Seasonal Hair Glucocorticoid Fluctuations in Wild Mice (Phyllotis darwini) within a Semi-Arid Landscape in North-Central Chile
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Study Design
2.3. Morphometric Data and Sample Collection
2.4. Determination of Intrinsic Factors
2.5. Biochemical Validation of Enzyme Immunoassay (EIA)
2.6. Measurement of Hair Glucocorticoid Concentration
2.7. Statistical Analyses
3. Results
4. Discussion
4.1. No Difference in Hair Corticosterone Levels between Anthropized Areas and Areas Protected from Human Disturbance
4.2. Sex-Based Differences in Hair Corticosterone
4.3. Seasonal Variation in Hair Corticosterone
4.4. No Association between Hair Corticosterone and Body Condition Nor Ectoparasite Load
4.5. Potential Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fuller, A.; Mitchell, D.; Maloney, S.K.; Hetem, R.S.; Fonsca, V.F.C.; Meyer, L.C.R.; Ven, T.M.F.N.V.; Snelling, E.P. How Dryland Mammals Will Respond to Climate Change: The Effects of Body Size, Heat Load and a Lack of Food and Water. J. Exp. Biol. 2021, 224, jeb238113. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.; Correa-Cuadros, J.P.; Henríquez, S.; Jaksic, F.M. Variable Interspecific Competition under Megadrought Conditions: Rodent Population Dynamics in Semiarid Chile. Oikos 2023, 2023, e09848. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, H.; Dai, Y. Stronger Warming Amplification over Drier Ecoregions Observed since 1979. Environ. Res. Lett. 2015, 10, 064012. [Google Scholar] [CrossRef]
- Word, K.R.; Austin, S.H.; Wingfield, J.C. Allostasis Revisited: A Perception, Variation, and Risk Framework. Front. Ecol. Evol. 2022, 10, 954708. [Google Scholar] [CrossRef]
- Romero, L.M.; Wingfield, J.C. Tempests, Poxes, Predators, and People: Stress in Wild Animals and How They Cope; Oxford University Press: New York, NY, USA, 2016. [Google Scholar]
- Landys, M.M.; Ramenofsky, M.; Wingfield, J.C. Actions of Glucocorticoids at a Seasonal Baseline as Compared to Stress-Related Levels in the Regulation of Periodic Life Processes. Gen. Comp. Endocrinol. 2006, 148, 132–149. [Google Scholar] [CrossRef] [PubMed]
- Moberg, G.P. Biological Responses to Stress: Implications for Animal Welfare. In The Biology of Animal Stress: Basic Principles and Implications for Animal Welfare; Moberg, G.P., Mench, J., Eds.; CAB international: New York, NY, USA, 2000; pp. 1–21. [Google Scholar]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How Do Glucocorticoids Influence Stress Responses? Integrating Permissive, Suppressive, Stimulatory, and Preparative Actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.R.; Gobush, K.S.; Vynne, C.H. Review of Factors Influencing Stress Hormones in Fish and Wildlife. J. Nat. Conserv. 2013, 21, 309–318. [Google Scholar] [CrossRef]
- Dantzer, B.; Fletcher, Q.E.; Boonstra, R.; Sheriff, M.J. Measures of Physiological Stress: A Transparent or Opaque Window into the Status, Management and Conservation of Species? Conserv. Physiol. 2014, 2, cou023. [Google Scholar] [CrossRef]
- Azevedo, A.; Bailey, L.; Bandeira, V.; Dehnhard, M.; Fonseca, C.; De Sousa, L.; Jewgenow, K. Age, Sex and Storage Time Influence Hair Cortisol Levels in a Wild Mammal Population. PLoS ONE 2019, 14, e0221124. [Google Scholar] [CrossRef]
- Cavigelli, S.A.; Caruso, M.J. Sex, Social Status and Physiological Stress in Primates: The Importance of Social and Glucocorticoid Dynamics. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140103. [Google Scholar] [CrossRef]
- Zenth, F.; Corlatti, L.; Giacomelli, S.; Saleri, R.; Cavalli, V.; Andrani, M.; Donini, V. Hair Cortisol Concentration as a Marker of Long-Term Stress: Sex and Body Temperature Are Major Determinants in Wild-Living Alpine Marmots. Mamm. Biol. 2022, 102, 2083–2089. [Google Scholar] [CrossRef]
- Beery, A.K.; Kaufer, D. Stress, Social Behavior, and Resilience: Insights from Rodents. Neurobiol. Stress 2015, 1, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Heck, A.L.; Handa, R.J. Sex Differences in the Hypothalamic–Pituitary–Adrenal Axis’ Response to Stress: An Important Role for Gonadal Hormones. Neuropsychopharmacology 2019, 44, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Bauer, C.M.; Hayes, L.D.; Ebensperger, L.A.; Romero, L.M. Seasonal Variation in the Degu (Octodon degus) Endocrine Stress Response. Gen. Comp. Endocrinol. 2014, 197, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Guinet, C.; Servera, N.; Mangin, S.; Georges, J.Y.; Lacroix, A. Change in Plasma Cortisol and Metabolites during the Attendance Period Ashore in Fasting Lactating Subantarctic Fur Seals. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2004, 137, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Lalande, L.D.; Gilot-Fromont, E.; Carbillet, J.; Débias, F.; Duhayer, J.; Gaillard, J.M.; Lemaître, J.F.; Palme, R.; Pardonnet, S.; Pellerin, M.; et al. Glucocorticoids Negatively Relate to Body Mass on the Short-Term in a Free-Ranging Ungulate. Oikos 2023, 2023, e09769. [Google Scholar] [CrossRef]
- Pokharel, S.S.; Seshagiri, P.B.; Sukumar, R. Assessment of Season-Dependent Body Condition Scores in Relation to Faecal Glucocorticoid Metabolites in Free-Ranging Asian Elephants. Conserv. Physiol. 2017, 5, cox039. [Google Scholar] [CrossRef] [PubMed]
- Gladbach, A.; Gladbach, D.J.; Koch, M.; Kuchar, A.; Möstl, E.; Quillfeldt, P. Can Faecal Glucocorticoid Metabolites Be Used to Monitor Body Condition in Wild Upland Geese Chloephaga picta leucoptera? Behav. Ecol. Sociobiol. 2011, 65, 1491–1498. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, B.C.; Verhulst, S. Ecological Immunology: Costly Parasite Defences and Trade-Offs in Evolutionary Ecology. TREE 1996, 11, 317–321. [Google Scholar] [CrossRef]
- Booth, D.T.; Clayton, D.H.; Block, B.A. Experimental Demonstration of the Energetic Cost of Parasitism in Free-Ranging Hosts. Proc. R. Soc. B Biol. Sci. 1993, 253, 125–129. [Google Scholar] [CrossRef]
- Giorgi, M.S.; Arlettaz, R.; Christe, P.; Vogel, P. The Energetic Grooming Costs Imposed by a Parasitic Mite (Spinturnix myoti) upon Its Bat Host (Myotis myotis). Proc. R. Soc. B Biol. Sci. 2001, 268, 2071–2075. [Google Scholar] [CrossRef]
- Scantlebury, M.; Waterman, J.M.; Hillegass, M.; Speakman, J.R.; Bennett, N.C. Energetic Costs of Parasitism in the Cape Ground Squirrel Xerus inauris. Proc. R. Soc. B Biol. Sci. 2007, 274, 2169–2177. [Google Scholar] [CrossRef]
- Raveh, A.; Kotler, B.P.; Abramsky, Z.; Krasnov, B.R. Driven to Distraction: Detecting the Hidden Costs of Flea Parasitism through Foraging Behaviour in Gerbils. Ecol. Lett. 2011, 14, 47–51. [Google Scholar] [CrossRef]
- St. Juliana, J.R.; Khokhlova, I.S.; Wielebnowski, N.; Kotler, B.P.; Krasnov, B.R. Ectoparasitism and Stress Hormones: Strategy of Host Exploitation, Common Host-Parasite History and Energetics Matter. J. Anim. Ecol. 2014, 83, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Defolie, C.; Merkling, T.; Fichtel, C. Patterns and Variation in the Mammal Parasite–Glucocorticoid Relationship. Biol. Rev. 2020, 95, 74–93. [Google Scholar] [CrossRef] [PubMed]
- O’Dwyer, K.; Dargent, F.; Forbes, M.R.; Koprivnikar, J. Parasite Infection Leads to Widespread Glucocorticoid Hormone Increases in Vertebrate Hosts: A Meta-Analysis. J. Anim. Ecol. 2020, 89, 519–529. [Google Scholar] [CrossRef]
- Huang, J.; Yu, H.; Guan, X.; Wang, G.; Guo, R. Accelerated Dryland Expansion under Climate Change. Nat. Clim. Chang. 2016, 6, 166–171. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Wang, Y.; Yao, Y.; Wang, C. Land Cover Change in Global Drylands: A Review. Sci. Total Environ. 2023, 863, 160943. [Google Scholar] [CrossRef] [PubMed]
- Neely, C.L.; Bunning, S.; Wilkes, A. Review of Evidence on Drylands Pastoral Systems and Climate Change Implications and Opportunities for Mitigation and Adaptation; FAO: Rome, Italy, 2009. [Google Scholar]
- Boyle, S.A.; de la Sancha, N.U.; Pérez, P.; Kabelik, D. Small Mammal Glucocorticoid Concentrations Vary with Forest Fragment Size, Trap Type, and Mammal Taxa in the Interior Atlantic Forest. Sci. Rep. 2021, 11, 2111. [Google Scholar] [CrossRef]
- Martin, L.B. Stress and Immunity in Wild Vertebrates: Timing Is Everything. Gen. Comp. Endocrinol. 2009, 163, 70–76. [Google Scholar] [CrossRef]
- Davis, A.K.; Maney, D.L.; Maerz, J.C. The Use of Leukocyte Profiles to Measure Stress in Vertebrates: A Review for Ecologists. Funct. Ecol. 2008, 22, 760–772. [Google Scholar] [CrossRef]
- Rakotoniaina, J.H.; Kappeler, P.M.; Kaesler, E.; Hämäläinen, A.M.; Kirschbaum, C.; Kraus, C. Hair Cortisol Concentrations Correlate Negatively with Survival in a Wild Primate Population. BMC Ecol. 2017, 17, 1–13. [Google Scholar] [CrossRef]
- Wilkening, J.L.; Ray, C. Characterizing Predictors of Survival in the American Pika (Ochotona princeps). J. Mammal. 2016, 97, 1366–1375. [Google Scholar] [CrossRef]
- Gormally, B.M.G.; Romero, L.M. What Are You Actually Measuring? A Review of Techniques That Integrate the Stress Response on Distinct Time-Scales. Funct. Ecol. 2020, 34, 2030–2044. [Google Scholar] [CrossRef]
- Robertson, K.E.; Ellington, E.H.; Tonra, C.M.; Gehrt, S.D. Stress in the City? Coyote Hair Cortisol Varies with Intrinsic and Extrinsic Factors within a Heavily Urbanized Landscape. Sci. Total Environ. 2023, 901, 165965. [Google Scholar] [CrossRef] [PubMed]
- Carlitz, E.H.D.; Miller, R.; Kirschbaum, C.; Gao, W.; Hänni, D.C.; Van Schaik, C.P. Measuring Hair Cortisol Concentrations to Assess the Effect of Anthropogenic Impacts on Wild Chimpanzees (Pan troglodytes). PLoS ONE 2016, 11, e0151870. [Google Scholar] [CrossRef]
- Beltrami, E.; Verdugo, C.; Beldomenico, P.; González-Acuña, D.; Moreno, L.; Acosta-Jamett, G. Flea and Tick Infestation of a Wild Rodent in an Anthropogenic Landscape of the Semi-Arid Chile. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Montecinos, S.; Gutiérrez, J.R.; López-Cortés, F.; López, D. Climatic Characteristics of the Semi-Arid Coquimbo Region in Chile. J. Arid. Environ. 2016, 126, 7–11. [Google Scholar] [CrossRef]
- López-Cortés, F.; Lopez, D. Antecedentes Bioclimáticos Del Parque Nacional Bosque Fray Jorge. In Historia Natural del Parque Nacional Bosque Fray Jorge; Squeo, F.A., Gutiérrez, J.R., Hernández, I.R., Eds.; Ediciones Universidad la Serena: La Serena, Chile, 2004; Volume 2, pp. 45–60. [Google Scholar]
- Squeo, F.A.; Arancio, G.; Cavieres, L.A. Sitios Prioritarios Para La Conservación de La Flora Nativa Con Riesgos de Extinción En La IV Región de Coquimbo, Chile. In Libro Rojo de la Flora Nativa y de los Sitios Prioritarios para su Conservación: Región de Coquimbo; Squeo, F.A., Arancio, G., Gutiérrez, J.R., Eds.; Ediciones Universidad de la Serena: La Serena, Chile, 2001; Volume 11, pp. 171–193. [Google Scholar]
- Squeo, F.A.; Gutiérrez, J.R.; Hernández, I.R.; Historia Natural Del Parque Nacional Bosque Fray Jorge Ediciones Universidad de La Serena; Squeo, F.A. , Gutiérrez, J.R., Hernández, I.R., Eds.; Ediciones Universidad de La Serena: La Serena, Chile, 2004; ISBN 9567393214. [Google Scholar]
- Squeo, A.F.; Loayza, P.A.; Lopez, R.P.; Gutierrez, J.R. Vegetation of Bosque Fray Jorge National Park and Its Surrounding Matrix in the Coastal Desert of North-Central Chile. J. Arid Environ. 2016, 126, 12–22. [Google Scholar] [CrossRef]
- Kreeger, T.; Arnemo, J. Handbook of Wildlife Chemical Immobilization, 5th ed.; Center for Wildlife Studies Press: North Yarmouth, ME, USA, 2018. [Google Scholar]
- Acker, M.; Mastromonaco, G.; Schulte-Hostedde, A.I. The Effects of Body Region, Season and External Arsenic Application on Hair Cortisol Concentration. Conserv. Physiol. 2018, 6, coy037. [Google Scholar] [CrossRef]
- Heimbürge, S.; Kanitz, E.; Otten, W. The Use of Hair Cortisol for the Assessment of Stress in Animals. Gen. Comp. Endocrinol. 2019, 270, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.; Julliard, R.; Stenseth, N.C.; Jaksic, F.M. Demographic Dynamics of a Neotropical Small Rodent (Phyllotis darwini): Feedback Structure, Predation and Climatic Factors. J. Anim. Ecol. 2001, 70, 761–775. [Google Scholar] [CrossRef]
- Rosalino, L.M.; Martin, P.S.; Gheler-Costa, C.; Lopes, P.C.; Verdade, L.M. Allometric Relations of Neotropical Small Rodents (Sigmodontinae) in Anthropogenic Environments. Zool. Sci. 2013, 30, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Keay, J.M.; Singh, J.; Gaunt, M.C.; Kaur, T. Fecal Glucocorticoids and Their Metabolites as Indicators of Stress in Various Mammalian Species: A Literature Review. J. Zoo Wildl. Med. 2006, 37, 234–244. [Google Scholar] [CrossRef]
- Mastromonaco, G.F.; Gunn, K.; McCurdy-Adams, H.; Edwards, D.B.; Schulte-Hostedde, A.I. Validation and Use of Hair Cortisol as a Measure of Chronic Stress in Eastern Chipmunks (Tamias striatus). Conserv. Physiol. 2014, 2, cou055. [Google Scholar] [CrossRef] [PubMed]
- Stewart, N.D.; Mastromonaco, G.F.; Burness, G. No Island-Effect on Glucocorticoid Levels for a Rodent from a near-Shore Archipelago. PeerJ 2020, 8, e8590. [Google Scholar] [CrossRef] [PubMed]
- Kaisin, O.; Fuzessy, L.; Poncin, P.; Brotcorne, F.; Culot, L. A Meta-Analysis of Anthropogenic Impacts on Physiological Stress in Wild Primates. Conserv. Biol. 2021, 35, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Partecke, J.; Schwabl, I.; Gwinner, E. Stress and the City: Urbanization and Its Effects on the Stress Physiology in European Blackbirds. Ecology 2006, 87, 1945–1952. [Google Scholar] [CrossRef] [PubMed]
- Rich, E.L.; Romero, L.M. Exposure to Chronic Stress Downregulates Corticosterone Responses to Acute Stressors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, 1628–1636. [Google Scholar] [CrossRef]
- Blumstein, D.T. Habituation and Sensitization: New Thoughts about Old Ideas. Anim. Behav. 2016, 120, 255–262. [Google Scholar] [CrossRef]
- Schradin, C. Seasonal Changes in Testosterone and Corticosterone Levels in Four Social Classes of a Desert Dwelling Sociable Rodent. Horm. Behav. 2008, 53, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, M.D.; Sjodin, B.; Ray, C.; Erb, L.; Wilkening, J.; Russello, M.A. Individual-Based Analysis of Hair Corticosterone Reveals Factors Influencing Chronic Stress in the American Pika. Ecol. Evol. 2017, 7, 4099–4108. [Google Scholar] [CrossRef] [PubMed]
- Kenagy, G.J.; Place, N.J.; Veloso, C. Relation of Glucocorticosteroids and Testosterone to the Annual Cycle of Free-Living Degus in Semiarid Central Chile. Gen. Comp. Endocrinol. 1999, 115, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.R. The Physiological Costs of Reproduction in Small Mammals. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 375–398. [Google Scholar] [CrossRef] [PubMed]
- Meserve, P.L.; Le Boulengé, É. Population Dynamics and Ecology of Small Mammals in the Northern Chilean Semiarid Region. Fieldiana Zool. 1987, 39, 413–431. [Google Scholar]
- Levine, J.E. Editorial: Stressing the Importance of Sex. Endocrinology 2002, 143, 4502–4504. [Google Scholar] [CrossRef]
- Russell, E.; Koren, G.; Rieder, M.; Van Uum, S. Hair Cortisol as a Biological Marker of Chronic Stress: Current Status, Future Directions and Unanswered Questions. Psychoneuroendocrinology 2012, 37, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Heimbürge, S.; Kanitz, E.; Tuchscherer, A.; Otten, W. Is It Getting in the Hair?—Cortisol Concentrations in Native, Regrown and Segmented Hairs of Cattle and Pigs after Repeated ACTH Administrations. Gen. Comp. Endocrinol. 2020, 295, 113534. [Google Scholar] [CrossRef]
- Colding-Jørgensen, P.; Hestehave, S.; Abelson, K.S.P.; Kalliokoski, O. Hair Glucocorticoids Are Not a Historical Marker of Stress—Exploring the Time-Scale of Corticosterone Incorporation into Hairs in a Rat Model. Gen. Comp. Endocrinol. 2023, 341, 114335. [Google Scholar] [CrossRef]
- Romero, L.M. Seasonal Changes in Plasma Glucocorticoid Concentrations in Free-Living Vertebrates. Gen. Comp. Endocrinol. 2002, 128, 1–24. [Google Scholar] [CrossRef]
- Gesquiere, L.R.; Khan, M.; Shek, L.; Wango, T.L.; Wango, E.O.; Alberts, S.C.; Altmann, J. Coping with a Challenging Environment: Effects of Seasonal Variability and Reproductive Status on Glucocorticoid Concentrations of Female Baboons (Papio cynocephalus). Horm. Behav. 2008, 54, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Garber, P.A.; McKenney, A.; Bartling-John, E.; Bicca-Marques, J.C.; de la Fuente, M.F.; Abreu, F.; Schiel, N.; Souto, A.; Phillips, K.A. Life in a Harsh Environment: The Effects of Age, Sex, Reproductive Condition, and Season on Hair Cortisol Concentration in a Wild Non-Human Primate. PeerJ 2020, 8, e9365. [Google Scholar] [CrossRef] [PubMed]
- INIA Agrometeorología. Available online: https://www.agrometeorologia.cl (accessed on 20 February 2024).
- Lima, M.; Previtali, M.A.; Meserve, P.L. Climate and Small Rodent Dynamics in Semi-Arid Chile: The Role of Lateral and Vertical Perturbations and Intra-Specific Processes. Clim. Res. 2006, 30, 125–132. [Google Scholar] [CrossRef]
- Lima, M.; Jaksic, F.M. Population Rate of Change in the Leaf-Eared Mouse: The Role of Density-Dependence, Seasonality and Rainfall. Austral. Ecol. 1999, 24, 110–116. [Google Scholar] [CrossRef]
- Lima, M.; Bozinovic, F.; Jaksic Fabian, M. Body Mass Dynamics and Growth Patterns of Leaf-Eared Mice Phyllotis darwini in a Semi-Arid Region of the Neotropics. Acta Theriol. 1997, 42, 15–24. [Google Scholar] [CrossRef]
- Sheriff, M.J.; Dantzer, B.; Delehanty, B.; Palme, R.; Boonstra, R. Measuring Stress in Wildlife: Techniques for Quantifying Glucocorticoids. Oecologia 2011, 166, 869–887. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.; Fandos Esteruelas, N.; Nakamura, M.; Rio-Maior, H.; Krofel, M.; Di Blasio, A.; Zoppi, S.; Robetto, S.; Llaneza, L.; García, E.; et al. Hair Cortisol Concentration Reflects the Life Cycle and Management of Grey Wolves across Four European Populations. Sci. Rep. 2022, 12, 5697. [Google Scholar] [CrossRef] [PubMed]
- Matzke, C.C.; Kusch, J.M.; Janz, D.M.; Lane, J.E. Perceived Predation Risk Predicts Glucocorticoid Hormones, but Not Reproductive Success in a Colonial Rodent. Horm. Behav. 2022, 143, 105200. [Google Scholar] [CrossRef] [PubMed]
- Kam, M.; Degen, A.A.; Khokhlova, I.S.; Krasnov, B.R.; Geffen, E. Do Fleas Affect Energy Expenditure of Their Free-Living Hosts? PLoS ONE 2010, 5, e13686. [Google Scholar] [CrossRef]
- Graham, S.P.; Freidenfelds, N.A.; McCormick, G.L.; Langkilde, T. The Impacts of Invaders: Basal and Acute Stress Glucocorticoid Profiles and Immune Function in Native Lizards Threatened by Invasive Ants. Gen. Comp. Endocrinol. 2012, 176, 400–408. [Google Scholar] [CrossRef]
- Shimamoto, T. Validation and Utility of Hair Cortisol Analysis as a Measure of Long-Term Physiological Stress in the Pallas’s Squirrel Callosciurus erythraeus. Gen. Comp. Endocrinol. 2022, 316, 113944. [Google Scholar] [CrossRef] [PubMed]
- Crill, C.; Janz, D.M.; Kusch, J.M.; Santymire, R.M.; Heyer, G.P.; Shury, T.K.; Lane, J.E. Investigation of the Utility of Feces and Hair as Non-Invasive Measures of Glucocorticoids in Wild Black-Tailed Prairie Dogs (Cynomys ludovicianus). Gen. Comp. Endocrinol. 2019, 275, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Ashley, N.T.; Barboza, P.S.; Macbeth, B.J.; Janz, D.M.; Cattet, M.R.L.; Booth, R.K.; Wasser, S.K. Glucocorticosteroid Concentrations in Feces and Hair of Captive Caribou and Reindeer Following Adrenocorticotropic Hormone Challenge. Gen. Comp. Endocrinol. 2011, 172, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Jewgenow, K.; Azevedo, A.; Albrecht, M.; Kirschbaum, C.; Dehnhard, M. Hair Cortisol Analyses in Different Mammal Species: Choosing the Wrong Assay May Lead to Erroneous Results. Conserv Physiol 2020, 8, coaa009. [Google Scholar] [CrossRef] [PubMed]
- Arbor Assays Corticosterone Enzyme Immunoassay Kit. Available online: https://www.arborassays.com/documentation/inserts/K014-H.pdf (accessed on 20 February 2024).


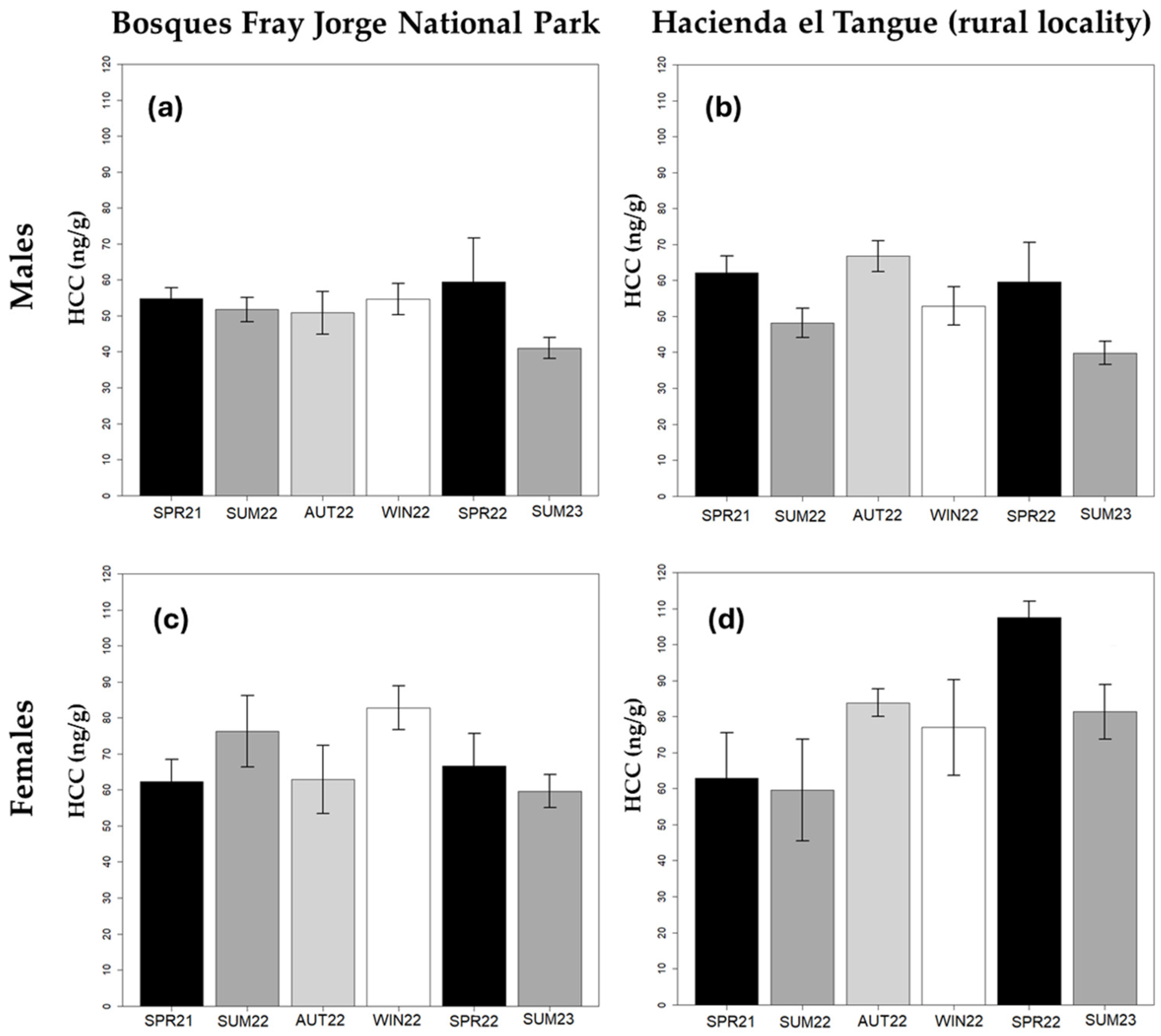
| Factor | BFJNP | El Tangue | ||||||
|---|---|---|---|---|---|---|---|---|
| N | HCC ng/g | SMI | Ectoparasitic Load | N | HCC ng/g | SMI | Ectoparasitic Load | |
| Sex | ||||||||
| Male | 81 | 51.6 (16.6) | 52.5 (9.0) | 2.9 (3.1) | 53 | 51.8 (16.5) | 53.5 (8.2) | 3.9 (4.5) |
| Female | 36 | 66.0 (20.0) | 45.9 (8.2) | 2.2 (2.5) | 29 | 75.0 (27.1) | 45.4 (6.7) | 1.9 (2.0) |
| Season | ||||||||
| Spring 2021 | 42 | 56.7 (17.8) | 49.4 (8.2) | 2.0 (1.5) | 14 | 62.5 (21.4) | 46.9 (8.2) | 4.1 (1.9) |
| Summer 2022 | 17 | 59.0 (18.8) | 47.8 (6.4) | 3.8 (3.3) | 23 | 51.2 (22.5) | 48.3 (6.0) | 2.1 (4.1) |
| Autumn 2022 | 19 | 55.3 (22.4) | 49.6 (9.0) | 2.7 (3.2) | 11 | 73.0 (13.0) | 47.7 (8.2) | 6.4 (5.2) |
| Winter 2022 | 10 | 63.1 (17.2) | 49.9 (9.4) | 4.9 (3.2) | 9 | 63.6 (22.2) | 52.6 (9.7) | 4.6 (5.1) |
| Spring 2022 | 8 | 62.2 (22.6) | 48.2 (8.4) | 1.2 (1.8) | 5 | 78.7 (29.8) | 54.2 (10.6) | 5.0 (1.9) |
| Summer 2023 | 21 | 47.2 (14.3) | 56.8 (11.5) | 3.3 (4.2) | 20 | 54.4 (25.1) | 55.4 (8.7) | 1.2 (1.6) |
| Variable | Estimate | Std. Error | t Value | Pr (>|t|) |
|---|---|---|---|---|
| Global Model | ||||
| (Intercept) | 62.417 | 3.462 | 18.031 | <0.001 * |
| Sex (Male) | −17.788 | 2.832 | −6.282 | <0.001 * |
| Season (Summer 2023) | ||||
| Spring 2021 | 8.154 | 3.840 | 2.123 | 0.035 * |
| Summer 2022 | 4.962 | 4.155 | 1.194 | 0.234 |
| Autumn 2022 | 10.644 | 4.487 | 2.372 | 0.019 * |
| Winter 2022 | 12.165 | 5.183 | 2.347 | 0.020 * |
| Spring 2022 | 17.072 | 5.945 | 2.872 | 0.005 * |
| Male Model | ||||
| (Intercept) | 40.432 | 2.989 | 13.528 | <0.001 * |
| Season (Summer 2023) | ||||
| Spring 2021 | 15.838 | 3.888 | 4.073 | <0.001 * |
| Summer 2022 | 9.231 | 4.153 | 2.223 | 0.028 * |
| Autumn 2022 | 16.286 | 4.650 | 3.502 | 0.001 * |
| Winter 2022 | 13.501 | 5.388 | 2.506 | 0.013 * |
| Spring 2022 | 19.031 | 6.251 | 3.044 | 0.003 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veloso-Frías, J.; Soto-Gamboa, M.; Mastromonaco, G.; Acosta-Jamett, G. Seasonal Hair Glucocorticoid Fluctuations in Wild Mice (Phyllotis darwini) within a Semi-Arid Landscape in North-Central Chile. Animals 2024, 14, 1260. https://doi.org/10.3390/ani14091260
Veloso-Frías J, Soto-Gamboa M, Mastromonaco G, Acosta-Jamett G. Seasonal Hair Glucocorticoid Fluctuations in Wild Mice (Phyllotis darwini) within a Semi-Arid Landscape in North-Central Chile. Animals. 2024; 14(9):1260. https://doi.org/10.3390/ani14091260
Chicago/Turabian StyleVeloso-Frías, Joseline, Mauricio Soto-Gamboa, Gabriela Mastromonaco, and Gerardo Acosta-Jamett. 2024. "Seasonal Hair Glucocorticoid Fluctuations in Wild Mice (Phyllotis darwini) within a Semi-Arid Landscape in North-Central Chile" Animals 14, no. 9: 1260. https://doi.org/10.3390/ani14091260
APA StyleVeloso-Frías, J., Soto-Gamboa, M., Mastromonaco, G., & Acosta-Jamett, G. (2024). Seasonal Hair Glucocorticoid Fluctuations in Wild Mice (Phyllotis darwini) within a Semi-Arid Landscape in North-Central Chile. Animals, 14(9), 1260. https://doi.org/10.3390/ani14091260






