Simple Summary
Beaches are important habitats for migratory birds, but human pressure and sea level rise threaten their availability. This study reveals post-reproductive habitat-use strategies of the Kentish plover (Charadrius alexandrinus) in the Iberian Peninsula by analysing C and N isotopes in feathers. Birds from the northwest of the Iberian Peninsula exhibit greater fidelity to a single habitat type, while those from the Mediterranean coast and the Atlantic coast of Andalusia show variety in their dispersal. The lack of alternative habitats, the reduction of beaches due to sea level rise, and human pressure threaten the survival of the species in the northwest of the Iberian Peninsula.
Abstract
Beaches are among the habitats most frequented by migratory birds for breeding and/or wintering. However, threats such as human pressure and sea level rise can reduce the availability of these habitats for different species. The presence of alternative areas, such as salt pans and brackish habitats, is essential for many migratory shorebird populations. This study addresses the post-breeding dispersal of the Kentish plover (Charadrius alexandrinus) in the Iberian Peninsula by analysing C and N isotopes in feathers. The study was conducted at six locations along the Iberian coast, which were categorized into three areas: the NW Atlantic coast, the Atlantic coast of Andalusia, and the Mediterranean coast. Although linear mixed models did not reveal any significant effects of sex or coastal area on isotopic levels, the variability in the data suggests different habitat-use strategies in the post-reproductive period. Isotopic levels in birds from the northwest of the Iberian Peninsula exhibit greater fidelity to a single habitat type, while those from the Mediterranean coast and the Atlantic coast of Andalusia show greater variability, indicating different individual dispersal strategies. The lack of alternative habitats for the northwest Iberian population, the reduction in available habitat due to rising sea levels, and human pressure together pose a serious threat to the survival of this species, already with an unfavourable conservation status.
1. Introduction
Migratory shorebirds migrate between breeding and staging areas along generally consistent routes [1], frequently using beaches and saline and/or brackish habitats [2,3]. Beaches are important breeding and foraging habitats for shorebirds, but they are threatened by resort development, road construction, coastal erosion, sea level rise, and human disturbance [4,5,6]. Increasing global temperatures will result in increases in sea level due to the expansion of oceanic water and the melting of glaciers and ice sheets [7]. Inundations due to sea level rise could lead to the conversion of intertidal to subtidal habitats and, therefore, a reduction in the availability of habitats to shorebirds [6,8]. Moreover, overwash and the associated consequences are expected to increase because of both sea level rise and the intensification of coastal occupation [9]. The impacts of such habitat changes on shorebird populations will depend on the availability of alternative areas that the birds can use and where similar levels of survivorship and fecundity can be reached [10]. Salt pans and wetlands are included among alternative areas that can support large populations of migratory waterbirds, such as shorebirds [11,12,13,14].
Unravelling migratory connectivity between breeding, stopover, and wintering areas is important to predict the influence of habitat change on population demographics [15,16]. Stable isotope analysis has been used to investigate dispersal and migratory movements in shorebirds [3,16,17,18], as natural patterns of geographic variation have been observed in isotopic ratios on land [19].
The Kentish plover (Charadrius alexandrinus) is a common shorebird in the temperate and subtropical belt of Eurasia and N Africa [20]. However, most breeding populations in Europe have undergone a marked decline since the early part of the twentieth century [21].
The Iberian population is the largest in Europe [20] and is concentrated along the coast, being less common in inland wetlands [22,23]. The species has undergone a strong decline in the area it occupies, and it has disappeared from some coastal areas, especially from the Mediterranean coast [23] and also the coast of Portugal [24]. In the northwest Iberian peninsula, the birds breed exclusively on beaches, but along the Portuguese coast, southern Spain and the Mediterranean coast, they also occupy salt pans and salt marshes, as well as sparsely vegetated salt flats and coastal grasslands [2,22,23,25]. Inland populations of Kentish plovers typically nest on the sandy margins of brackish lagoons, but also on the shores of reservoirs or on islands and rice fields [2,22,26,27]. In the context of climate change, the rise in sea level and the increase in the rate or severity of maritime storms are restricting the useful strip of beaches available [28]. This situation increases the importance of supratidal habitats such as salt pans for the species.
The objective of this study was to unravel the post-breeding dispersal strategy in Iberian Kentish plovers through the analysis of the isotopic levels of C and N in the feathers of breeding adults.
2. Materials and Methods
2.1. Study Area
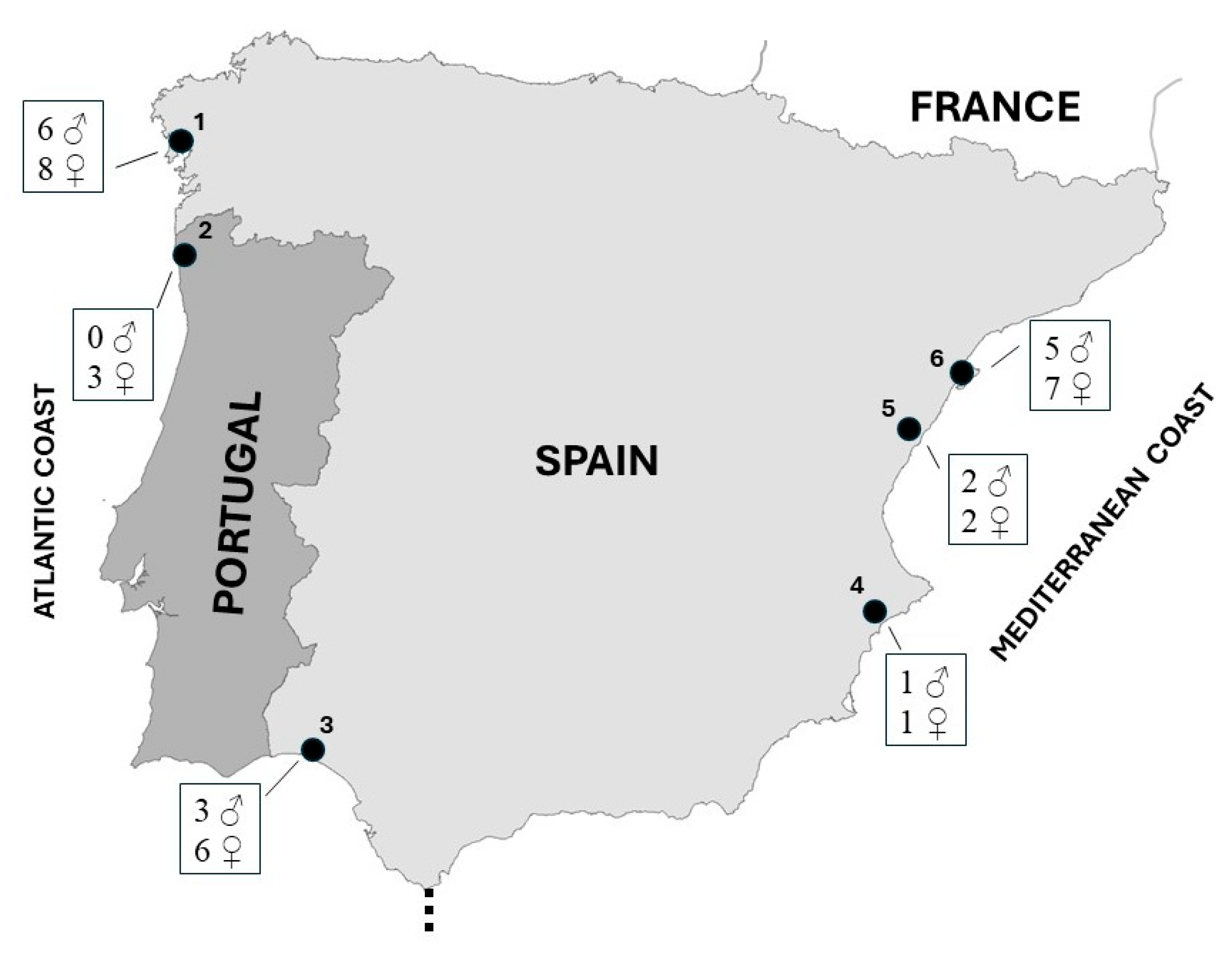
Samples were collected from six breeding locations situated along the Iberian coast. Among these, three sites are positioned along the Mediterranean coast, while the remaining three are located on the Atlantic coast, with one in southern Spain and two in northern Portugal and northwestern Spain (Figure 1).
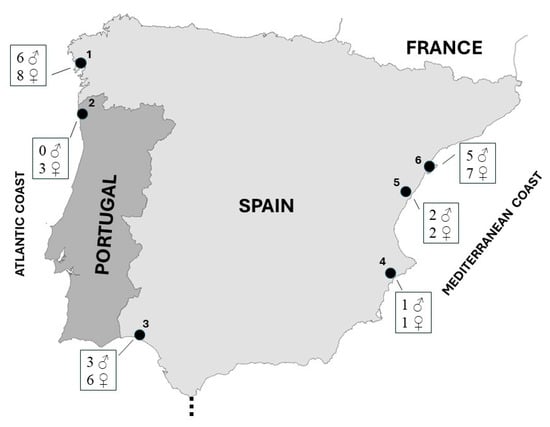
Figure 1.
Kentish plover sampling sites used in this study. Locations: 1, Galician beaches (Rostro, Carnota, and Balieiros beaches); 2, Carreço and Ancora beaches; 3; Tinto-Odiel estuary; 4, Laguna de la Mata; 5, Serradal beach; 6, Delta del Ebro. Sample size by sex is shown for each locality.
The Mediterranean localities include the Ebro Delta wetland, Laguna de la Mata, and Serradal beach (Figure 1). Within the Ebro Delta, recognized as a significant breeding area for the Kentish plover in Spain [29], nests were predominantly observed in zones adjacent to brackish water and coastal beaches. Similarly, nests in Laguna de la Mata were found along areas bordering brackish water, whereas on Serradal beach, nests were situated in deep sandy areas amidst dry interdunal depressions.
The Atlantic location in Andalusia (southern Spain) was the Tinto-Odiel estuary, where breeding areas were located on beaches of the marine zone, while in northern Portugal (Carreço and Ancora beaches) and northwestern Spain (Rostro, Carnota, and Balieiros beaches), nests were found exclusively on coastal sandy beaches (Figure 1).
2.2. Methods
Plovers were captured from nests using a funnel-trap and were sexed by dichromatic plumage characteristics [30].
Feathers were collected between March and June 2009 from 44 adult Kentish plovers (17 males and 27 females) breeding on the Mediterranean coast (18 birds), the Atlantic coast of southern Spain (9 birds), and northern Portugal and northwestern Spain (17 birds).
The inner first primary feather of each bird was clipped. Although little is known about temporal and spatial moulting patterns in the Iberian Kentish plover [27], the moulting of primary feathers in European populations usually takes place in the post-breeding period (August-October), although it may begin in June [31,32]. Even so, our samples were mostly obtained between April and May, and only 3 samples were obtained in June. Each feather sample was placed in a (separate) plastic bag and stored at −20 °C prior to analysis.
2.3. Isotopic Analysis
The feathers were washed in a solution of 0.25 M sodium hydroxide and pure water to remove waxes and oils. The washed feathers were placed in clean, screw-top vials and dried overnight at 60 °C, in the oven of an elemental analyser. The dried feathers were cut into small sections (<1 mm sections) in the sample vials with surgical scissors.
Stable isotope ratios were measured in a continuous-flow isotope-ratio mass spectrometer (Deltaplus, ThermoFinnigan, SUERC-East Kilbride, UK) coupled to an elemental analyser (FlashEA1112, ThermoFinnigan Instruments, CE Elantech, Lakewood, NJ, USA) through a Conflo II interface (ThermoFinnigan, Breman, Germany). Tin-encapsulated samples were combusted at 1020 °C in a quartz column containing chromium oxide and silvered cobaltous/cobaltic oxide. After combustion, excess oxygen and nitrogen oxides were reduced in a reduction column (reduced copper at 650 °C). N2 and CO2 were separated on a GC column prior to isotope-ratio mass spectrometry. A series of international reference materials for δ15N (IAEA-N-1, IAEA-N-2, and USGS25) and δ13C (NBS 22, IAEA-CH-6, and USGS24) were also analysed along with some test batches. Replicate assays of the laboratory standard acetanilide indicated measurement errors of ±0.15‰ for δ13C and δ15N.
Delta values are expressed relative to international standards: Vienna Pee Dee Belemnite (VPDB) for δ13C and Atmospheric Air for δ15N.
2.4. Statistical Analysis
Locations were clustered by geographical proximity in three coastal areas: the Iberian NW coast (locations 1–2), the Atlantic coast of Andalusia (3), and the Mediterranean coast (4–6) (Figure 1).
We employed Linear Mixed Models (LMMs) to address the statistical non-independence present in the data [33]. These models were constructed with sex and coastal area as fixed categorical predictors, while location (nested within coastal area) was treated as a random factor. The dependent variables considered were δ13C and δ15N. In cases where it was necessary to ensure data normality and homoscedasticity, a log transformation was applied. Significance testing for each fixed term was conducted using the F-statistic, employing the restricted maximum likelihood (REML) approach. The coefficient of variation was used to visualize the data dispersion in the three coastal areas.
Mean values ± SE are presented throughout the paper. Statistical analyses were performed using IBM SPSS Statistics 29 software. The biplot graph was made using the SIBER and ggplot2 packages in the R programming environment version 4.3.3.
3. Results
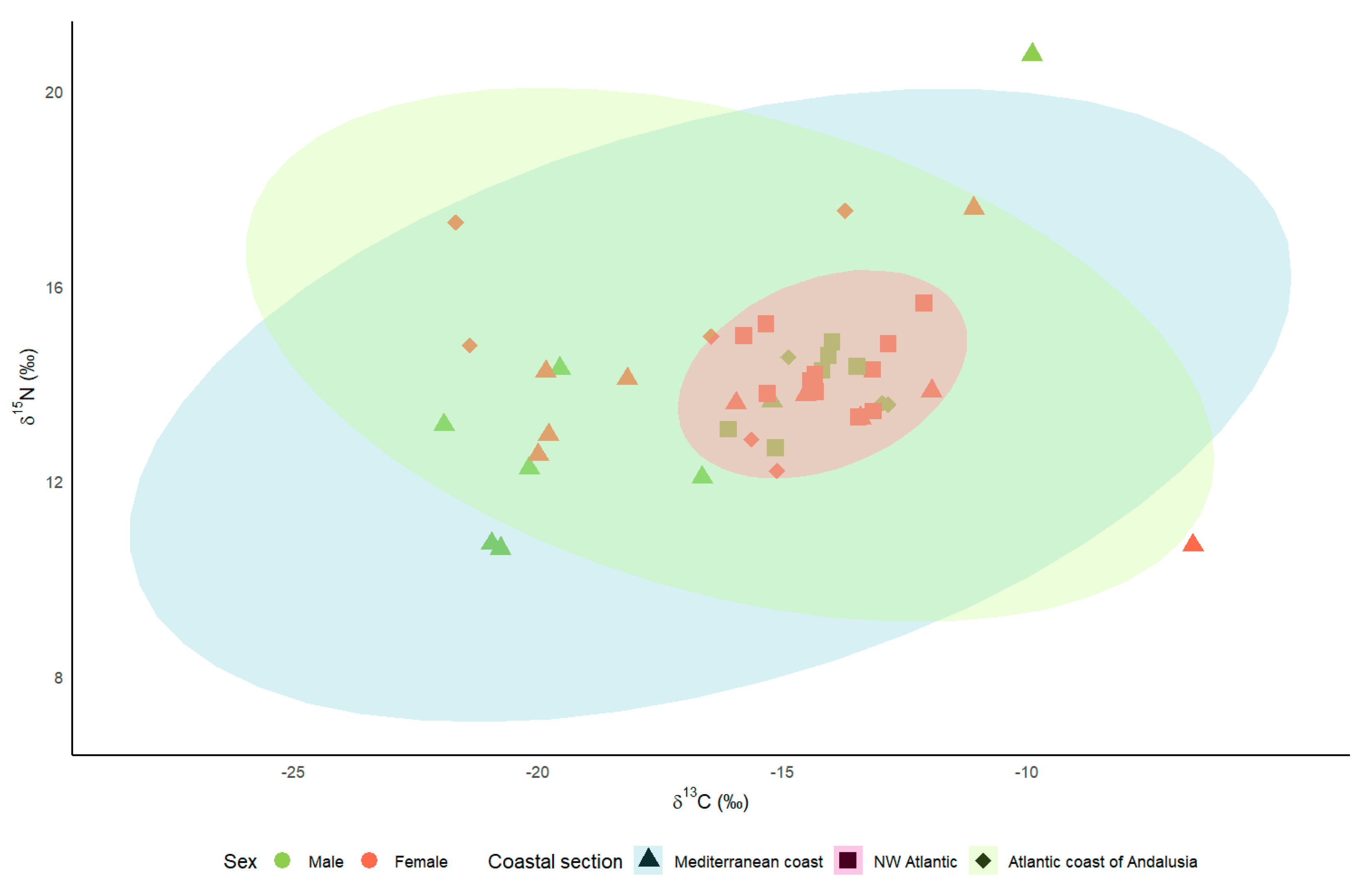
The mean δ13C values were −16.47 ± 1.05‰ (n = 18) for Kentish plovers breeding on the Mediterranean coast, −16.07 ± 1.11‰ (n = 9) for those breeding on the Atlantic coast of Andalusia, and −14.18 ± 0.27‰ (n = 17) for those breeding on the NW Iberian coast. Regarding the δ15N, the mean values for the Mediterranean coast, the Atlantic coast of Andalusia, and the NW Iberian coast were, respectively, 13.57 ± 0.57‰, 14.60 ± 0.61‰, and 14.20 ± 0.19‰. The values for the Mediterranean coast and the Atlantic coast of Andalusia were much more widely dispersed than those corresponding to the NW Iberian coast (Figure 2), with coefficients of variation in δ13C and δ15N of 8% and 6% for the NW Iberian coast, 20% and 12% for the Atlantic coast of Andalusia, and 27% and 18% for the Mediterranean coast.
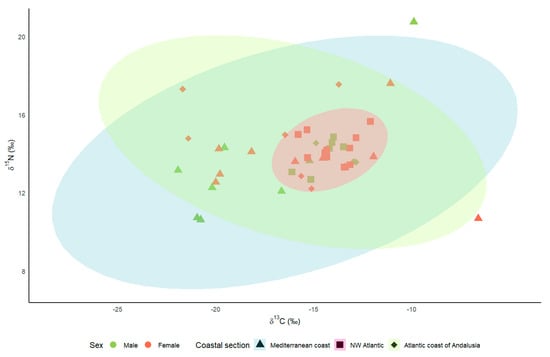
Figure 2.
Stable isotope Bayesian ellipses encompassing 95% of δ13C and δ15N data for Kentish plover by coastal section.
The LMMs showed that neither δ13C nor δ15N concentrations were significantly affected by sex or coastal section (Table 1).

Table 1.
Linear Mixed Models using δ13C and δ15N in Iberian Kentish plover feathers as response variables, coastal section and sex as fixed categorical predictors, and location (nested within coastal section) as random factor.
4. Discussion
In the Kentish plover adults, the post-breeding moult of primaries is complete and descendent [30]. Temporal moulting patterns in the Iberian Kentish plovers is little-known [27]. In other European, Mediterranean, and north African areas, moult mostly starts in the breeding area between June and mid-July [30,31], with most birds completing the process from mid-August to October [31,32]. In our study, 93.2% of the primaries were collected in April and May, and only three plovers were sampled in June. This collection period minimised the risk of obtaining already moulted feathers, so it is reasonable to assume that the isotopic levels reflected in the study corresponded to the habitats used in the post-reproductive period.
Although the LMMs did not show any significant influence of sex and coastal area on isotopic levels, the coefficients of variation suggest different strategies regarding habitat use in the post-breeding period. Kentish plovers breeding on the Mediterranean coast and the Atlantic coast of Andalusia showed greater variability in isotope values than birds in the NW Iberian Peninsula, both in δ13C and δ15N. Trophic web carbon and nitrogen values are known to differ between habitats [34,35]. Thus, the size of carbon and nitrogen isotopic niches seems to be related to different individual strategies, such as birds making latitudinal movements in coastal environments and others moving to freshwater wetlands in the post-breeding season, implying that δ13C and δ15N isotope values are lower than in coastal areas [36,37,38]. The coefficient of variation for the Mediterranean birds reveals very marked habitat changes in plover populations. The high δ13C values (−6.59‰ or −9.88‰) may indicate that these birds frequented hypersaline environments [37,39] at the time of feather growth. Conversely, low δ13C values (−21.92‰ or −20.95‰) indicate that the birds frequented environments with low salinity [37,39], including inland lakes [40]. Such migratory movements have been reported by other authors [27,40]. By contrast, the results obtained for the northwestern Iberian plover indicate the greater fidelity of this population to a single habitat type, i.e., coastal beaches, with a lower coefficient of variation. In this population, most ringed birds exclusively occupied coastal beaches throughout the annual cycle [41] (unpublished data), although the tracking of some tagged plovers revealed that they use freshwater wetlands in winter (unpublished data). Combining isotopic levels with other techniques, such as tracking birds using telemetry, would be necessary to better identify the areas used by the Iberian population in autumn and winter.
In recent years, beaches have been subjected to strong anthropogenic pressure (urban planning, industrial development, and tourism) [6,42]. The rise in sea level due to climate change is an additional threat [43,44,45]. The most widely accepted projection for the next 100 years is a global sea level rise of 0.63–1.02 m under a scenario of very high greenhouse gas emissions [46]. More specifically, a sea level rise of 0.6–0.8 m is expected in the northwest Iberian Peninsula by 2100 [47]. The inundation of the intertidal zone will result in the conversion of intertidal habitats to subtidal habitats [6] and lead to reductions in habitat availability for many shorebirds. Species that use a wide range of habitats may be better able to cope with these rapid climatic or habitat changes [48,49,50]. By contrast, 90% of the Kentish plovers in the Iberian Peninsula are concentrated on coastal sandy beaches, although a wide variety of habitats are occupied by this species, including salt pans, salt marshes, and endorheic lagoons [2,23,27]. Specifically, in the NW Iberian Peninsula, beaches are the only habitat available for the species [51,52]. In the absence of alternative habitats, the expected rise in sea level due to the effect of climate change will therefore pose a serious problem for the survival of this population, as it will cause a gradual reduction in the availability of suitable habitats for the species. A similar problem has been observed in other coastal populations of this species [53] and of the Snowy plover (Charadrius nivosus) [54]. Population decline can be slowed down by implementing effective conservation measures such as increasing survival rates or nest success by implementing actions such as nest protection measures, surveillance, headstarting, or predator control [51,54], as well as the protection and conservation of coastal areas with dune vegetation, for example, by restricting access to sensitive areas, and the restoration of dune environments [28,51]. However, habitat loss can only be compensated for by the creation of alternative habitats.
5. Conclusions
This study highlights the heterogeneity in the post-breeding dispersal strategies of Iberian Kentish plovers, with populations in the southern Iberian Peninsula and the Mediterranean showing a wider range of habitats than those in the NW Iberian Peninsula, which are much more dependent on coastal environments and therefore more vulnerable to rising sea levels.
Author Contributions
Conceptualization, J.D. and M.V.; methodology, J.D. and M.V.; software, J.D. and A.G.; validation, J.D., M.V. and A.G.; formal analysis, A.G.; investigation, J.D. and M.V.; resources, J.D.; data curation, J.D.; writing—original draft preparation, A.G.; writing—review and editing, J.D., M.V. and A.G.; visualization, J.D.; supervision, J.D. and M.V.; project administration, J.D.; funding acquisition, J.D. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by the POCTEP 0123_IBERALEX_6_E project “Sustainable management of Iberian beaches and wetlands: conservation of the Kentish Plover as a tool to make human uses and biodiversity compatible”, financed by the European Regional Development Fund by 75%, within the framework of the EP-Interreg VI program Spain Portugal (POCTEP) 2021–2027.
Institutional Review Board Statement
All applicable national and institutional guidelines for the use of wild birds were followed in this research. Permission to trap and handle Kentish Plovers was granted through the authorisations of the Spanish Autonomous Communities (Generalitat de Catalunya (SF/331; date of approval:14 April 2009), Generalitat Valenciana (2009/10976; date of approval: 20 March 2009), Junta de Andalucía (SGYB-AFR-CMM; date of approval 03 March 2009) and Xunta de Galicia (55/2009; date of approval: 02 April 2009) and the Portuguese Government (ICNB, Licença 94/2008CAPT; date of approval: 20 February 2008).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated and/or analysed during the current study are available upon request to the corresponding author.
Acknowledgments
The isotopic analyses were conducted by the Servicios de Apoio á Investigación (SAI) of the A Coruña University.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Delany, S.; Scott, D.A.; Dodman, T.; Stroud, D.A. An Atlas of Wader Populations in Africa and Western Eurasia; Wetlands International: Wageningen, The Netherlands, 2009; ISBN 9789058820471. [Google Scholar]
- Rocha, A.D.; Fonseca, D.; Masero, J.A.; Ramos, J.A. Coastal saltpans are a good alternative breeding habitat for Kentish plover Charadrius alexandrinus when umbrella species are present. J. Avian Biol. 2016, 47, 824–833. [Google Scholar] [CrossRef]
- Robin, F.; Delaporte, P.; Rousseau, P.; Meunier, F.; Bocher, P. Tracing changes in the diet and habitat use of black-tailed godwits in Western France, using a stable isotope approach. Isot. Environ. Health Stud. 2018, 54, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.C.; McLachlan, A. The Ecology of Sandy Shores; Elsevier: Amsterdam, The Netherlands, 2006; ISBN 978-0-12-372569-1. [Google Scholar]
- Defeo, O.; Mclachlan, A.; Schoeman, D.S.; Schlacher, T.A.; Dugan, J.; Jones, A.; Lastra, M.; Scapini, F. Threats to sandy beach ecosystems: A review. Estuar. Coast. Shelf Sci. 2009, 81, 1–12. [Google Scholar] [CrossRef]
- Vousdoukas, M.I.; Ranasinghe, R.; Mentaschi, L.; Plomaritis, T.A.; Athanasiou, P.; Luijendijk, A.; Feyen, L. Sandy coastlines under threat of erosion. Nat. Clim. Change 2020, 10, 260–263. [Google Scholar] [CrossRef]
- Dangendorf, S.; Hay, C.; Calafat, F.M.; Marcos, M.; Piecuch, C.G.; Berk, K.; Jensen, J. Persistent acceleration in global sea-level rise since the 1960s. Nat. Clim. Change 2019, 9, 705–710. [Google Scholar] [CrossRef]
- Galbraith, H.; Jones, R.; Park, R.; Clough, J.; Herrod-Julius, S.; Harrington, B.; Page, G. Global climate change and sea level rise: Potential losses of intertidal habitat for shorebirds. Waterbirds 2002, 25, 173–183. [Google Scholar] [CrossRef]
- Ferreira, Ó.; Kupfer, S.; Costas, S. Implications of sea-level rise for overwash enhancement at South Portugal. Nat. Hazards 2021, 109, 2221–2239. [Google Scholar] [CrossRef]
- Smart, J.; Gill, J.A. Non-intertidal habitat use by shorebirds: A reflection of inadequate intertidal resources? Biol. Conserv. 2003, 111, 359–369. [Google Scholar] [CrossRef]
- Yasué, M.; Patterson, A.; Dearden, P. Are saltflats suitable supplementary nesting habitats for Malaysian Plovers Charadrius peronii threatened by beach habitat loss in Thailand? Bird Conserv. Int. 2007, 17, 211–223. [Google Scholar] [CrossRef]
- Jackson, M.V.; Choi, C.Y.; Amano, T.; Estrella, S.M.; Lei, W.; Moores, N.; Mundkur, T.; Rogers, D.I.; Fuller, R.A. Navigating coasts of concrete: Pervasive use of artificial habitats by shorebirds in the Asia-Pacific. Biol. Conserv. 2020, 247, 108–591. [Google Scholar] [CrossRef]
- Lei, W.; Masero, J.A.; Dingle, C.; Liu, Y.; Chai, Z.; Zhu, B.; Peng, H.; Zhang, Z.; Piersma, T. The value of coastal saltpans for migratory shorebirds: Conservation insights from a stable isotope approach based on feeding guild and body size. Anim. Conserv. 2021, 24, 1071–1083. [Google Scholar] [CrossRef]
- Jourdan, C.; Fort, J.; Robin, F.; Pinaud, D.; Delaporte, P.; Desmots, D.; Gentric, A.; Lagrange, P.; Gernigon, J.; Jomat, L.; et al. Combination of marine and artificial freshwater habitats provides wintering Black-tailed Godwits with landscape supplementation. Wader Study 2022, 129, 86–99. [Google Scholar] [CrossRef]
- Iwamura, T.; Possingham, H.P.; Chadès, I.; Minton, C.; Murray, N.J.; Rogers, D.I.; Treml, E.A.; Fuller, R.A. Migratory connectivity magnifies the consequences of habitat loss from sea-level rise for shorebird populations. Proc. R. Soc. B Biol. Sci. 2013, 280, 20130325. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.M.; Kim, K.; Ki, J.; Kim, H.; Yoo, J.C. Use of stable isotopes (δ2H, δ13C and δ15N) to infer the migratory connectivity of Terek Sandpipers (Xenus cinereus) at stopover sites in the East Asian-Australasian Flyway. Avian Biol. Res. 2020, 13, 10–17. [Google Scholar] [CrossRef]
- Catry, T.; Lourenco, P.M.; Lopes, R.J.; Bocher, P.; Carneiro, C.; Alves, J.A.; Delaporte, P.; Bearhop, S.; Piersma, T.; Granadeiro, J.P. Use of stable isotope fingerprints to assign wintering origin and trace shorebird movements along the East Atlantic Flyway. Basic Appl. Ecol. 2016, 17, 177–187. [Google Scholar] [CrossRef]
- Ulman, S.E.; Van Wilgenburg, S.L.; Morton, J.M.; Williams, C.K. Geographic Origins of Shorebirds Using an Alaskan Estuary during Migration. Waterbirds 2023, 46, 47–56. [Google Scholar] [CrossRef]
- Hobson, K.A. Isotopic ornithology: A perspective. J. Ornithol. 2011, 152, 49–66. [Google Scholar] [CrossRef]
- Bronskov, O.; Keller, V. Charadrius alexandrinus, Kentish Plover. In European Breeding Bird Atlas 2: Distribution, Abundance and Change; Keller, V., Herrando, S., Voříšek, P., Franch, M., Kipson, M., Milanesi, P., Martí, D., Anton, M., Klvaňová, A., Kalyakin, M.V., et al., Eds.; European Bird Census Council & Lynx Edicions: Barcelona, Spain, 2020; pp. 300–301. ISBN 978-84-16728-38-1. [Google Scholar]
- Meininger, P.; Székely, T.; Scott, D.A. Kentish Plover (Charadrius alexandrinus). In An Atlas of Wader Populations in Africa and Western Eurasia; Delany, S., Scott, D., Dodman, T., Stroud, S., Eds.; Wetlands International: Wageningen, The Netherlands, 2009; pp. 230–235. ISBN 9789058820471. [Google Scholar]
- De Juana, E.; García, E. The Birds of the Iberian Peninsula; Christopher Helm: London, UK, 2015; ISBN 978-1-4081-2480-2. [Google Scholar]
- Gómez-Serrano, M.A.; Hortas, F. Chorlitejo patinegro Charadrius alexandrinus. In III Atlas de las Aves en Época de Reproducción en España; Molina, B., Nebreda, A., Muñoz, R.A., Seoane, J., Real, R., Bustamante, J., Del Moral, J.C., Eds.; SEO/BirdLife: Madrid, Spain, 2022; ISBN 978-84-126555-6-8. [Google Scholar]
- Rocha, A. Censo nacional de Borrelho-de-coleira-interrompida. In O Estado das Aves em Portugal, 2022; Alonso, H., Andrade, J., Teodósio, J., Lopes, A., Eds.; Sociedade Portuguesa para o Estudo das Aves: Lisboa, Portugal, 2022; pp. 70–75. [Google Scholar]
- Catry, P.; Costa, H.; Elias, G.; Matías, R. Aves de Portugal. Ornitología do Território Continental; Assirio & Alvim: Lisboa, Portugal, 2010; ISBN 978-84-16728-11-4. [Google Scholar]
- Toral, G.M.; Figuerola, J. Nest success of Black-winged Stilt Himantopus himantopus and Kentish Plover Charadrius alexandrinus in rice fields, southwest Spain. Ardea 2012, 100, 29–36. [Google Scholar] [CrossRef]
- Amat, J.A. Chorlitejo patinegro. In Enciclopedia Virtual de los Vertebrados Españoles; Salvador, A., Morales, M.B., Eds.; Museo Nacional de Ciencias Naturales: Madrid, Spain, 2016. [Google Scholar]
- Gómez-Serrano, M.Á.; Castro, E.M.; Domínguez, J.; Pérez-Hurtado, A.; Tejera, G.; Vidal, M. Chorlitejo patinegro Charadrius alexandrinus. In Libro Rojo de las Aves de España; López-Jiménez, A.N., Ed.; SEO/Birdlife: Madrid, Spain, 2021; pp. 375–385. ISBN 978-84-124888-2-1. [Google Scholar]
- Molina, B. Chorlitejo Patinegro. In Aves acuáticas reproductoras. Población en 2007 y Método de Censo; Palomino, D., Molina, B., Eds.; SEO/BirdLife: Madrid, Spain, 2009; pp. 115–125. ISBN 9788493644192. [Google Scholar]
- Cramp, S.; Simmons, K.E.L. The Birds of the Western Palearctic, Vol. III; Oxford University Press: Oxford, UK, 1982; ISBN 019857506. [Google Scholar]
- Pienkowski, M.; Knight, P.; Stanyard, D.; Argyle, F. The primary moult of waders on the Atlantic coast of Morocco. IBIS 1976, 118, 347–365. [Google Scholar] [CrossRef]
- Blasco-Zumeta, J.; Heinze, G.M. Chorlitejo patinegro. Sociedad de Ciencias Aranzadi. 2023. Available online: http://blascozumeta.com/?page_id=19 (accessed on 14 February 2024).
- Pinheiro, J.C.; Bates, D.M. Linear mixed-effects models: Basic concepts and examples. In Mixed-effects models in S and S-Plus; Springer: New York, NY, USA, 2000; pp. 3–56. ISBN 978-0387989570. [Google Scholar]
- Sabat, P.; Martínez del Rio, C. Inter-and intraspecific variation in the use of marine food resources by three Cinclodes (Furnariidae, Aves) species: Carbon isotopes and osmoregulatory physiology. Zoology 2002, 105, 247–256. [Google Scholar] [CrossRef]
- Martínez del Rio, C.; Sabat, P.; Anderson-Sprecher, R.; Gonzalez, S.P. Dietary and isotopic specialization: The isotopic niche of three Cinclodes ovenbirds. Oecologia 2009, 161, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Schoeninger, M.J.; DeNiro, M.J.; Tauber, H. Stable nitrogen isotope ratios of bone collagen reflect marine and terrestrial components of prehistoric human diet. Science 1983, 220, 1381–1383. [Google Scholar] [CrossRef] [PubMed]
- Chmura, G.; Aharon, P. Stable carbon isotope signatures of sedimentary carbon in coastal wetlands as indicators of salinity regime. J. Coast. Res. 1995, 11, 124–135. [Google Scholar]
- Jiménez-Morillo, N.T.; Moreno, J.; Moreno, F.; Fatela, F.; Leorri, E.; De la Rosa, J.M. Composition and sources of sediment organic matter in a western Iberian salt marsh: Developing a novel prediction model of the bromine sedimentary pool. Sci. Total Environ. 2024, 907, 167931. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, S.; Dehairs, F.; Velimirov, B.; Abril, G.; Borges, A.V. Dynamics of organic and inorganic carbon across contiguous mangrove and seagrass systems (Gazi Bay, Kenya). J. Geophys. Res-Biogeosci. 2007, 112. [Google Scholar] [CrossRef]
- Gonçalves, M.S.S.; Gil-Delgado, J.A.; Gosalvez, R.U.; Lopez-Iborra, G.M.; Ponz, A.; Velasco, A. Spatial synchrony of wader populations in inland lakes of the Iberian Peninsula. Ecol. Res. 2016, 31, 947–956. [Google Scholar] [CrossRef]
- De Souza, J.A.; Caeiro, M.L.; Rosende, F.; Monteagudo, A.; Fafián, J.M. Estacionamientos, estructura y patrones de residencia de la población invernante del Chorlitejo patinegro (Charadrius alexandrinus) en Galicia: Un análisis preliminar. Chioglossa 1999, 1, 23–45. [Google Scholar]
- Sardá, R.; Ariza, E.; Jimenez, J.A. Buscando el uso sostenible de las playas. In La Gestión Integrada de Playas y Dunas: Experiencias en Latinoamérica, Norte de Africa y Europa; Rodríguez-Perea, A., Pons, G.X., Roig-Munar, F.X., Martín-Prieto, J.A., Mir-Gual, M., Cabrera, J.A., Eds.; Sociedad Historia Natural Balears: Palma de Mallorca, Spain, 2012; Volume 19, pp. 33–44. ISBN 978-84-616-2240-5. [Google Scholar]
- Losada, I.J.; Méndez, F.J.; Olabarrieta, M.; Liste, M.; Menéndez, M.; Tomás, A.; Abascal, A.J.; Agudelo, P.; Guanche, R. Fase II: Evaluación de efectos en la costa española. In Impactos en la Costa Española por Efecto del Cambio Climático; Ministerio de Medio Ambiente: Madrid, Spain, 2004. [Google Scholar]
- Caetano, E.; Innocentini, V.; Magaña, V.; Martins, S.; Méndez, B. Cambio climático y el aumento del nivel del mar. In Vulnerabilidad de las Zonas Costeras Mexicanas Ante el Cambio Climático; Botello, A.V., Villanueva-Fragoso, S., Gutiérrez, J., Rojas-Galaviz, J.L., Eds.; Semarnat-INE, UNAM-ICMYL, Universidad Autónoma de Campeche: México, Spain, 2011; pp. 283–304. ISBN 978-92-0-053196-5. [Google Scholar]
- Cooley, S.; Schoeman, D.; Bopp, L.; Boyd, P.; Donner, S.; Ito, S.-I.; Kiessling, W.; Martinetto, P.; Ojea, E.; Racault, M.-F.; et al. Oceans and coastal ecosystems and their services. In Climate Change 2022: Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar] [CrossRef]
- Melet, A.; Van de Wal, R.; Amores, A.; Arns, A.; Chaigneau, A.A.; Dinu, I.; Haigh, I.D.; Hermans, T.H.; Lionello, P.; Marcos, M. Sea level rise in Europe: Observations and projections. State Planet Discuss. 2023, preprint. [Google Scholar] [CrossRef]
- Vousdoukas, M.I.; Mentaschi, L.; Voukouvalas, E.; Verlaan, M.; Feyen, L. Extreme sea levels on the rise along Europe’s coasts. Earth’s Future 2017, 5, 304–323. [Google Scholar] [CrossRef]
- Phillips, R.A.; Bearhop, S.; Mcgill, R.A.; Dawson, D.A. Stable isotopes reveal individual variation in migration strategies and habitat preferences in a suite of seabirds during the nonbreeding period. Oecologia 2009, 160, 795–806. [Google Scholar] [CrossRef]
- Dias, M.P.; Granadeiro, J.P.; Phillips, R.A.; Alonso, H.; Catry, P. Breaking the routine: Individual Cory’s shearwaters shift winter destinations between hemispheres and across ocean basins. Proc. R. Soc. B Biol. Sci. 2011, 278, 1786–1793. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A.B.; Magioli, M.; Bogoni, J.A.; Moreira, M.Z.; Silveira, L.F.; Alexandrino, E.R.; da Luz, D.T.A.; Pizo, M.A.; Silva, W.R.; de Oliveira, V.C. Human-modified landscapes narrow the isotopic niche of neotropical birds. Oecologia 2021, 196, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, J.; Vidal, M. Plan de Conservación del Chorlitejo Patinegro (Charadrius alexandrinus) en Galicia; Consellería de Medio Ambiente e Desenvolvemento Sostible: Santiago de Compostela, Spain, 2008. [Google Scholar]
- Vidal, M.; Domínguez, J. Long-term population trends of breeding Kentish Plovers (Charadrius alexandrinus) in Northwestern Spain under the effects of a major oil spill. Bird Conserv. Int. 2013, 23, 386–397. [Google Scholar] [CrossRef]
- AlRashidi, M.; Shobrak, M.; Al-Eissa, M.S.; Székely, T. Integrating spatial data and shorebird nesting locations to predict the potential future impact of global warming on coastal habitats: A case study on Farasan Islands, Saudi Arabia. Saudi J. Biol. Sci. 2012, 19, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Aiello-Lammens, M.E.; Chu-Agor, M.L.; Convertino, M.; Fischer, R.A.; Linkov, I.; Akcakaya, H.R. The impact of sea-level rise on Snowy Plovers in Florida: Integrating geomorphological, habitat, and metapopulation models. Glob. Change Biol. 2011, 17, 3644–3654. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).