The Influence of Three Commercial Soy Lecithin-Based Semen Extenders and Two Spermatozoa Concentrations on the Quality of Pre-Freeze and Post-Thaw Ram Epididymal Spermatozoa
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Media, Reagents, and Materials
2.2. Study Location and Testicle Collection
2.3. Epididymal Sperm Collection
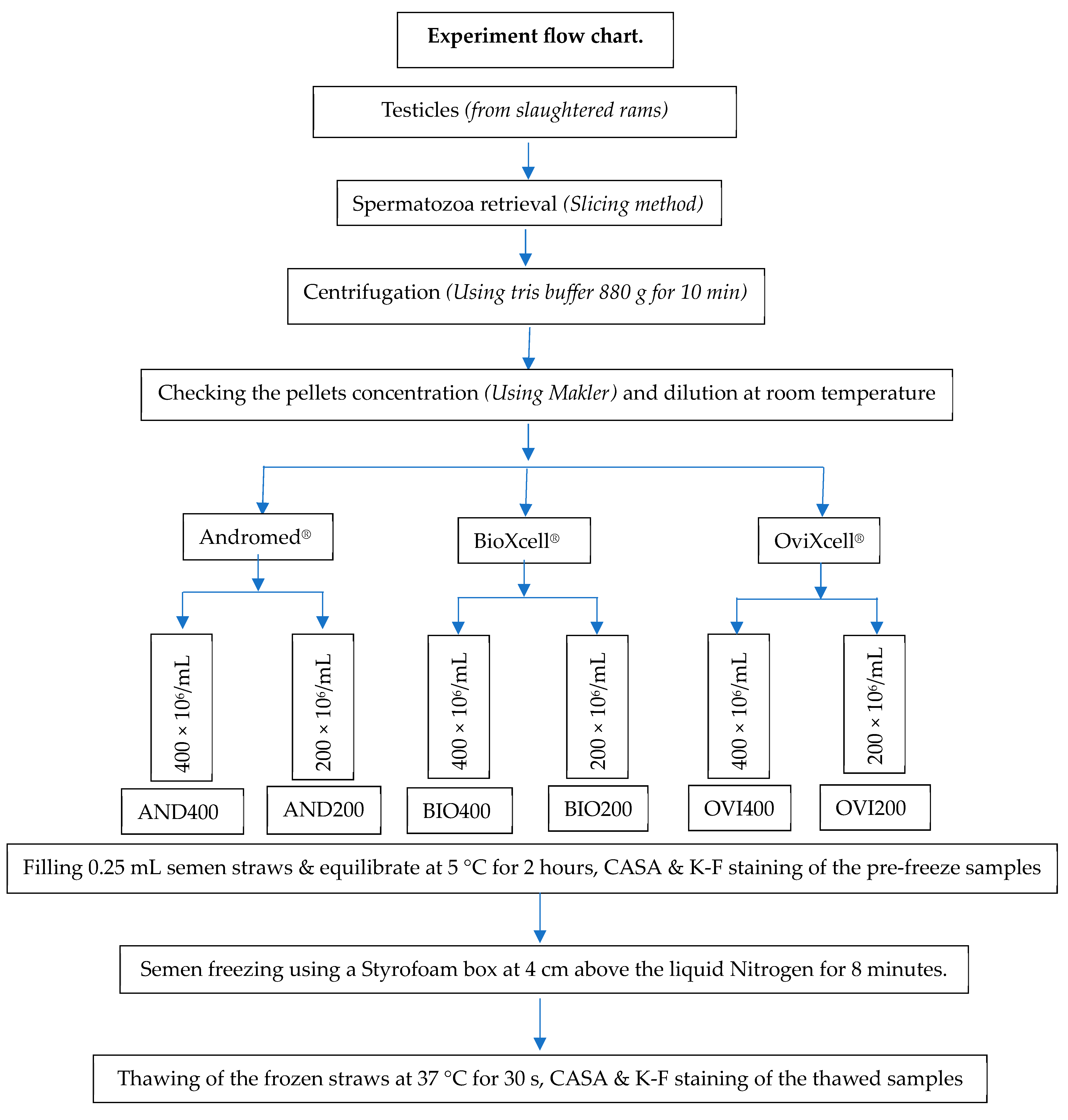
2.4. Sample Dilution, Equilibration and Freezing
2.5. Sample Quality Assessment
2.5.1. Standard Motility and Kinematic Parameters
2.5.2. Viability and Morphology Assessment
2.6. Data Analysis
3. Results
3.1. General Parameters of Ram Epididymal Spermatozoa
3.2. Effects of Three Different Commercial Soy Lecithin-Based Semen Extenders and Two Spermatozoa Concentrations on the Standard Motility and Kinematic Parameters of Pre-Freeze Ram Epididymal Spermatozoa
3.3. Effects of Three Different Commercial Soy Lecithin-Based Extenders and Two Spermatozoa Concentrations on Standard Motility and Kinematic Parameters of Post-Thaw Ram Epididymal Spermatozoa
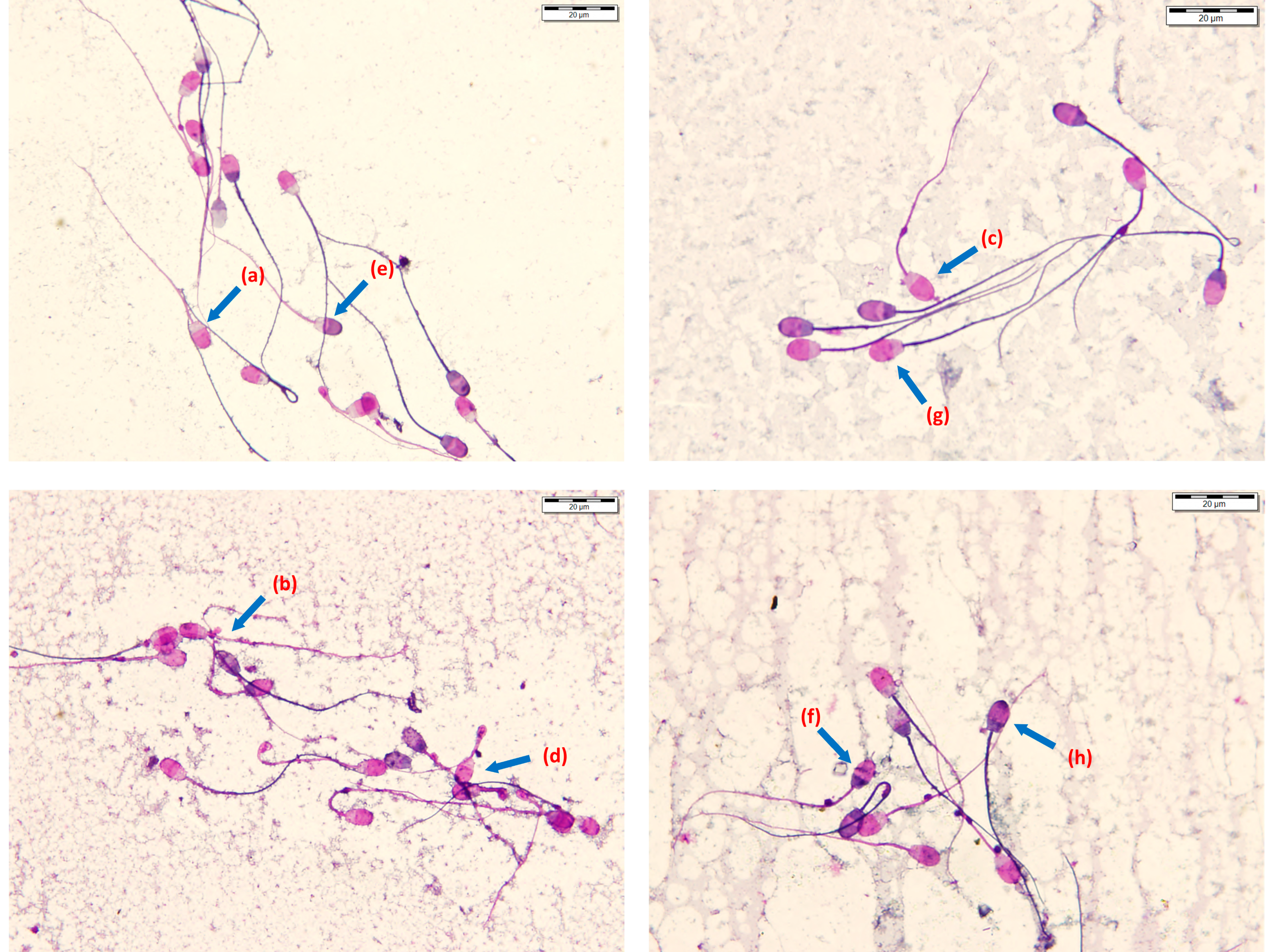
3.4. Effects of Different Soy Lecithin-Based Commercial Semen Extenders and the Two Spermatozoa Concentrations on the Post-Thaw Viability and Morphological Characteristics of Ram Epididymal Spermatozoa
3.5. Effect of Freezing with Different Commercial Soy Lecithin-Based Semen Extenders on All Distal Droplets and Tail Defects of Ram Epididymal Spermatozoa
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kang, S.-S.; Kim, U.-H.; Jeon, M.-H.; Lee, M.-S.; Cho, S.-R. Comparison of Spermatozoa Recovery Methods on Cauda Epididymal Sperm of Hanwoo Bulls. J. Anim. Reprod. Biotechnol. 2018, 33, 321–326. [Google Scholar] [CrossRef]
- Mamani-Mango, G.; Moina Gonzales, M.; Ramos Hidalgo, M.; Mendoza Mallma, J.; Ruiz Béjar, J.; Rivas Palma, V.; Mellisho Salas, E. Effect of Extender and Freezing Rate on Quality Parameters and In Vitro Fertilization Capacity of Alpaca Spermatozoa Recovered from Cauda Epididymis. Biopreserv. Biobank. 2019, 17, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Akiyoshi, T.; Kan, M.; Mori, M.; Teshima, H.; Shimada, M. Artificial Insemination with Seminal Plasma Improves the Reproductive Performance of Frozen-Thawed Boar Epididymal Spermatozoa. J. Androl. 2012, 33, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-S.; Kim, U.-H.; Ahn, J.S.; Won, J.I.; Cho, S.-R. Improvement of Pregnancy Rate after Deep Uterine Artificial Insemination with Frozen-Thawed Cauda Epididymal Spermatozoa in Hanwoo Cattle. J. Anim. Reprod. Biotechnol. 2021, 36, 82–90. [Google Scholar] [CrossRef]
- Ehling, C.; Rath, D.; Struckmann, C.; Frenzel, A.; Schindler, L.; Niemann, H. Utilization of Frozen–Thawed Epididymal Ram Semen to Preserve Genetic Diversity in Scrapie Susceptible Sheep Breeds. Theriogenology 2006, 66, 2160–2164. [Google Scholar] [CrossRef] [PubMed]
- Rickard, J.P.; Pini, T.; Soleilhavoup, C.; Cognie, J.; Bathgate, R.; Lynch, G.W.; Evans, G.; Maxwell, W.M.C.; Druart, X.; de Graaf, S.P. Seminal Plasma Aids the Survival and Cervical Transit of Epididymal Ram Spermatozoa. Reproduction 2014, 148, 469–478. [Google Scholar] [CrossRef]
- Fernández Abella, D.; Da Costa, M.; Guérin, Y.; Dacheux, J.L. Fertility of Undiluted Ram Epididymal Spermatozoa Stored for Several Days at 4 °C. Animal 2015, 9, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Ocampo, L.C.; DelaRosa, I.C.J.; Lofranco, J.O.C.; Ortiz, J.G.M.; Ocampo, M.B. Live Birth after Artificial Insemination Using Cryopreserved Epididymal Sperm Recovered from the Cauda Epididymis of Slaughtered Non-Descript Bucks in the Philippines. Int. J. Agric. Technol. 2021, 17, 2183–2196. [Google Scholar]
- Miró, J.; Morató, R.; Vilagran, I.; Taberner, E.; Bonet, S.; Yeste, M. Preservation of Epididymal Stallion Sperm in Liquid and Frozen States: Effects of Seminal Plasma on Sperm Function and Fertility. J. Equine Vet. Sci. 2020, 88, 102940. [Google Scholar] [CrossRef]
- Heise, A.; Kähn, W.; Volkmann, D.H.; Thompson, P.N.; Gerber, D. Influence of Seminal Plasma on Fertility of Fresh and Frozen-Thawed Stallion Epididymal Spermatozoa. Anim. Reprod. Sci. 2010, 118, 48–53. [Google Scholar] [CrossRef]
- Soler, A.J.; García, A.J.; Fernández-Santos, M.R.; Esteso, M.C.; Garde, J.J. Effects of Thawing Procedure on Postthawed In Vitro Viability and In Vivo Fertility of Red Deer Epididymal Spermatozoa Cryopreserved at −196 °C. J. Androl. 2003, 24, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, M.; Tamayo-Canul, J.; Martínez-Rodríguez, C.; López-Urueña, E.; Gomes-Alves, S.; Anel, L.; Martínez-Pastor, F.; de Paz, P. Specificity of the Extender Used for Freezing Ram Sperm Depends of the Spermatozoa Source (Ejaculate, Electroejaculate or Epididymis). Anim. Reprod. Sci. 2012, 132, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Lone, F.; Islam, R.; Khan, M.; Sofi, K. Effect of Different Egg Yolk-Based Extenders on the Quality of Ovine Cauda Epididymal Spermatozoa during Storage at 4 °C: Effect of Extenders on Ovine Epididymal Sperm. Reprod. Domest. Anim. 2012, 47, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Rastegarnia, A.; Shahverdi, A.; topraggaleah, T.R.; Shafiepour, V. In Vitro Comparison of Soybean Lecithin-Based Extenders for Cryopreservation of Buffalo (Bubalus Bubalis) Semen. Comp. Clin. Pathol. 2014, 23, 893–900. [Google Scholar] [CrossRef]
- Abdussamad, A.M.; Detterer, J.; Gauly, M.; Holtz, W. Comparison of Various Semen Extenders and Addition of Prostaglandin F2 on Pregnancy Rate in Cows. Animal 2016, 10, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Aires, V.A.; Hinsch, K.-D.; Mueller-Schloesser, F.; Bogner, K.; Mueller-Schloesser, S.; Hinsch, E. In Vitro and in Vivo Comparison of Egg Yolk-Based and Soybean Lecithin-Based Extenders for Cryopreservation of Bovine Semen. Theriogenology 2003, 60, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Khatun, A.; Fazili, M.R.; Malik, A.A.; Shah, R.A.; Khan, H.M.; Choudhury, A.R.; Malik, A. In Vitro Assessment of Tris Egg Yolk and Soybean Lecithin Based Extenders for Cryopreservation of Crossbred Ram Semen. Cryoletters 2021, 42, 73–80. [Google Scholar] [PubMed]
- Murphy, E.M.; Murphy, C.; O’Meara, C.; Dunne, G.; Eivers, B.; Lonergan, P.; Fair, S. A Comparison of Semen Diluents on the in Vitro and in Vivo Fertility of Liquid Bull Semen. J. Dairy. Sci. 2017, 100, 1541–1554. [Google Scholar] [CrossRef] [PubMed]
- Braga, M.Q.; Franco, R.V.R.; Rodrigues, L.F.; Galeli, G.; Oliveira, K.M.; Reis, F.A.C.; Nishikawa, M.F.A.; Moura, E.P. 110 Comparison Of Andromed®, Bioxcell®, And Botu-Bov® Extenders For Cryopreservation Of Bull Sexed Semen. Reprod. Fertil. Dev. 2007, 19, 172. [Google Scholar] [CrossRef]
- Crespilho, A.M.; Sá Filho, M.F.; Dell’Aqua, J.A.; Nichi, M.; Monteiro, G.A.; Avanzi, B.R.; Martins, A.; Papa, F.O. Comparison of in Vitro and in Vivo Fertilizing Potential of Bovine Semen Frozen in Egg Yolk or New Lecithin Based Extenders. Livest. Sci. 2012, 149, 1–6. [Google Scholar] [CrossRef]
- Meena, G.S.; Raina, V.S.; Gupta, T.K.; Mohanty; Bishist, R. Comparative Performance of Biociphos and Egg Yolk Based Extenders for Buffalo Semen Cryopreservation. Indian. J. Anim. Sci. 2010, 80, 414–417. [Google Scholar]
- Jiménez-Rabadán, P.; Ramón, M.; García-Álvarez, O.; Maroto-Morales, A.; del Olmo, E.; Pérez-Guzmán, M.D.; Bisbal, A.; Fernández-Santos, M.R.; Garde, J.J.; Soler, A.J. Effect of Semen Collection Method (Artificial Vagina vs. Electroejaculation), Extender and Centrifugation on Post-Thaw Sperm Quality of Blanca-Celtibérica Buck Ejaculates. Anim. Reprod. Sci. 2012, 132, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Gil, J.; Lundeheim, N.; Söderquist, L.; Rodríguez-Martínez, H. Influence of Extender, Temperature, and Addition of Glycerol on Post-Thaw Sperm Parameters in Ram Semen. Theriogenology 2003, 59, 1241–1255. [Google Scholar] [CrossRef]
- Fukui, Y.; Kohno, H.; Togari, T.; Hiwasa, M.; Okabe, K. Fertility after Artificial Insemination Using a Soybean-Based Semen Extender in Sheep. J. Reprod. Dev. 2008, 54, 286–289. [Google Scholar] [CrossRef]
- Kulaksiz, R.; Çebi, Ç.; Akçay, E. The Effect of Different Extenders on the Motility and Morphology of Ram Sperm Frozen or Stored at 4 °C. Turk. J. Vet. Anim. Sci. 2012. [Google Scholar] [CrossRef]
- Akçay, E.; Kulaksiz, R.; Daşkin, A.; Çebi, C.; Tekin, K. The Effect of Different Dilution Rates on Post-Thaw Quality of Ram Semen Frozen in Two Different Egg-Yolk Free Extenders. Slov. Vet. Res. 2012, 42, 97–102. [Google Scholar]
- Fernandes, M.; Hernández, P.R.; Simões, J.; Barbas, J.P. Effects of Three Semen Extenders, Breeding Season Month and Freezing–Thawing Cycle on Spermatozoa Preservation of Portuguese Merino Sheep. Animals 2021, 11, 2619. [Google Scholar] [CrossRef]
- Ari, U.; Kulaksiz, R.; Öztürkler, Y. Freezability of Tushin Ram Semen Extended with Goat or Cow Milk Based Extenders: Freezability of Tushin Ram Semen Extended with Goat or Cow Milk. Reprod. Domest. Anim. 2011, 46, 975–979. [Google Scholar] [CrossRef] [PubMed]
- Bohlooli, S.; Cedden, F.; PishJang, J.; Razzaghzadeh, S.; Bozoğlu, S. The Effectof Different Extenders on Post-Thaw Sperm Viability, Motility and Membrane Integrity in Cryopreserved Semen of ZandiRam. J. Basic. Appl. Sci. Res. 2012, 2, 1120–1123. [Google Scholar]
- Khalifa, T.; Lymberopoulos, A.; Theodosiadou, E. Association of Soybean-Based Extenders with Field Fertility of Stored Ram (Ovis Aries) Semen: A Randomized Double-Blind Parallel Group Design. Theriogenology 2013, 79, 517–527. [Google Scholar] [CrossRef]
- Sharafi, M.; Forouzanfar, M.; Hosseini, S.M.; Hajian, M.; Ostadhosseini, S.; Hosseini, L.; Abedi, P.; Nili, N.; Rahmani, H.R.; Javaheri, A.R.; et al. In Vitro Comparison of Soybean Lecithin Based-Extender with Commercially Available Extender for Ram Semen Cryopreservation. Int. J. Fertil. Steril. 2009, 3, 149–152. [Google Scholar] [CrossRef]
- Emamverdi, M.; Zhandi, M.; Shahneh, A.Z.; Sharafi, M.; Akhlaghi, A.; Motlagh, M.K.; Dadkhah, F.; Davachi, N.D. Flow Cytometric and Microscopic Evaluation of Post-Thawed Ram Semen Cryopreserved in Chemically Defined Home-Made or Commercial Extenders. Anim. Prod. Sci. 2015, 55, 551. [Google Scholar] [CrossRef]
- Lone, F.A.; Islam, R.; Khan, M.Z.; Sofi, K.A. Effect of Transportation Temperature on the Quality of Cauda Epididymal Spermatozoa of Ram. Anim. Reprod. Sci. 2011, 123, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Kaabi, M.; Paz, P.; Alvarez, M.; Anel, E.; Boixo, J.C.; Rouissi, H.; Herraez, P.; Anel, L. Effect of Epididymis Handling Conditions on the Quality of Ram Spermatozoa Recovered Post-Mortem. Theriogenology 2003, 60, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Tamayo-Canul, J.; Alvarez, M.; López-Urueña, E.; Nicolas, M.; Martinez-Pastor, F.; Anel, E.; Anel, L.; de Paz, P. Undiluted or Extended Storage of Ram Epididymal Spermatozoa as Alternatives to Refrigerating the Whole Epididymes. Anim. Reprod. Sci. 2011, 126, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Islam, R.; Lone, F.A.; Malik, A.A. Effect of Washing on the Post-Thaw Quality of Cryopreserved Ram Epididymal Spermatozoa. Vet. World 2016, 9, 519–523. [Google Scholar] [CrossRef]
- Ahmed, T. Cryopreservation of Ram Cauda Epididymal Spermatozoa Using Different Buffers and Sugar Combinations. JAR 2019, 9, 927–933. [Google Scholar] [CrossRef]
- Leahy, T.; Marti, J.I.; Mendoza, N.; Pérez-Pé, R.; Muiño-Blanco, T.; Cebrián-Pérez, J.A.; Evans, G.; Maxwell, W.M.C. High Pre-Freezing Dilution Improves Post-Thaw Function of Ram Spermatozoa. Anim. Reprod. Sci. 2010, 119, 137–146. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, A.G.; Martemucci, G.; Colonna, M.A.; Bellitti, A. Post-Thaw Survival of Ram Spermatozoa and Fertility after Insemination as Affected by Prefreezing Sperm Concentration and Extender Composition. Theriogenology 2001, 55, 1159–1170. [Google Scholar] [CrossRef]
- Nascimento, J.; Raphael, C.F.; Andrade, A.F.C.; Alonso, M.A.; Celeghini, E.C.C.; Arruda, R.P. Effects of Sperm Concentration and Straw Volume on Motion Characteristics and Plasma, Acrosomal, and Mitochondrial Membranes of Equine Cryopreserved Spermatozoa. J. Equine Vet. Sci. 2008, 28, 351–358. [Google Scholar] [CrossRef]
- Maxwell, W.M.; Johnson, L.A. Physiology of Spermatozoa at High Dilution Rates: The Influence of Seminal Plasma. Theriogenology 1999, 52, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- Penitente-Filho, J.M.; Dias, J.C.; Oliveira, F.A.; Silveira, C.O.; Torres, C.A. Correlation between Sperm Motility and Hypoosmotic Swelling Test on Cryopreserved Goat Semen. Magistra Cruz Das Almas 2017, 27, 468–472. [Google Scholar]
- Egerszegi, I.; Sarlos, P.; Ratky, J. Cryopreservation of Epididymal Spermatozoa of Black Racka Rams from Hortobágy-a Possible Means of Gene Preservation. Magyar Állatorvosok Lapja 2012, 134, 524–528. [Google Scholar]
- Goovaerts, I.G.F.; Hoflack, G.G.; Van Soom, A.; Dewulf, J.; Nichi, M.; de Kruif, A.; Bols, P.E.J. Evaluation of Epididymal Semen Quality Using the Hamilton–Thorne Analyser Indicates Variation between the Two Caudae Epididymides of the Same Bull. Theriogenology 2006, 66, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Bergstein-Galan, T.G.; Weiss, R.R.; Kozicki, L.E.; Bicudo, S.D. Sperm Subpopulations in Ejaculated Sperm and Spermatozoa Recovered from Ovine Epididymides up to 48 h after Death. Anim. Reprod. Sci. 2017, 187, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Kútvölgyi, G.; Stefler, J.; Kovács, A. Viability and Acrosome Staining of Stallion Spermatozoa by Chicago Sky Blue and Giemsa. Biotech. Histochem. 2006, 81, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Kovács, A.; Foote, R.H. Viability and Acrosome Staining of Bull, Boar and Rabbit Spermatozoa. Biotech. Histochem. 1992, 67, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.F. The Causes of Reduced Fertility with Cryopreserved Semen. Anim. Reprod. Sci. 2000, 60–61, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Salamon, S.; Maxwell, W.M.C. Storage of Ram Semen. Anim. Reprod. Sci. 2000, 62, 77–111. [Google Scholar] [CrossRef]
- Van Der Horst, G. Computer Aided Sperm Analysis (CASA) in Domestic Animals: Current Status, Three D Tracking and Flagellar Analysis. Anim. Reprod. Sci. 2020, 220, 106350. [Google Scholar] [CrossRef]
- Nagy, Á.; Polichronopoulos, T.; Gáspárdy, A.; Solti, L.; Cseh, S. Correlation between Bull Fertility and Sperm Cell Velocity Parameters Generated by Computer-Assisted Semen Analysis. Acta Vet. Hung. 2015, 63, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Robayo, I.; Montenegro, V.; Valdés, C.; Cox, J. CASA Assessment of Kinematic Parameters of Ram Spermatozoa and Their Relationship to Migration Efficiency in Ruminant Cervical Mucus. Reprod. Domest. Anim. 2008, 43, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Holt, W.V. Basic Aspects of Frozen Storage of Semen. Anim. Reprod. Sci. 2000, 62, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Sinapov, B.; Yotov, S. Relationship between Some Kinematic Parameters of Ram Semen and Reproductive Performance of Dairy Sheep after Application of Assisted Reproductive Technologies. Int. J. Curr. Microbiol. App. Sci. 2023, 12, 307–317. [Google Scholar] [CrossRef]
- Ondřej, Š.; Jiří, Š.; Jan, B.; Pavla, M.; Lucie, T.; Dole~alová, M.; Petra, F.; Ludk, S.; Radko, R. Low Density Lipoprotein - Important Player in Increasing Cryoprotective Efficiency of Soybean Lecithin-Based Bull Semen Extenders. Anim. Reprod. 2019, 16, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Tanga, B.M.; Qamar, A.Y.; Raza, S.; Bang, S.; Fang, X.; Yoon, K.; Cho, J. Semen Evaluation: Methodological Advancements in Sperm Quality-Specific Fertility Assessment - A Review. Anim. Biosci. 2021, 34, 1253–1270. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Montanero, J.; Calero, R.; Roy, T.J. Identification of Sperm Subpopulations with Defined Motility Characteristics in Ejaculates from Ile de France Rams. Anim. Reprod. Sci. 2011, 129, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Dorado, J.; Rodríguez, I.; Hidalgo, M. Cryopreservation of Goat Spermatozoa: Comparison of Two Freezing Extenders Based on Post-Thaw Sperm Quality and Fertility Rates after Artificial Insemination. Theriogenology 2007, 68, 168–177. [Google Scholar] [CrossRef]
- Domingo, P.; Olaciregui, M.; González, N.; De Blas, I.; Gil, L. Comparison of Different Semen Extenders and Cryoprotectant Agents to Enhance Cryopreservation of Rabbit Spermatozoa. Czech J. Anim. Sci. 2019, 64, 59–66. [Google Scholar] [CrossRef]
- Henning, H.; Luther, A.-M.; Höfner-Schmiing, L.; Waberski, D. Compensability of an Enhanced Incidence of Spermatozoa with Cytoplasmic Droplets in Boar Semen for Use in Artificial Insemination: A Single Cell Approach. Sci. Rep. 2022, 12, 21833. [Google Scholar] [CrossRef]
- Henning, H.; Luther, A.-M.; Waberski, D. A High Incidence of Sperm with Cytoplasmic Droplets Affects the Response to Bicarbonate in Preserved Boar Semen. Animals 2021, 11, 2570. [Google Scholar] [CrossRef] [PubMed]
- Mandal, D.K.; Kumar, M.; Tyagi, S. Effect of Age on Spermiogram of Holstein Friesian × Sahiwal Crossbred Bulls. Animal 2010, 4, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.; Saleh, R.A.; Sharma, R.K.; Lewis-Jones, I.; Esfandiari, N.; Thomas, A.J.; Agarwal, A. Novel Association between Sperm Reactive Oxygen Species Production, Sperm Morphological Defects, and the Sperm Deformity Index. Fertil. Steril. 2004, 81, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.A.; Van Leyen, K.; Lovercamp, K.W.; Manandhar, G.; Sutovsky, M.; Feng, D.; Safranski, T.; Sutovsky, P. 15-Lipoxygenase Is a Component of the Mammalian Sperm Cytoplasmic Droplet. Reproduction 2005, 130, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Kuster, C.E.; Hess, R.A.; Althouse, G.C. Immunofluorescence Reveals Ubiquitination of Retained Distal Cytoplasmic Droplets on Ejaculated Porcine Spermatozoa. J. Androl. 2004, 25, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yuan, S.-Q.; Zheng, Z.-H.; Yan, W. The Cytoplasmic Droplet May Be Indicative of Sperm Motility and Normal Spermiogenesis. Asian J. Androl. 2013, 15, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Nagy, S.; Házas, G.; Bali Papp, A.; Iváncsics, J.; Szász, F.; Szász, F.; Kovács, A.; Foote, R.H. Evaluation of Sperm Tail Membrane Integrity by Light Microscopy. Theriogenology 1999, 52, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Nagy, S.; Kovács, A.; Zubor, T.; Zomborszky, Z.; Tóth, J.; Horn, P. Evaluation of Membrane Integrity of Frozen/Thawed Deer Spermatozoa: Short Communication. Acta Vet. Hung. 2001, 49, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Kútvölgyi, G. Development of Qualification of Fresh and Frozen Stallion Semen, Investigation of Factors Affecting Sperm Quality Using a New Evaluation Method [A Friss És Mélyhűtött Ménsperma Minősítésének Fejlesztése, a Sperma Minőségét Befolyásoló Tényezők Vizsgálata Egy Új Bírálati Módszer Alkalmazásával]. Ph. D. Thesis, Kaposvári Egyetem, Kaposvár, Hungary, 2013. [Google Scholar] [CrossRef]
- Turri, F.; Madeddu, M.; Gliozzi, T.M.; Gandini, G.; Pizzi, F. Effect of Testicle Postmortem Storage on Goat Frozen-Thawed Epididymal Sperm Quality as a Tool to Improve Genebanking in Local Breeds. Animal 2014, 8, 440–447. [Google Scholar] [CrossRef]
- Bezerra, J.A.B.; da Silva, A.M.; Peixoto, G.C.X.; da Silva, M.d.A.; Franco de Oliveira, M.; Silva, A.R. Influence of Recovery Method and Centrifugation on Epididymal Sperm from Collared Peccaries (Pecari Tajacu Linnaeus, 1758). Zool. Sci. 2014, 31, 338–342. [Google Scholar] [CrossRef]
- Tebet, J.M.; Martins, M.I.M.; Chirinea, V.H.; Souza, F.F.; Campagnol, D.; Lopes, M.D. Cryopreservation Effects on Domestic Cat Epididymal versus Electroejaculated Spermatozoa. Theriogenology 2006, 66, 1629–1632. [Google Scholar] [CrossRef] [PubMed]

| AndroMed® (100 mL) | BioXcell® (1000 mL) | OviXcell® (100 mL) |
|---|---|---|
| Phospholipids | Glycine (0.2 g/L) | Amino acid |
| TRIS | TRIS (2.3 g/L) | Buffers |
| Citric acid | Monohydrate citric acid (2.5 g/L) | |
| Sodium citrate (6.2 g/L) Potassium chloride (0.8 g/L) Hydrate of calcium lactate (0.7 g/L) | Salts | |
| Sugars | Fructose (1.2 g/L) Monohydrate lactose (0.8 g/L) Anhydrous glucose (0.5 g/L) | Sugars |
| Antioxidants | Taurine (0.005 g/L) | Taurine |
| Glycerol (6.7%) | Glycerol (7.0%/40.2 g/L) | Glycerol |
| Tylosin (5.7 mg) | Tylosin tartrate (0.33 g/L) | Tylosin tartrate |
| Gentamicin (28.6 mg) | Gentamycin sulphate (0.24 g/L) | Gentamicin |
| Spectinomycin (34.3 mg) | Spectinomycin | Spectinomycin sulfate (<0.2%) |
| Lincomycin (17.2 mg) | Licospectin 100 (0.385 g/L) | Lincomycin hydrochloride |
| Soy lecithin | Soy lecithin (1.5 g/L) | Soy lecithin |
| Ultrapure water | Ultrapure water (ad 1000 mL) | Ultrapure water |
| Parameters | Range | Mean ± SE |
|---|---|---|
| Testicular weight (g) | 113.07–308.09 | 157.78 ± 22.15 |
| Cauda epididymal weight (g) | 7.89–20.39 | 14.25 ± 1.38 |
| Spermatozoa concentration (106/mL) | 5800–14,240 | 9061.44 ± 845.53 |
| Extenders | Standard Motility and Kinematic Parameters | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TM (%) | PM (%) | VCL (μm/s) | VAP (μm/s) | VSL (μm/s) | LIN (%) | STR (%) | BCF (Hz) | WOB (%) | ALH (μm) | |
| AndroMed® | 72.22 ± 3.2 | 64.89 ± 3.4 | 163.94 ± 5.8 | 76.85 ± 2.3 | 54.05 ± 2.4 | 32.83 ± 1.3 | 70.00 ± 2.2 | 26.80 ± 0.8 a | 46.61 ± 0.5 | 5.55 ± 0.2 |
| BioXcell® | 69.00 ± 3.8 | 62.44 ± 4.0 | 168.11 ± 3.9 | 82.21 ± 2.3 | 60.64 ± 3.1 | 35.50 ± 1.6 | 72.83 ± 2.4 | 30.18 ± 1.1 b | 48.44 ± 0.6 | 5.21 ± 0.2 |
| OviXcell® | 67.61 ± 3.7 | 60.78 ± 3.9 | 169.06 ± 3.2 | 83.16 ± 2.2 | 62.00 ± 3.4 | 35.94 ± 1.5 | 73.22 ± 2.2 | 29.99 ± 1.0 b | 48.56 ± 0.7 | 5.27 ± 0.1 |
| p-value | 0.633 | 0.727 | 0.863 | 0.336 | 0.215 | 0.267 | 0.463 | 0.020 | 0.080 | 0.695 |
| Conc. (106/mL) | ||||||||||
| 200 | 67.85 ± 3.3 | 61.26 ± 3.4 | 167.48 ± 3.7 | 81.14 ± 1.9 | 59.80 ± 2.5 | 35.26 ± 1.2 | 72.85 ± 1.9 | 29.24 ± 0.9 | 48.04 ± 0.6 | 5.25 ± 0.2 |
| 400 | 71.37 ± 2.5 | 64.15 ± 2.8 | 166.59 ± 3.6 | 80.34 ± 1.9 | 58.00 ± 2.5 | 34.56 ± 1.2 | 71.19 ± 1.9 | 28.71 ± 0.8 | 47.70 ± 0.5 | 5.42 ± 0.2 |
| p-value | 0.170 | 0.231 | 0.556 | 0.379 | 0.302 | 0.808 | 0.584 | 0.834 | 0.985 | 0.181 |
| p-value Ext. * Conc. | 0.619 | 0.643 | 0.852 | 0.744 | 0.659 | 0.887 | 0.840 | 0.854 | 0.946 | 0.712 |
| Extenders | Standard Motility and Kinematic Parameters (Mean ± SE) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TM (%) | PM (%) | VCL (μm/s) | VAP (μm/s) | VSL (μm/s) | LIN (%) | STR (%) | BCF (Hz) | WOB (%) | ALH (μm) | |
| AndroMed® | 34.89 ± 3.9 | 27.11 ± 3.4 | 139.55 ± 6.3 | 67.72 ± 3.5 a | 50.58 ± 3.3 | 35.72 ± 1.4 | 74.06 ± 2.3 | 28.72 ± 0.9 a | 47.67 ± 0.7 a | 4.41 ± 0.2 |
| BioXcell® | 38.83 ± 3.5 | 31.50 ± 3.1 | 156.72 ± 5.0 | 77.78 ± 3.2 b | 58.96 ± 3.9 | 37.11 ± 1.8 | 74.28 ± 2.5 | 32.81 ± 1.1 b | 49.56 ± 0.9 ab | 4.42 ± 0.2 |
| OviXcell® | 37.61 ± 3.7 | 31.56 ± 3.5 | 157.39 ± 5.4 | 80.48 ± 3.1 b | 61.46 ± 3.9 | 38.33 ± 1.7 | 75.00 ± 2.4 | 32.46 ± 1.0 b | 50.56 ± 0.8 b | 4.55 ± 0.2 |
| p-value | 0.893 | 0.509 | 0.191 | 0.024 | 0.154 | 0.554 | 0.816 | 0.012 | 0.044 | 0.849 |
| Concentrations (106/mL) | ||||||||||
| 200 | 34.33 ± 2.3 | 27.33 ± 2.2 | 150.40 ± 5.3 | 75.43 ± 3.2 | 57.92 ± 3.4 | 37.74 ± 1.4 | 75.41 ± 1.9 | 31.83 ± 0.9 | 49.37 ± 0.8 | 4.33 ± 0.1 |
| 400 | 39.89 ± 3.5 | 32.78 ± 3.1 | 152.04 ± 4.3 | 75.22 ± 2.4 | 56.07 ± 2.8 | 36.37 ± 1.3 | 73.48 ± 1.9 | 30.83 ± 0.8 | 49.15 ± 0.6 | 4.58 ± 0.2 |
| p-value | 0.170 | 0.249 | 0.878 | 0.957 | 0.534 | 0.486 | 0.566 | 0.400 | 0.815 | 0.250 |
| p-value Ext. * Conc. | 0.723 | 0.946 | 0.648 | 0.855 | 0.913 | 0.976 | 0.959 | 0.827 | 0.882 | 0.927 |
| Extenders | Viability and Morphological Parameters (Mean ± SE) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IHITIA (%) | IPD (%) | IDD (%) | IBT (%) | IHITDA (%) | DHIT (%) | IHDT (%) | DHDTDA (%) | All Intact (%) | All Intact Head (%) | All Intact Tail (%) | All Distal Droplets (%) | All Bent Tails (%) | |
| AndroMed® | 5.92 ± 1.2 | 0.87 ± 0.3 | 9.72 ± 1.4 | 2.56 ± 0.6 a | 0.04 ± 0.0 | 6.31 ± 1.1 | 15.52 ± 1.8 | 59.06 ± 3.4 | 19.08 ± 2.2 | 34.64 ± 3.2 a | 25.42 ± 2.9 | 28.44 ± 2.9 | 9.74 ± 1.4 a |
| BioXcell® | 6.55 ± 1.1 | 0.91 ± 0.2 | 9.44 ± 1.4 | 8.14 ± 1.5 b | 0.03 ± 0.0 | 2.91 ± 0.7 | 20.27 ± 2.5 | 51.73 ± 3.3 | 25.03 ± 1.5 | 45.33 ± 3.3 b | 27.97 ± 1.9 | 21.89 ± 2.7 | 18.33 ± 2.4 b |
| OviXcell® | 7.46 ± 1.3 | 0.68 ± 0.2 | 9.33 ± 1.5 | 7.19 ± 1.3 b | 0.02 ± 0.0 | 2.53 ± 0.4 | 20.00 ± 1.8 | 52.79 ± 3.0 | 24.66 ± 2.4 | 44.68 ± 2.9 b | 27.21 ± 2.6 | 20.33 ± 2.4 | 17.39 ± 1.7 b |
| p-value | 0.717 | 0.613 | 0.981 | 0.003 | 0.866 | 0.155 | 0.143 | 0.242 | 0.094 | 0.030 | 0.771 | 0.100 | 0.0001 |
| Con. (106/mL) | |||||||||||||
| 200 | 5.43 ± 1.3 | 0.70 ± 0.3 | 9.03 ± 2.1 | 6.39 ± 1.9 | 0.04 ± 0.0 | 4.83 ± 0.8 | 16.36 ± 2.2 | 57.23 ± 4.1 | 21.55 ± 2.8 | 37.95 ± 3.4 A | 26.41 ± 3.7 | 24.15 ± 4.1 | 16.81 ± 2.9 |
| 400 | 7.86 ± 1.9 | 0.94 ± 0.3 | 9.96 ± 2.1 | 5.53 ± 1.1 | 0.02 ± 0.0 | 3.00 ± 0.9 | 20.83 ± 4.0 | 51.83 ± 5.1 | 24.29 ± 2.9 | 45.15 ± 5.1 B | 27.32 ± 3.4 | 22.96 ± 3.7 | 13.49 ± 2.1 |
| p-value | 0.186 | 0.079 | 0.587 | 0.820 | 0.703 | 0.165 | 0.151 | 0.160 | 0.267 | 0.049 | 0.760 | 0.713 | 0.118 |
| p-value Ext. * Conc. | 0.337 | 0.692 | 0.946 | 0.819 | 0.374 | 0.876 | 0.783 | 0.918 | 0.750 | 0.724 | 0.984 | 0.833 | 0.277 |
| All Distal Droplets | p-Values | All Bent Tails | p-Values | |||
|---|---|---|---|---|---|---|
| Extender | Pre-Freeze | Post-Thaw | Pre-Freeze | Post-Thaw | ||
| AND | 38.51 ± 4.8 a | 28.17 ± 2.9 b | 0.002 | 5.52 ± 1.3 a | 9.74 ± 1.4 b | 0.003 |
| BIO | 32.92 ± 5.5 a | 21.72 ± 2.8 b | 0.009 | 11.24 ± 2.7 a | 18.33 ± 2.4 b | 0.003 |
| OVI | 26.62 ± 3.6 a | 20.33 ± 2.5 b | 0.032 | 11.31 ± 2.4 a | 17.39 ± 1.7 b | 0.002 |
| Overall | 32.69 ± 2.7 a | 23.41 ± 1.6 b | 0.0001 | 9.29 ± 1.3 a | 15.15 ± 1.2 b | 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mujitaba, M.A.; Kútvölgyi, G.; Radnai Szentpáli, J.; Debnár, V.J.; Tokár, A.; Vass, N.; Bodó, S. The Influence of Three Commercial Soy Lecithin-Based Semen Extenders and Two Spermatozoa Concentrations on the Quality of Pre-Freeze and Post-Thaw Ram Epididymal Spermatozoa. Animals 2024, 14, 1237. https://doi.org/10.3390/ani14081237
Mujitaba MA, Kútvölgyi G, Radnai Szentpáli J, Debnár VJ, Tokár A, Vass N, Bodó S. The Influence of Three Commercial Soy Lecithin-Based Semen Extenders and Two Spermatozoa Concentrations on the Quality of Pre-Freeze and Post-Thaw Ram Epididymal Spermatozoa. Animals. 2024; 14(8):1237. https://doi.org/10.3390/ani14081237
Chicago/Turabian StyleMujitaba, Malam Abulbashar, Gabriella Kútvölgyi, Judit Radnai Szentpáli, Viktória Johanna Debnár, Alexandra Tokár, Nóra Vass, and Szilárd Bodó. 2024. "The Influence of Three Commercial Soy Lecithin-Based Semen Extenders and Two Spermatozoa Concentrations on the Quality of Pre-Freeze and Post-Thaw Ram Epididymal Spermatozoa" Animals 14, no. 8: 1237. https://doi.org/10.3390/ani14081237
APA StyleMujitaba, M. A., Kútvölgyi, G., Radnai Szentpáli, J., Debnár, V. J., Tokár, A., Vass, N., & Bodó, S. (2024). The Influence of Three Commercial Soy Lecithin-Based Semen Extenders and Two Spermatozoa Concentrations on the Quality of Pre-Freeze and Post-Thaw Ram Epididymal Spermatozoa. Animals, 14(8), 1237. https://doi.org/10.3390/ani14081237






