte | Precursor Ion (m/z) | Product Ion (m/z) | Capillary (V) | Detector (V) | CE (V) |
|---|---|---|---|---|---|
| ERY | 735.3 | 158.2 a 577.5 | 65 | 1750 | 29 19 |
| AZI | 750.2 | 158.2 a 592.1 | 65 | 1750 | 28 36 |
| IS | 739.2 | 162.3 a 83.3 | 65 | 1750 | 28 18 |
| Antibiotic | Concentration (ng/g) | Repeatability (RSD %) | Recovery (%) ± SD | Matrix Effect (%) | |
|---|---|---|---|---|---|
| Intra-Day | Inter-Day | ||||
| ERY | 100 200 1000 | 8.11 10.97 14.75 | 13.92 12.51 11.89 | 67.12 ± 5.03 73.55 ± 2.61 62.93 ± 2.92 | 15.55 −27.27 27.84 |
| AZI | 200 500 1000 | 14.05 7.76 9.47 | 14.37 14.93 14.7 | 83.09 ± 3.08 60.53 ± 0.03 63.69 ± 3.43 | −20 −0.36 −27.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dessì, F.; Varoni, M.V.; Baralla, E.; Nieddu, M.; Pasciu, V.; Piras, G.; Lorenzoni, G.; Demontis, M.P. Contaminants of Emerging Concern: Antibiotics Research in Mussels from the Coasts of the Tyrrhenian Sea (Sardinia, Italy). Animals 2024, 14, 1205. https://doi.org/10.3390/ani14081205
Dessì F, Varoni MV, Baralla E, Nieddu M, Pasciu V, Piras G, Lorenzoni G, Demontis MP. Contaminants of Emerging Concern: Antibiotics Research in Mussels from the Coasts of the Tyrrhenian Sea (Sardinia, Italy). Animals. 2024; 14(8):1205. https://doi.org/10.3390/ani14081205
Chicago/Turabian StyleDessì, Filomena, Maria Vittoria Varoni, Elena Baralla, Maria Nieddu, Valeria Pasciu, Gabriella Piras, Giuseppa Lorenzoni, and Maria Piera Demontis. 2024. "Contaminants of Emerging Concern: Antibiotics Research in Mussels from the Coasts of the Tyrrhenian Sea (Sardinia, Italy)" Animals 14, no. 8: 1205. https://doi.org/10.3390/ani14081205






