The Effect of κ-Carrageenan on Porcine Sperm Cryo-Survival
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Extender Preparation
2.2. Semen Collection
2.3. Sperm Cryopreservation Process
2.4. Evaluation of FT Sperm Kinematics
2.5. Evaluation of Sperm Viability
2.6. Assessment of Acrosome Status
2.7. Evaluation of Sperm Mitochondrial Activity
2.8. Measurement of Intracellular Caspase Activities
2.9. Measurement of Lipid Peroxidation
2.10. Statistical Analysis
3. Results
3.1. Effects of κ-Carrageenan on Post-Thawing Kinematic Parameters
3.2. Effects of κ-Carrageenan on FT Sperm Viability
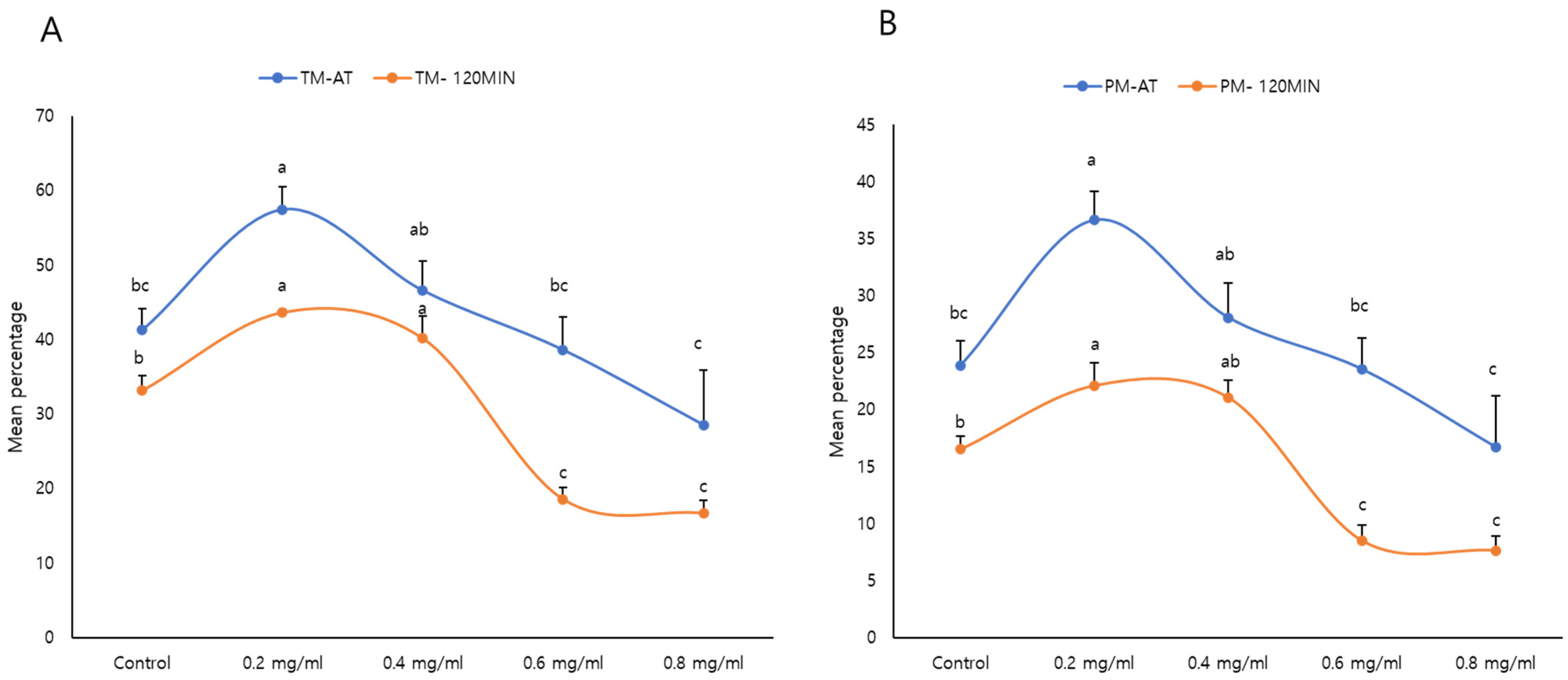
3.3. Effects of κ-Carrageenan on Post-Thawing Acrosome Integrity
3.4. Effects of κ-Carrageenan on FT Sperm Mitochondrial Activity
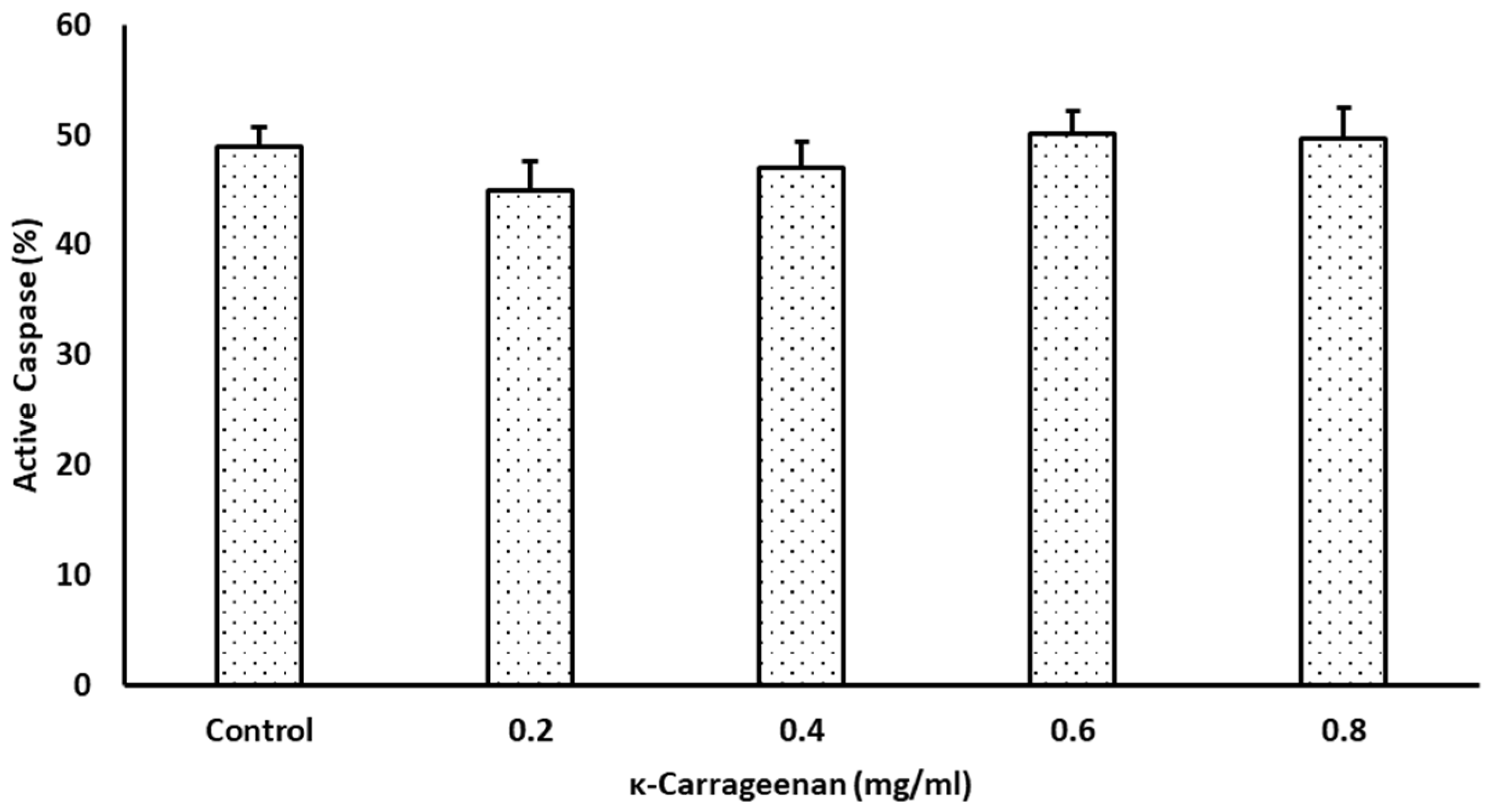
3.5. Effects of κ-Carrageenan on Intracellular Caspase Activities
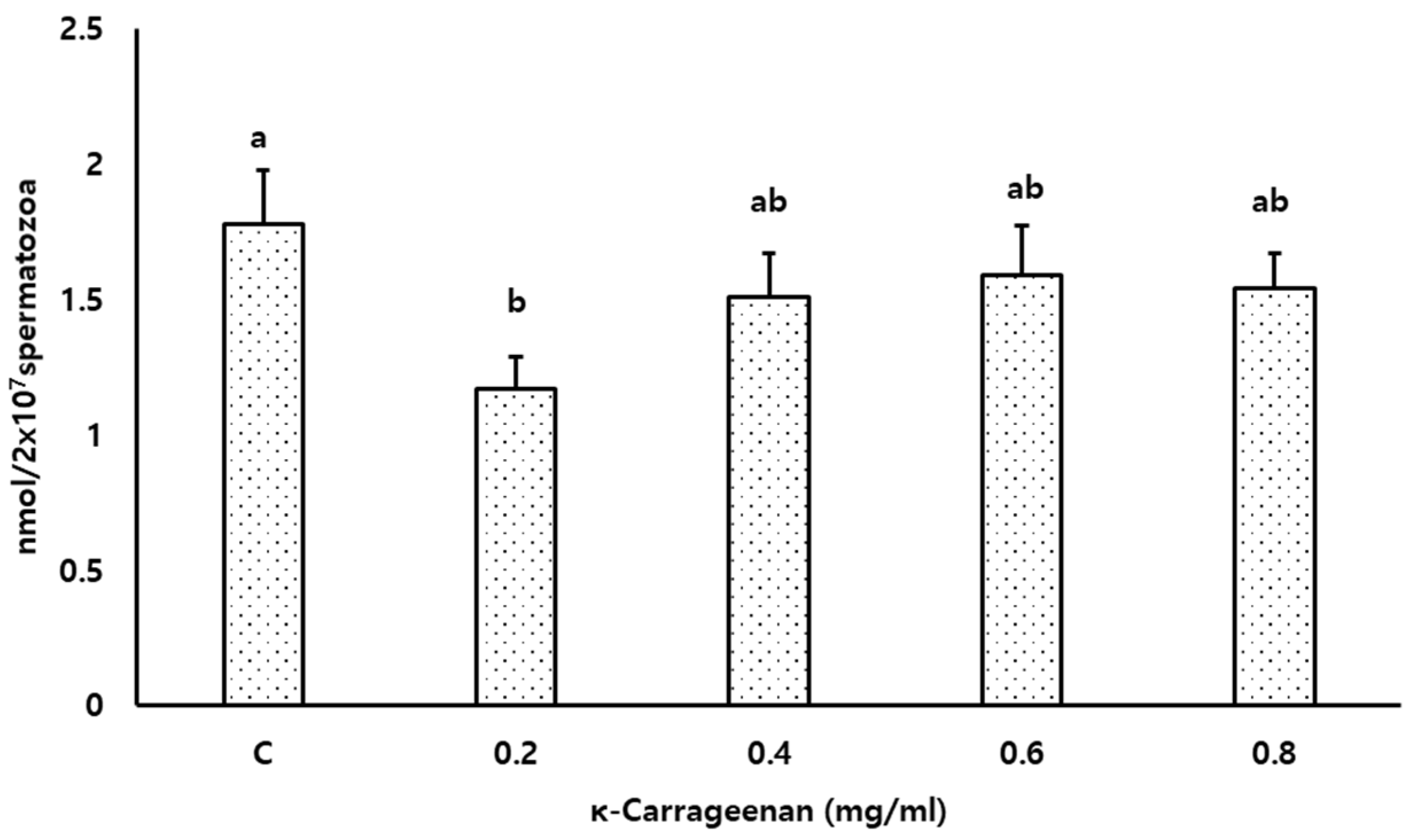
3.6. Effects of κ-Carrageenan on Lipid Peroxidation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Großfeld, R.; Sieg, B.; Struckmann, C.; Frenzel, A.; Maxwell, W.M.C.; Rath, D. New aspects of boar semen freezing strategies. Theriogenology 2008, 70, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Mara, L.; Casu, S.; Carta, A.; Dattena, M. Cryobanking of farm animal gametes and embryos as a means of conserving livestock genetics. Anim. Reprod. Sci. 2013, 138, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Knox, R. The Fertility of Frozen Boar Sperm When used for Artificial Insemination. Reprod. Domest. Anim. 2015, 50, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Yeste, M.; Rodríguez-Gil, J.E.; Bonet, S. Artificial insemination with frozen-thawed boar sperm. Mol. Reprod. Dev. 2017, 84, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.G.; Cunha, E.R.; Blume, G.R.; Malaquias, J.V.; Bao, S.N.; Martins, C.F. Cryopreservation of boar sperm comparing different cryoprotectants associated in media based on powdered coconut water, lactose and trehalose. Cryobiology 2015, 70, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Malo, C.; Gil, L.; Gonzalez, N.; Cano, R.; de Blas, I.; Espinosa, E. Comparing sugar type supplementation for cryopreservation of boar semen in egg yolk based extender. Cryobiology 2010, 61, 17–21. [Google Scholar] [CrossRef]
- Kim, D. Evaluation of Antifreeze Proteins on Miniature Pig Sperm Viability, DNA Damage, and Acrosome Status during Cryopreservation. J. Emb. Trans. 2016, 31, 355–365. [Google Scholar] [CrossRef]
- Yang, C.H.; Wu, T.W.; Cheng, F.P.; Wang, J.H.; Wu, J.T. Effects of different cryoprotectants and freezing methods on post-thaw boar semen quality. Reprod. Biol. 2016, 16, 41–46. [Google Scholar] [CrossRef]
- Park, C.H.; Kim, S.W.; Hwang, Y.J.; Kim, D.Y. Cryopreservation with Trehalose Reduced Sperm Chromatin Damage in Miniature Pig. J. Emb. Trans. 2012, 27, 107–111. [Google Scholar]
- Malo, C.; Gil, L.; Gonzalez, N.; Martinez, F.; Cano, R.; de Blas, I.; Espinosa, E. Anti-oxidant supplementation improves boar sperm characteristics and fertility after cryopreservation: Comparison between cysteine and rosemary (Rosmarinus officinalis). Cryobiology 2010, 61, 142–147. [Google Scholar] [CrossRef]
- Sun, L.; Fan, X.; Zeng, Y.; Wang, L.; Zhu, Z.; Li, R.; Tian, X.; Wang, Y.; Lin, Y.; Wu, D.; et al. Resveratrol protects boar sperm in vitro via its antioxidant capacity. Zygote 2020, 28, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Martinez, H.; Wallgren, M. Advances in Boar Semen Cryopreservation. Vet. Med. Int. 2011, 2011, 396181. [Google Scholar] [CrossRef] [PubMed]
- Jovičić, M.; Chmelíková, E.; Sedmíková, M. Cryopreservation of boar semen. Czech J. Anim. Sci. 2020, 65, 115–123. [Google Scholar] [CrossRef]
- Walczak-Jedrzejowska, R.; Wolski, J.K.; Slowikowska-Hilczer, J. The role of oxidative stress and antioxidants in male fertility. Cent. Eur. J. Urol. 2013, 66, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Virk, G.; Ong, C.; du Plessis, S.S. Effect of Oxidative Stress on Male Reproduction. World J. Men’s Health 2014, 32, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Alahmar, A.T. Role of Oxidative Stress in Male Infertility: An Updated Review. J. Hum. Reprod. Sci. 2019, 12, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Mandal, R.; Badyakar, D.; Chakrabarty, J. Role of Membrane Lipid Fatty Acids in Sperm Cryopreservation. Adv. Androl. 2014, 2014, 190542. [Google Scholar] [CrossRef]
- Yeste, M. Recent Advances in Boar Sperm Cryopreservation: State of the Art and Current Perspectives. Reprod. Domest. Anim. 2015, 50 (Suppl. S2), 71–79. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.J.; Kim, M.K.; Song, H.J.; Kang, E.J.; Ock, S.A.; Kumar, B.M.; Balasubramanian, S.; Rho, G.J. Effect of alpha-tocopherol supplementation during boar semen cryopreservation on sperm characteristics and expression of apoptosis related genes. Cryobiology 2009, 58, 181–189. [Google Scholar] [CrossRef]
- Estrada, E.; Rodríguez-Gil, J.E.; Rocha, L.G.; Balasch, S.; Bonet, S.; Yeste, M. Supplementing cryopreservation media with reduced glutathione increases fertility and prolificacy of sows inseminated with frozen-thawed boar semen. Andrology 2014, 2, 88–99. [Google Scholar] [CrossRef]
- Kaeoket, K.; Chanapiwat, P.; Tummaruk, P.; Techakumphu, M. Supplemental effect of varying L-cysteine concentrations on the quality of cryopreserved boar semen. Asian J. Androl. 2010, 12, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Luno, V.; Gil, L.; Olaciregui, M.; Gonzalez, N.; Jerez, R.A.; de Blas, I. Rosmarinic acid improves function and in vitro fertilising ability of boar sperm after cryopreservation. Cryobiology 2014, 69, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Rocha de Souza, M.C.; Marques, C.T.; Guerra Dore, C.M.; Ferreira da Silva, F.R.; Oliveira Rocha, H.A.; Leite, E.L. Antioxidant activities of sulfated polysaccharides from brown and red seaweeds. J. Appl. Phycol. 2007, 19, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Necas, J.; Bartosikova, L. Carrageenan: A review. Veterinární Medicína 2013, 58, 187–205. [Google Scholar] [CrossRef]
- Ullah, S.; Khalil, A.A.; Shaukat, F.; Song, Y. Sources, Extraction and Biomedical Properties of Polysaccharides. Foods 2019, 8, 304. [Google Scholar] [CrossRef] [PubMed]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical Structures and Bioactivities of Sulfated Polysaccharides from Marine Algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Almubarak, A.; Talha, N.; Yu, I.-J.; Jeon, Y. The Use of κ-Carrageenan in Egg Yolk Free Extender Improves the Efficiency of Canine Semen Cryopreservation. Animals 2022, 12, 88. [Google Scholar]
- Li, W.; Appiah, M.O.; Zhao, J.; Liu, H.; Wang, J.; Lu, W. Effects of k-carrageenan supplementation or in combination with cholesterol-loaded cyclodextrin following freezing-thawing process of rooster spermatozoa. Cryobiology 2020, 95, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Pettitt, M.J.; Buhr, M.M. Extender Components and Surfactants Affect Boar Sperm Function and Membrane Behavior During Cryopreservation. J. Androl. 1998, 19, 736–746. [Google Scholar] [CrossRef]
- Park, S.-H.; Yu, I.-J. Effect of Antioxidant Supplementation in Freezing Extender on Porcine Sperm Viability, Motility and Reactive Oxygen Species. J. Emb. Trans. 2017, 32, 9–15. [Google Scholar] [CrossRef]
- Córdova, A.; Ducolomb, Y.; Jiménez, I.; Casas, E.; Bonilla, E.; Betancourt, M. In vitro fertilizing capacity of frozen-thawed boar semen. Theriogenology 1997, 47, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Almubarak, A.M.; Kim, W.; Abdelbagi, N.H.; Balla, S.E.; Yu, I.-J.; Jeon, Y. Washing solution and centrifugation affect kinematics of cryopreserved boar semen. J. Anim. Reprod. Biotechnol. 2021, 36, 65–75. [Google Scholar] [CrossRef]
- Yu, I.; Leibo, S.P. Recovery of motile, membrane-intact spermatozoa from canine epididymides stored for 8 days at 4 °C. Theriogenology 2002, 57, 1179–1190. [Google Scholar] [CrossRef] [PubMed]
- Najafi, A.; Taheri, R.A.; Mehdipour, M.; Farnoosh, G.; Martínez-Pastor, F. Lycopene-loaded nanoliposomes improve the performance of a modified Beltsville extender broiler breeder roosters. Anim. Reprod. Sci. 2018, 195, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Tatemoto, H.; Osokoshi, N.; Hirai, M.; Masuda, Y.; Konno, T.; Yamanaka, K. Addition of l-carnitine to the freezing extender improves post-thaw sperm quality of Okinawan native Agu pig. Theriogenology 2021, 188, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.K.; Bilaspuri, G.S. Impacts of oxidative stress and antioxidants on semen functions. Vet. Med. Int. 2010, 2010, 686137. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Gao, X.; Cheng, C.; Liu, C.; Wang, Q.; Han, X. The Structural Characteristics of Seaweed Polysaccharides and Their Application in Gel Drug Delivery Systems. Mar. Drugs 2020, 18, 658. [Google Scholar] [CrossRef] [PubMed]
- Hentati, F.; Tounsi, L.; Djomdi, D.; Pierre, G.; Delattre, C.; Ursu, A.V.; Fendri, I.; Abdelkafi, S.; Michaud, P. Bioactive Polysaccharides from Seaweeds. Molecules 2020, 25, 3152. [Google Scholar] [CrossRef] [PubMed]
- Tesfay, H.H.; Sun, Y.; Li, Y.; Shi, L.; Fan, J.; Wang, P.; Zong, Y.; Ni, A.; Ma, H.; Mani, A.I.; et al. Comparative studies of semen quality traits and sperm kinematic parameters in relation to fertility rate between 2 genetic groups of breed lines. Poult. Sci. 2020, 99, 6139–6146. [Google Scholar] [CrossRef]
- Vizcarra, J.A.; Ford, J.J. Validation of the sperm mobility assay in boars and stallions. Theriogenology 2006, 66, 1091–1097. [Google Scholar] [CrossRef]
- Ritagliati, C.; Baro Graf, C.; Stival, C.; Krapf, D. Regulation mechanisms and implications of sperm membrane hyperpolarization. Mech. Dev. 2018, 154, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Boguenet, M.; Bouet, P.-E.; Spiers, A.; Reynier, P.; May-Panloup, P. Mitochondria: Their role in spermatozoa and in male infertility. Hum. Reprod. Update 2021, 27, 697–719. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.; Lourenço, B.; Marques, M.; Ramalho-Santos, J. Mitochondria functionality and sperm quality. Reproduction 2013, 146, R163–R174. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-J.; Pang, M.-G. Mitochondrial Functionality in Male Fertility: From Spermatogenesis to Fertilization. Antioxidants 2021, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.-H.; Li, Q.-W.; Zhang, T.; Jiang, Z.-L. Effect of Gynostemma Pentaphyllum Polysaccharide on boar spermatozoa quality following freezing–thawing. Cryobiology 2009, 59, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Zhang, X.; Wang, J.; Jia, S.; Zhou, Y.; Tian, J.; Wang, H.; Tang, Y. Inhibitory effect of Lycium barbarum polysaccharide on sperm damage during cryopreservation. Exp. Ther. Med. 2020, 20, 3051–3063. [Google Scholar] [CrossRef] [PubMed]
- Stramová, X.; Hampl, R.; Stěpán, J.; Kanďár, R. Role of fatty acids in sperm membrane. Ceska Gynekol. 2014, 79, 103–106. [Google Scholar] [PubMed]
- Sanocka, D.; Kurpisz, M. Reactive oxygen species and sperm cells. Reprod. Biol. Endocrinol. 2004, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Gibb, Z.; Baker, M.A.; Drevet, J.; Gharagozloo, P. Causes and consequences of oxidative stress in spermatozoa. Reprod. Fertil. Dev. 2015, 28, 1–10. [Google Scholar] [CrossRef]
- Aitken, R.J.; Baker, M.A. Oxidative stress, sperm survival and fertility control. Mol. Cell. Endocrinol. 2006, 250, 66–69. [Google Scholar] [CrossRef]
- Yuan, H.; Song, J.; Zhang, W.; Li, X.; Li, N.; Gao, X. Antioxidant activity and cytoprotective effect of κ-carrageenan oligosaccharides and their different derivatives. Bioorganic Med. Chem. Lett. 2006, 16, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jiang, N.; Li, B.; Wan, M.; Chang, X.; Liu, H.; Zhang, L.; Yin, S.; Qi, H.; Liu, S. Antioxidant activity of purified ulvan in hyperlipidemic mice. Int. J. Biol. Macromol. 2018, 113, 971–975. [Google Scholar] [CrossRef]
- Costa, L.S.; Fidelis, G.P.; Cordeiro, S.L.; Oliveira, R.M.; Sabry, D.A.; Câmara, R.B.G.; Nobre, L.T.D.B.; Costa, M.S.S.P.; Almeida-Lima, J.; Farias, E.H.C.; et al. Biological activities of sulfated polysaccharides from tropical seaweeds. Biomed. Pharmacother. 2010, 64, 21–28. [Google Scholar] [CrossRef]
- Thornberry, N.A.; Lazebnik, Y. Caspases: Enemies Within. Science 1998, 281, 1312–1316. [Google Scholar] [CrossRef] [PubMed]
- Thornberry, N.A. Caspases: Key mediators of apoptosis. Chem. Biol. 1998, 5, R97–R103. [Google Scholar] [CrossRef]
- Kim, S.-H.; Yu, D.-H.; Kim, Y.-J. Effects of cryopreservation on phosphatidylserine translocation, intracellular hydrogen peroxide, and DNA integrity in canine sperm. Theriogenology 2010, 73, 282–292. [Google Scholar] [CrossRef]
- Aitken, R.J.; Jones, K.T.; Robertson, S.A. Reactive Oxygen Species and Sperm Function—In Sickness and in Health. J. Androl. 2012, 33, 1096–1106. [Google Scholar] [CrossRef]
- Zribi, N.; Chakroun, N.F.; Ben Abdallah, F.; Elleuch, H.; Sellami, A.; Gargouri, J.; Rebai, T.; Fakhfakh, F.; Keskes, L.A. Effect of freezing–thawing process and quercetin on human sperm survival and DNA integrity. Cryobiology 2012, 65, 326–331. [Google Scholar] [CrossRef]
- Thomson, L.K.; Fleming, S.D.; Aitken, R.J.; De Iuliis, G.N.; Zieschang, J.-A.; Clark, A.M. Cryopreservation-induced human sperm DNA damage is predominantly mediated by oxidative stress rather than apoptosis. Hum. Reprod. 2009, 24, 2061–2070. [Google Scholar] [CrossRef] [PubMed]
- Stanic, P.; Tandara, M.; Sonicki, Z.; Simunic, V.; Radakovic, B.; Suchanek, E. Comparison of protective media and freezing techniques for cryopreservation of human semen. Eur. J. Obstet. Gynecol. Reprod. Biol. 2000, 91, 65–70. [Google Scholar] [CrossRef]
- Di Santo, M.; Tarozzi, N.; Nadalini, M.; Borini, A. Human Sperm Cryopreservation: Update on Techniques, Effect on DNA Integrity, and Implications for ART. Adv. Urol. 2012, 2012, 854837. [Google Scholar] [CrossRef] [PubMed]



| Group (mg/mL) | MP (%) | VCL (µm/s) | VAP (µm/s) | VSL (µm/s) | ALH (µm) |
|---|---|---|---|---|---|
| Control–AT | 12.77 ± 1.90 ab | 50.43 ± 2.98 ab | 38.67 ± 1.38 a | 31.13 ± 1.51 ab | 1.82 ± 0.15 ab |
| Post 120 min | 6.92 ± 1.91 a | 38.73 ± 4.73 a | 31.51 ± 4.68 a | 25.73 ± 5.15 a | 1.42 ± 0.15 a |
| 0.2–AT | 18.69 ± 2.01 a | 56.03 ± 1.66 a | 43.67 ± 1.59 a | 34.41 ± 2.14 a | 1.96 ± 0.10 a |
| Post 120 min | 9.58 ± 3.55 a | 40.72 ± 5.88 a | 32.76 ± 5.63 a | 26.83 ± 5.94 a | 1.49 ± 0.16 a |
| 0.4–AT | 15.37 ± 1.86 ab | 50.87 ± 1.51 ab | 40.26 ± 1.43 a | 32.46 ± 1.50 ab | 1.78 ± 0.07 ab |
| Post 120 min | 9.70 ± 1.60 a | 45.73 ± 6.75 a | 34.33 ± 3.63 a | 27.93 ± 4.39 a | 1.51 ± 0.06 a |
| 0.6–AT | 11.56 ± 2.10 bc | 48.85 ± 1.56 b | 39.42 ± 1.91 a | 31.69 ± 2.40 ab | 1.65 ± 0.08 b |
| Post 120 min | 3.31 ± 0.94 a | 37.49 ± 4.93 a | 28.48 ± 3.89 a | 22.14 ± 3.96 a | 1.47 ± 0.18 a |
| 0.8–AT | 6.42 ± 1.96 c | 43.03 ± 1.86 c | 33.39 ± 2.20 b | 26.25 ± 2.54 b | 1.58 ± 0.07 b |
| Post 120 min | 2.95 ± 1.11 a | 37.56 ± 4.06 a | 29.71 ± 3.65 a | 23.78 ± 4.34 a | 1.41 ± 0.09 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almubarak, A.; Kim, E.; Yu, I.-J.; Park, H.; Jeon, Y. The Effect of κ-Carrageenan on Porcine Sperm Cryo-Survival. Animals 2024, 14, 1387. https://doi.org/10.3390/ani14091387
Almubarak A, Kim E, Yu I-J, Park H, Jeon Y. The Effect of κ-Carrageenan on Porcine Sperm Cryo-Survival. Animals. 2024; 14(9):1387. https://doi.org/10.3390/ani14091387
Chicago/Turabian StyleAlmubarak, Areeg, Eunji Kim, Il-Jeoung Yu, Hanseul Park, and Yubyeol Jeon. 2024. "The Effect of κ-Carrageenan on Porcine Sperm Cryo-Survival" Animals 14, no. 9: 1387. https://doi.org/10.3390/ani14091387
APA StyleAlmubarak, A., Kim, E., Yu, I. -J., Park, H., & Jeon, Y. (2024). The Effect of κ-Carrageenan on Porcine Sperm Cryo-Survival. Animals, 14(9), 1387. https://doi.org/10.3390/ani14091387






