Increasing the Understanding of Nutrient Transport Capacity of the Ovine Placentome
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. RNA Extraction
2.3. Gene Expression Profile
2.4. Data Processing
2.5. Statistical Analyses
3. Results
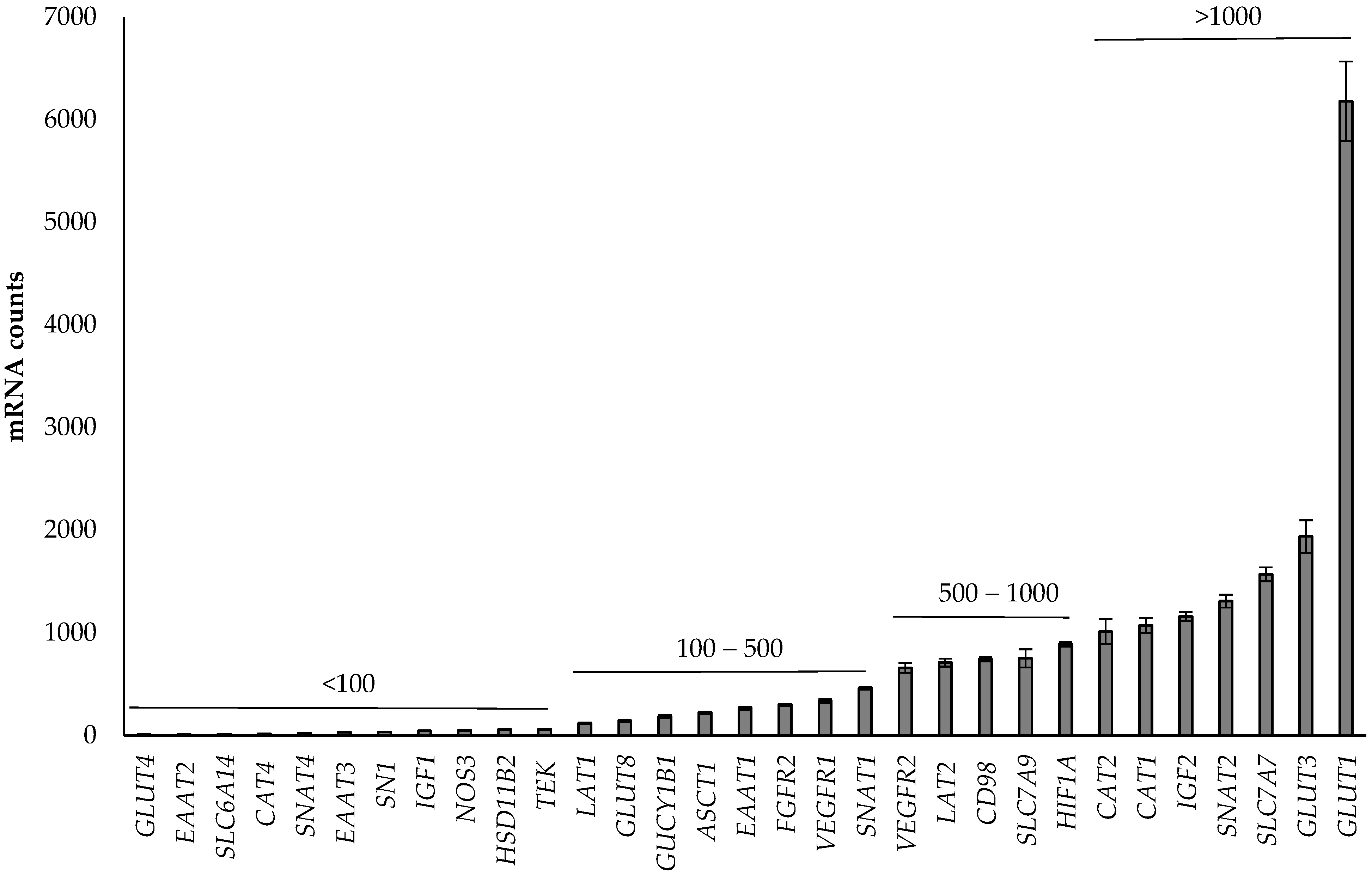
3.1. Abundance of Genes in the Placenta
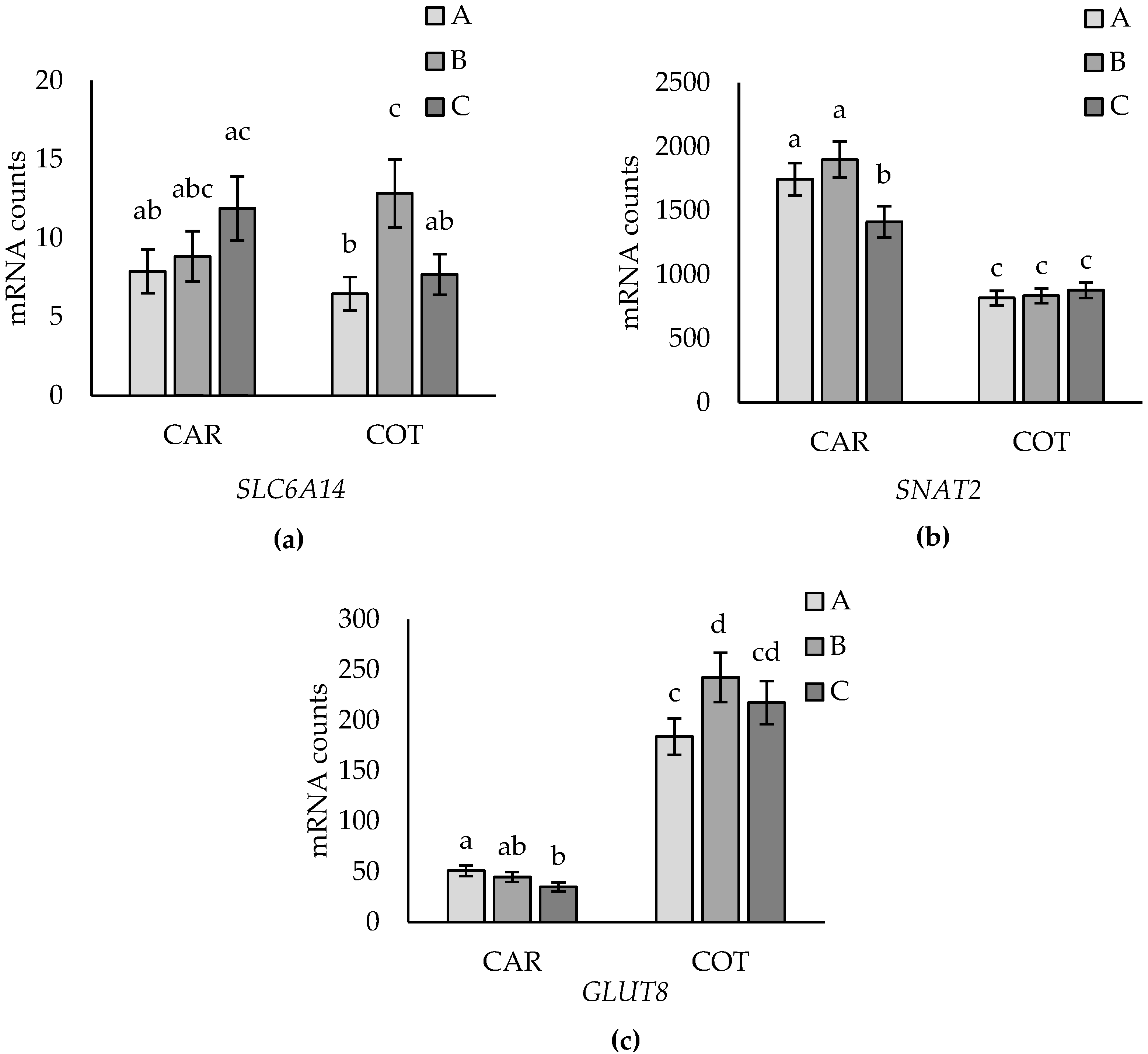
3.2. Tissue Effects
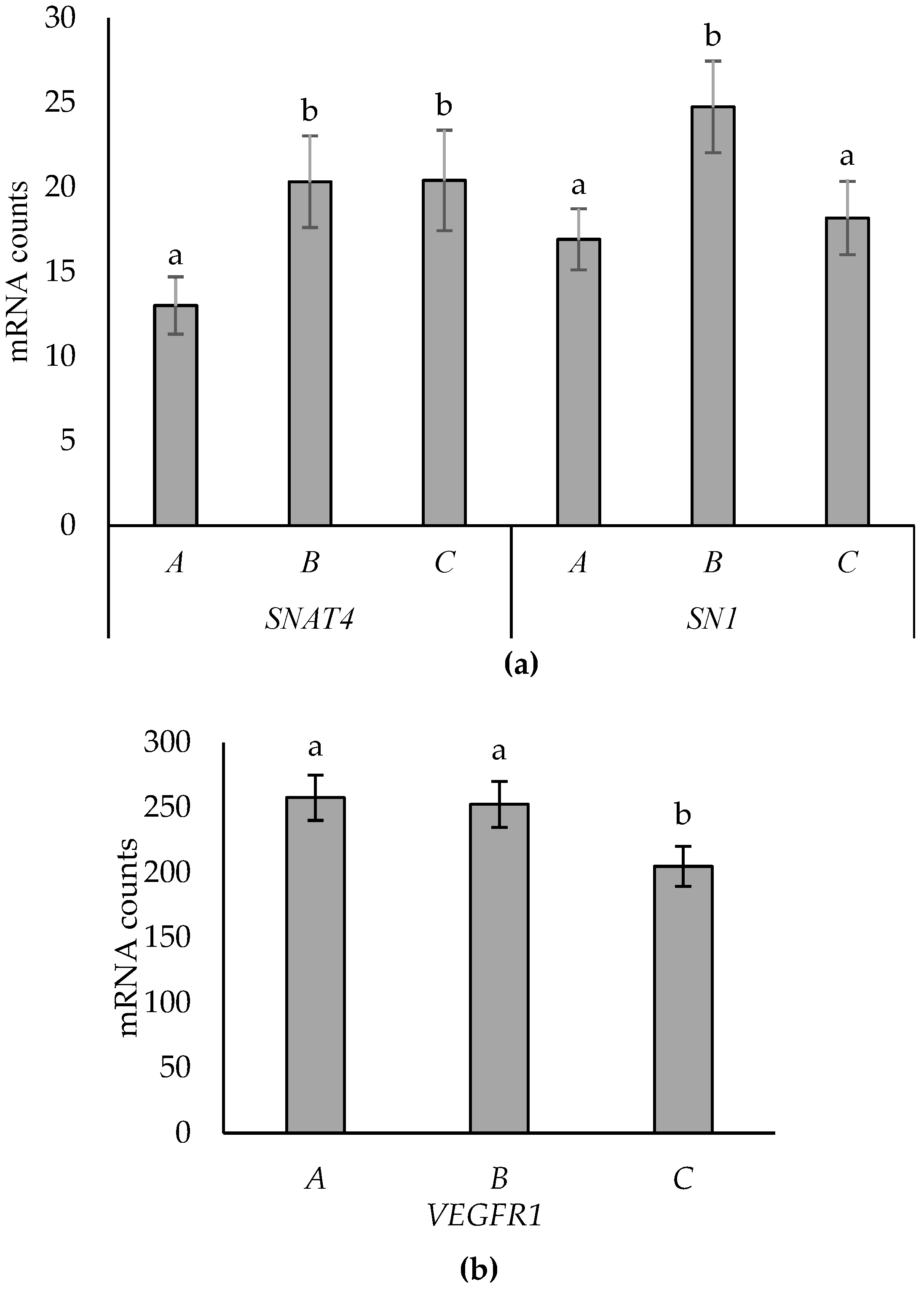
3.3. Effects of Placentome Subtype
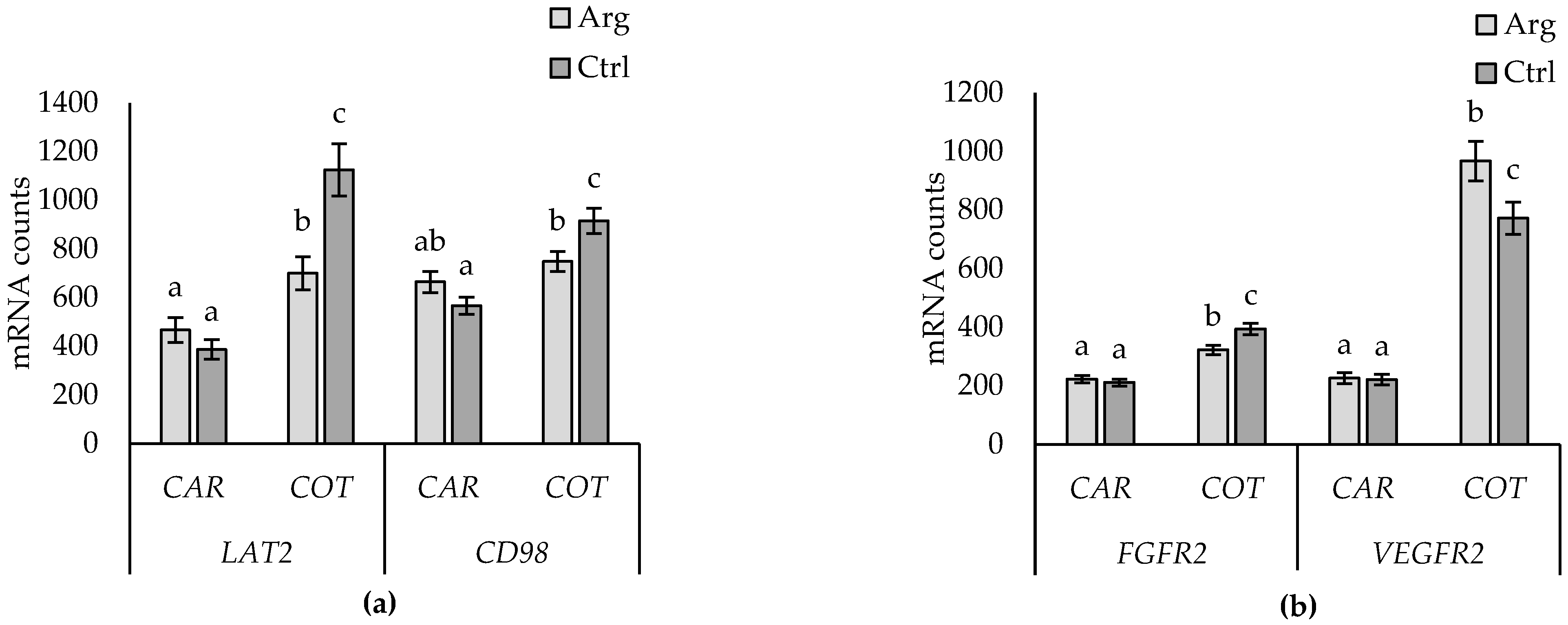
3.4. Supplementation Effects
3.5. Supplementation-by-Subtype Interactions
4. Discussion
4.1. Abundance of Genes in the Placenta
4.2. Tissue Differences
4.3. Placentome Morphology
4.4. Arginine Supplementation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bell, A.W.; Ehrhardt, R.A. Regulation of Placental Nutrient Transport and Implications for Fetal Growth. Nutr. Res. Rev. 2002, 15, 211. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, L.P.; Redmer, D.A. Angiogenesis in the Placenta. Biol. Reprod. 2001, 64, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Redmer, D.A.; Wallace, J.M.; Reynolds, L.P. Effect of Nutrient Intake during Pregnancy on Fetal and Placental Growth and Vascular Development. Domest. Anim. Endocrinol. 2004, 27, 199–217. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Bazer, F.W.; Wallace, J.M.; Spencer, T.E. Board-Invited Review: Intrauterine Growth Retardation: Implications for the Animal Sciences. J. Anim. Sci. 2006, 84, 2316–2337. [Google Scholar] [CrossRef] [PubMed]
- Björkman, N. An Atlas of Placental Fine Structure; Bailliere, Tindall & Cassell: London, UK, 1970. [Google Scholar]
- Bell, A.W.; Hay, W.W.J.; Ehrhardt, R.A. Placental Transport of Nutrients and Its Implications for Fetal Growth. J. Reprod. Fertil. Suppl. 1999, 54, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Fowden, A.L.; Ward, J.W.; Wooding, F.P.B.; Forhead, A.J.; Constancia, M. Programming Placental Nutrient Transport Capacity. J. Physiol. 2006, 572, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Regnault, T.R.H.; Barker, P.L.; Botting, K.J.; McMillen, I.C.; McMillan, C.M.; Roberts, C.T.; Morrison, J.L. Placental Adaptations in Growth Restriction. Nutrients 2015, 7, 360–389. [Google Scholar] [CrossRef]
- Vaughan, O.R.; Davies, K.L.; Ward, J.W.; de Blasio, M.J.; Fowden, A.L. A Physiological Increase in Maternal Cortisol Alters Uteroplacental Metabolism in the Pregnant Ewe. J. Physiol. 2016, 594, 6407–6418. [Google Scholar] [CrossRef]
- Wallace, J.M.; Milne, J.S.; Aitken, R.P. Maternal Growth Hormone Treatment from Day 35 to 80 of Gestation Alters Nutrient Partitioning in Favor of Uteroplacental Growth in the Overnourished Adolescent Sheep1. Biol. Reprod. 2004, 70, 1277–1285. [Google Scholar] [CrossRef]
- Vonnahme, K.A.; Hess, B.W.; Nijland, M.J.; Nathanielsz, P.W.; Ford, S.P. Placentomal Differentiation May Compensate for Maternal Nutrient Restriction in Ewes Adapted to Harsh Range Conditions. J. Anim. Sci. 2006, 84, 3451–3459. [Google Scholar] [CrossRef]
- van der Linden, D.S.; Sciascia, Q.; Sales, F.; McCoard, S.A. Placental Nutrient Transport Is Affected by Pregnancy Rank in Sheep. J. Anim. Sci. 2013, 91, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Vatnick, I.; Schoknecht, P.A.; Darrigrand, R.; Bell, A.W. Growth and Metabolism of the Placenta after Unilateral Fetectomy in Twin-Pregnant Ewes. J. Dev. Physiol. 1991, 15, 351–356. [Google Scholar] [PubMed]
- Penningaa, L.; Longo, L.D. Ovine Hypoxia Placentome Morphology: Effect of High Altitude. Placenta 1998, 19, 187–193. [Google Scholar] [CrossRef] [PubMed]
- McCoard, S.; Haack, N.; Heiser, A.; Maclean, P. Effect of Birth Rank, and Placentome Subtype on Expression of Genes Involved in Placental Nutrient Transport in Sheep. Theriogenology 2023, 203, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Amino Acids: Metabolism, Functions, and Nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Bazer, F.W.; Satterfield, M.C.; Gilbreath, K.R.; Posey, E.A.; Sun, Y. L-Arginine Nutrition and Metabolism in Ruminants. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2022; Volume 1354, pp. 177–206. [Google Scholar]
- van der Linden, D.S.; Sciascia, Q.; Sales, F.; Wards, N.J.; Oliver, M.H.; McCoard, S.A. Intravenous Maternal L-Arginine Administration to Twin-Bearing Ewes during Late Pregnancy Enhances Placental Growth and Development. J. Anim. Sci. 2015, 93, 4917–4925. [Google Scholar] [CrossRef]
- McCoard, S.; Wards, N.; Koolaard, J.; Salerno, M.S. The Effect of Maternal Arginine Supplementation on the Development of the Thermogenic Program in the Ovine Fetus. Anim. Prod. Sci. 2014, 54, 1843–1847. [Google Scholar] [CrossRef]
- McCoard, S.A.; Sales, F.; Wards, N.; Sciascia, Q.; Oliver, M.; Koolaard, J.; van der Linden, D. Parenteral Administration of Twin-Bearing Ewes with L-Arginine Enhances the Birth Weight and Brown Fat Stores in Sheep. Springerplus 2013, 2, 684. [Google Scholar] [CrossRef]
- Geiss, G.K.; Bumgarner, R.E.; Birditt, B.; Dahl, T.; Dowidar, N.; Dunaway, D.L.; Fell, H.P.; Ferree, S.; George, R.D.; Grogan, T.; et al. Direct Multiplexed Measurement of Gene Expression with Color-Coded Probe Pairs. Nat. Biotechnol. 2008, 26, 317–325. [Google Scholar] [CrossRef]
- Heiser, A.; Leblanc, S.J.; McDougall, S. Pegbovigrastim Treatment Affects Gene Expression in Neutrophils of Pasture-Fed, Periparturient Cows. J. Dairy Sci. 2018, 101, 8194–8207. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. LmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Luo, D.; Ganesh, S.; Koolard, J. Package ‘Predictmeans’ Comprehensive R Archive Network, Version 1.1.0. 2018. Available online: https://CRAN.R-project.org/package=predictmeans (accessed on 22 June 2020).
- Bell, A.W.; Kennaugh, J.M.; Battaglia, F.C.; Makowski, E.L.; Meschia, G. Metabolic and Circulatory Studies of Fetal Lamb at Midgestation. Am. J. Physiol. 1986, 250, E538–E544. [Google Scholar] [CrossRef] [PubMed]
- Molina, R.D.; Meschia, G.; Battaglia, F.C.; Hay, W.W. Gestational Maturation of Placental Glucose Transfer Capacity in Sheep. Am. J. Physiol. 1991, 261, R697–R704. [Google Scholar] [CrossRef] [PubMed]
- Currie, M.J.; Bassett, N.S.; Gluckman, P.D. Ovine Glucose Transporter-1 and -3: CDNA Partial Sequences and Developmental Gene Expression in the Placenta. Placenta 1997, 18, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Das, U.G.; Sadiq, H.F.; Soares, M.J.; Hay, W.W.; Devaskar, S.U. Time-Dependent Physiological Regulation of Rodent and Ovine Placental Glucose Transporter (GLUT-1) Protein. Am. J. Physiol. Integr. Comp. Physiol. 1998, 274, R339–R347. [Google Scholar] [CrossRef] [PubMed]
- Anthony, R.V.; Limesand, S.W.; Fanning, M.D.; Liang, R. Placental Lactogen and Growth Hormone—Regulation and Action. In Endocrinology of Pregnancy; Humana Press: Totowa, NJ, USA, 1998; pp. 461–462. [Google Scholar]
- Wali, J.A.; de Boo, H.A.; Derraik, J.G.B.; Phua, H.H.; Oliver, M.H.; Bloomfield, F.H.; Harding, J.E. Weekly Intra-Amniotic Igf-1 Treatment Increases Growth of Growth-Restricted Ovine Fetuses and up-Regulates Placental Amino Acid Transporters. PLoS ONE 2012, 7, e37899. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, K.A.; Brown, J.D.; Keith, A.B.; Satterfield, M.C. Factors Controlling Nutrient Availability to the Developing Fetus in Ruminants. J. Anim. Sci. Biotechnol. 2015, 6, 16. [Google Scholar] [CrossRef]
- Satterfield, M.C.; Edwards, A.K.; Bazer, F.W.; Dunlap, K.A.; Steinhauser, C.B.; Wu, G. Placental Adaptation to Maternal Malnutrition. Reproduction 2021, 162, R73–R83. [Google Scholar] [CrossRef] [PubMed]
- Fowden, A.L.; Sferruzzi-Perri, A.N.; Coan, P.M.; Constancia, M.; Burton, G.J. Placental Efficiency and Adaptation: Endocrine Regulation. J. Physiol. 2009, 587, 3459–3472. [Google Scholar] [CrossRef]
- McCoard, S.A.; Sales, F.A.; Sciascia, Q.L. Amino Acids in Sheep Production. Front. Biosci. Elit. 2016, 8, 264–288. [Google Scholar] [CrossRef]
- Vonnahme, K.A.; Evoniuk, J.; Johnson, M.L.; Borowicz, P.P.; Luther, J.S.; Pant, D.; Redmer, D.A.; Reynolds, L.P.; Grazul-Bilska, A.T. Placental Vascularity and Growth Factor Expression in Singleton, Twin, and Triplet Pregnancies in the Sheep. Endocrine 2008, 33, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Regnault, T.R.H.; De Vrijer, B.; Battaglia, F.C. Transport and Metabolism of Amino Acids in Placenta. Endocrine 2002, 19, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Desforges, M.; Sibley, C.P. Placental Nutrient Supply and Fetal Growth. Int. J. Dev. Biol. 2009, 54, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wu, G.; Spencer, T.E.; Johnson, G.A.; Bazer, F.W. Select Nutrients in the Ovine Uterine Lumen. IV. Expression of Neutral and Acidic Amino Acid Transporters in Ovine Uteri and Peri-Implantation Conceptuses. Biol. Reprod. 2009, 80, 1196–1208. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Dai, Z.; Zhang, Y.; Chen, J.; Yang, Y.; Wu, G.; Tso, P.; Wu, Z. Maternal L-Proline Supplementation Enhances Fetal Survival, Placental Development, and Nutrient Transport in Mice. Biol. Reprod. 2019, 100, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Aliaga, I.; Wagner, C.A. Regulation and Function of the SLC38A3/SNAT3 Glutamine Transporter. Channels 2016, 10, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, F.C. Glutamine and Glutamate Exchange between the Fetal Liver and the Placenta. J. Nutr. 2000, 130, 974S–977S. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xie, C.; Zhang, Y.; Fan, Z.; Yin, Y.; Blachier, F. Glutamate-Glutamine Cycle and Exchange in the Placenta-Fetus Unit during Late Pregnancy. Amino Acids 2015, 47, 45–53. [Google Scholar] [CrossRef]
- Vaughn, P.R.; Lobo, C.; Battaglia, F.C.; Fennessey, P.V.; Wilkening, R.B.; Meschia, G. Glutamine Glutamate Exchange between Placenta and Fetal Liver. Am. J. Physiol.-Endocrinol. Metab. 1995, 31, E705–E711. [Google Scholar] [CrossRef]
- Chung, M.; Teng, C.; Timmerman, M.; Meschia, G.; Battaglia, F.C. Production and Utilization of Amino Acids by Ovine Placenta in Vivo. Am. J. Physiol. 1998, 274, E13–E22. [Google Scholar] [CrossRef]
- Limesand, S.W.; Regnault, T.R.H.; Hay, W.W. Characterization of Glucose Transporter 8 (GLUT8) in the Ovine Placenta of Normal and Growth Restricted Fetuses. Placenta 2004, 25, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Vonnahme, K.A.; Arndt, W.J.; Johnson, M.L.; Borowicz, P.P.; Reynolds, L.P. Effect of Morphology on Placentome Size, Vascularity, and Vasoreactivity in Late Pregnant Sheep. Biol. Reprod. 2008, 79, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zheng, J. Signaling Regulation of Fetoplacental Angiogenesis. J. Endocrinol. 2012, 212, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Kingdom, J.; Huppertz, B.; Seaward, G.; Kaufmann, P. Development of the Placental Villous Tree and Its Consequences for Fetal Growth. Eur. J. Obstet. Gynecol. Reprod. Biol. 2000, 92, 35–43. [Google Scholar] [CrossRef]
- Malkov, V.A.; Serikawa, K.A.; Balantac, N.; Watters, J.; Geiss, G.; Mashadi-Hossein, A.; Fare, T. Multiplexed Measurements of Gene Signatures in Different Analytes Using the Nanostring NCounterTM assay System. BMC Res. Notes 2009, 2, 80. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Bazer, F.W.; Dai, Z.; Li, D.; Wang, J.; Wu, Z. Amino Acid Nutrition in Animals: Protein Synthesis and Beyond. Annu. Rev. Anim. Biosci. 2014, 2, 387–417. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.H.; Moe, A.J. Nutrient Transport Pathways across the Epithelium of the Placenta. Mol. Biol. 1992, 12, 183–206. [Google Scholar] [CrossRef] [PubMed]
- Cleal, J.K.; Lewis, R.M. The Mechanisms and Regulation of Placental Amino Acid Transport to the Human Foetus. J. Neuroendocrinol. 2008, 20, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Lager, S.; Powell, T.L. Regulation of Nutrient Transport across the Placenta. J. Pregnancy 2012, 2012, 179827. [Google Scholar] [CrossRef]
- Brennan, K.A.; Gopalakrishnan, G.S.; Kurlak, L.; Rhind, S.M.; Kyle, C.E.; Brooks, A.N.; Rae, M.T.; Olson, D.M.; Stephenson, T.; Symonds, M.E. Impact of Maternal Undernutrition and Fetal Number on Glucocorticoid, Growth Hormone and Insulin-like Growth Factor Receptor MRNA Abundance in the Ovine Fetal Kidney. Reproduction 2005, 129, 151–159. [Google Scholar] [CrossRef]
- Carr, J.M.; Owens, J.A.; Grant, P.A.; Walton, P.E.; Owens, P.C.; Wallace, J.C. Circulating Insulin-like Growth Factors (IGFs), IGF-Binding Proteins (IGFBPs) and Tissue MRNA Levels of IGFBP-2 and IGFBP-4 in the Ovine Fetus. J. Endocrinol. 1995, 145, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Owens, J.A. Endocrine and Substrate Control of Fetal Growth: Placental and Maternal Influences and Insulin-like Growth Factors. Reprod. Fertil. Dev. 1991, 3, 501–517. [Google Scholar] [CrossRef] [PubMed]
- Sferruzzi-Perri, A.N.; Sandovici, I.; Constancia, M.; Fowden, A.L. Placental Phenotype and the Insulin-like Growth Factors: Resource Allocation to Fetal Growth. J. Physiol. 2017, 595, 5057–5093. [Google Scholar] [CrossRef] [PubMed]
- Constância, M.; Hemberger, M.; Hughes, J.; Dean, W.; Ferguson-Smith, A.; Fundele, R.; Stewart, F.; Kelsey, G.; Fowden, A.; Sibley, C.; et al. Placental-Specific IGF-II Is a Major Modulator of Placental and Fetal Growth. Nature 2002, 417, 945–948. [Google Scholar] [CrossRef] [PubMed]
- Sibley, C.P.; Coan, P.M.; Ferguson-Smith, A.C.; Dean, W.; Hughes, J.; Smith, P.; Reik, W.; Burton, G.J.; Fowden, A.L.; Constância, M. Placental-Specific Insulin-like Growth Factor 2 (Igf2) Regulates the Diffusional Exchange Characteristics of the Mouse Placenta. Proc. Natl. Acad. Sci. USA 2004, 101, 8204–8208. [Google Scholar] [CrossRef] [PubMed]
- Bazer, F.W.; Wang, X.; Johnson, G.A.; Wu, G. Select Nutrients and Their Effects on Conceptus Development in Mammals. Anim. Nutr. 2015, 1, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, F.C.; Østergaard, L.; Nielsen, J.; Christiansen, J. Growth-Dependent Translation of IGF-II MRNA by a Rapamycin-Sensitive Pathway. Nature 1995, 377, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.Y. Vascular Endothelial Growth Factor: Possible Role in Fetal Development and Placental Function. J. Soc. Gynecol. Investig. 1997, 4, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Vonnahme, K.A.; Wilson, M.E.; Li, Y.; Rupnow, H.L.; Phernetton, T.M.; Ford, S.P.; Magness, R.R. Circulating Levels of Nitric Oxide and Vascular Endothelial Growth Factor throughout Ovine Pregnancy. J. Physiol. 2005, 565, 101–109. [Google Scholar] [CrossRef]
- Reynolds, L.P.; Redmer, D.A. Utero-Placental Vascular Development and Placental Function. J. Anim. Sci. 1995, 73, 1839–1851. [Google Scholar] [CrossRef]
- Murohara, T.; Horowitz, J.R.; Silver, M.; Tsurumi, Y.; Chen, D.; Sullivan, A.; Isner, J.M. Vascular Endothelial Growth Factor/Vascular Permeability Factor Enhances Vascular Permeability Via Nitric Oxide and Prostacyclin. Circulation 1998, 97, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Morris, S.M. Arginine Metabolism: Nitric Oxide and Beyond. Biochem. J. 1998, 336, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Sooranna, S.; Morris, N.; Steer, P. Placental Nitric Oxide Metabolism. Reprod. Fertil. Dev. 1995, 7, 1525–1531. [Google Scholar] [CrossRef] [PubMed]
- Kamath, S.G.; Furesz, T.C.; Way, B.A.; Smith, C.H. Identification of Three Cationic Amino Acid Transporters in Placental Trophoblast: Cloning, Expression, and Characterization of HCAT-1. J. Membr. Biol. 1999, 171, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Ayuk, P.T.Y.; Sibley, C.P.; Donnai, P.; D’Souza, S.; Glazier, J.D. Development and Polarization of Cationic Amino Acid Transporters and Regulators in the Human Placenta. Am. J. Physiol. Cell Physiol. 2000, 278, C1162–C1171. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, J.O.A.; Jiang, B.; Iyer, N.V.; Agani, F.; Leung, S.W. Activation of Vascular Endothelial Growth Factor Gene Transcription by Hypoxia-Inducible Factor Activation of Vascular Endothelial Growth Factor Gene Transcription by Hypoxia-Inducible Factor 1. Mol. Cell. Biol. 1996, 16, 4604–4612. [Google Scholar] [CrossRef]
- Semenza, G.L. Regulation of Mammalian O2 Homeostasis by Hypoxia-Inducible Factor 1. Annu. Rev. Cell Dev. Biol. 1999, 15, 551–578. [Google Scholar] [CrossRef]
- Sales, F.; Peralta, O.A.; Narbona, E.; McCoard, S.; De los Reyes, M.; González-Bulnes, A.; Parraguez, V.H. Hypoxia and Oxidative Stress Are Associated with Reduced Fetal Growth in Twin and Undernourished Sheep Pregnancies. Animals 2018, 8, 217. [Google Scholar] [CrossRef]



| Placentome Subtype | Arg | Control |
|---|---|---|
| A | 18 (7) | 21 (9) |
| B | 17 (8) | 19 (10) |
| C | 16 (6) | 17 (7) |
| Function | Gene ID | Accession | Name |
|---|---|---|---|
| Glucose transporter | SLC2A1 (GLUT1) | XM_015091913.1 | Glucose transporter 1; solute carrier family 2, member 1 |
| Glucose transporter | SLC2A3 (GLUT3) | XM_012159621.2 | Glucose transporter 3; solute carrier family 2, member 3 |
| Glucose transporter | SLC2A4 (GLUT4) | XM_012185632.1 | Glucose transporter 4; solute carrier family 2, member 4 |
| Glucose transporter | SLC2A8 (GLUT8) | XM_012162546.2 | Glucose transporter 8; solute carrier family 2, member 8 |
| AA transporter | SLC38A1 (SNAT1) | XM_012170444.1 | SNAT1 System A; solute carrier family 38, member 1 |
| AA transporter | SLC38A2 (SNAT2) | XM_012170436.2 | SNAT2 System A; solute carrier family 38, member 2 |
| AA transporter | SLC38A4 (SNAT4) | XM_012155123.2 | SNAT4 System A; solute carrier family 38, member 4 |
| AA transporter | SLC7A5 (LAT1) | XM_012159575.2 | LAT1 System L; Solute carrier family 7, member 5 |
| AA transporter | SLC7A8 (LAT2) | XM_012129264.1 | LAT2 System L; Solute carrier family 7, member 8 |
| AA transporter | SLC3A2/4F2hc (CD98) | XM_012146229.2 | Heavy chain transmembrane protein for systems L and y+L; solute carrier family 3, member 2 |
| AA transporter | SLC7A7 (y+LAT1) | XM_012129339.2 | y+LAT1 System y+L; solute carrier family 7, member 7 |
| AA transporter | SLC7A1 (CAT1) | XM_012142866.2 | System y+; solute carrier family 7, member 1 |
| AA transporter | SLC7A2 (CAT2) | XM_012159185.2 | System y+; solute carrier family 7, member 2 |
| AA transporter | SLC7A4 (CAT4) | XM_012164278.2 | System y+; solute carrier family 7, member 4 |
| AA transporter | SLC1A4 (ASCT1) | XM_012125788.2 | System ASC |
| AA transporter | SLC38A3 (SN1) | XM_012122049.1 | SN1 System N; solute carrier family 38, member 3 |
| AA transporter | SLC7A9 | XM_015104066.1 | B(0,+)AT System b; solute carrier family 7, member 9 |
| AA transporter | SLC6A14 | XM_004022406.3 | bBo+ System B; solute carrier family 6, member 14 |
| AA transporter | SLC1A3 (EAAT1) | XM_004017043.2 | EAAT1 System X; solute carrier family 1, member 3 |
| AA transporter | SLC1A2 (EAAT2) | XM_012096234.2 | EAAT2 System X; solute carrier family 1, member 2 |
| AA transporter | SLC1A1 (EAAT3) | XM_004004350.3 | EAAT1 System X; solute carrier family 1, member 1 |
| Angiogenic factor | VEGFR-1 (FLT1) | XM_015098156.1 | VEGF receptor 1; FMs-related receptor tyrosine kinase 1 |
| Angiogenic factor | VEGFR-2 (KDR) | NM_001278565.1 | VEGF receptor 2; Kinase insert domain receptor |
| Angiogenic factor | FGFR2 | XM_012103045.2 | FGF2 receptor |
| Angiogenic factor | TEK (Tie2) | XM_012153095.2 | Angiopoietin-1 receptor/TEK receptor tyrosine kinase |
| Angiogenic factor | NOS3 | XM_015095442.1 | Nitric oxide synthase 3 |
| Angiogenic factor | GUCY1B1 | XM_015101545.1 | Soluble guanylate cyclase NO receptor enzyme |
| Hypoxic marker | HIF1a | XM_015097105.1 | Hypoxia-inducible factor 1 |
| Growth factor | IGF-1 | NM_001009774.3 | Insulin-like growth factor 1 |
| Growth factor | IGF-2 | NM_001009311.1 | Insulin-like growth factor 2 |
| Stress marker | HSD11B2 | NM_001009460.1 | 11 beta-hydroxysteroid dehydrogenase type 2 |
| Reference gene | Cyclophilin A | NM_001308578.1 | Peptidylprolyl isomerase A |
| Reference gene | GADPH | NM_001190390.1 | Glyceraldehyde 3-phosphate dehydrogenase |
| Reference gene | RPL19 | XM_004012836.2 | Ribosomal protein L19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erichsen, C.; Heiser, A.; Haack, N.; Maclean, P.; Dwyer, C.M.; McCoard, S. Increasing the Understanding of Nutrient Transport Capacity of the Ovine Placentome. Animals 2024, 14, 1294. https://doi.org/10.3390/ani14091294
Erichsen C, Heiser A, Haack N, Maclean P, Dwyer CM, McCoard S. Increasing the Understanding of Nutrient Transport Capacity of the Ovine Placentome. Animals. 2024; 14(9):1294. https://doi.org/10.3390/ani14091294
Chicago/Turabian StyleErichsen, Cathrine, Axel Heiser, Neville Haack, Paul Maclean, Cathy Mary Dwyer, and Sue McCoard. 2024. "Increasing the Understanding of Nutrient Transport Capacity of the Ovine Placentome" Animals 14, no. 9: 1294. https://doi.org/10.3390/ani14091294
APA StyleErichsen, C., Heiser, A., Haack, N., Maclean, P., Dwyer, C. M., & McCoard, S. (2024). Increasing the Understanding of Nutrient Transport Capacity of the Ovine Placentome. Animals, 14(9), 1294. https://doi.org/10.3390/ani14091294






