Selective Breeding for Genetic Improvement of Nile tilapia (Oreochromis niloticus Linnaeus, 1758) in Uganda: Current Status, Challenges, and Future Perspectives
Abstract
Simple Summary
Abstract
1. Introduction
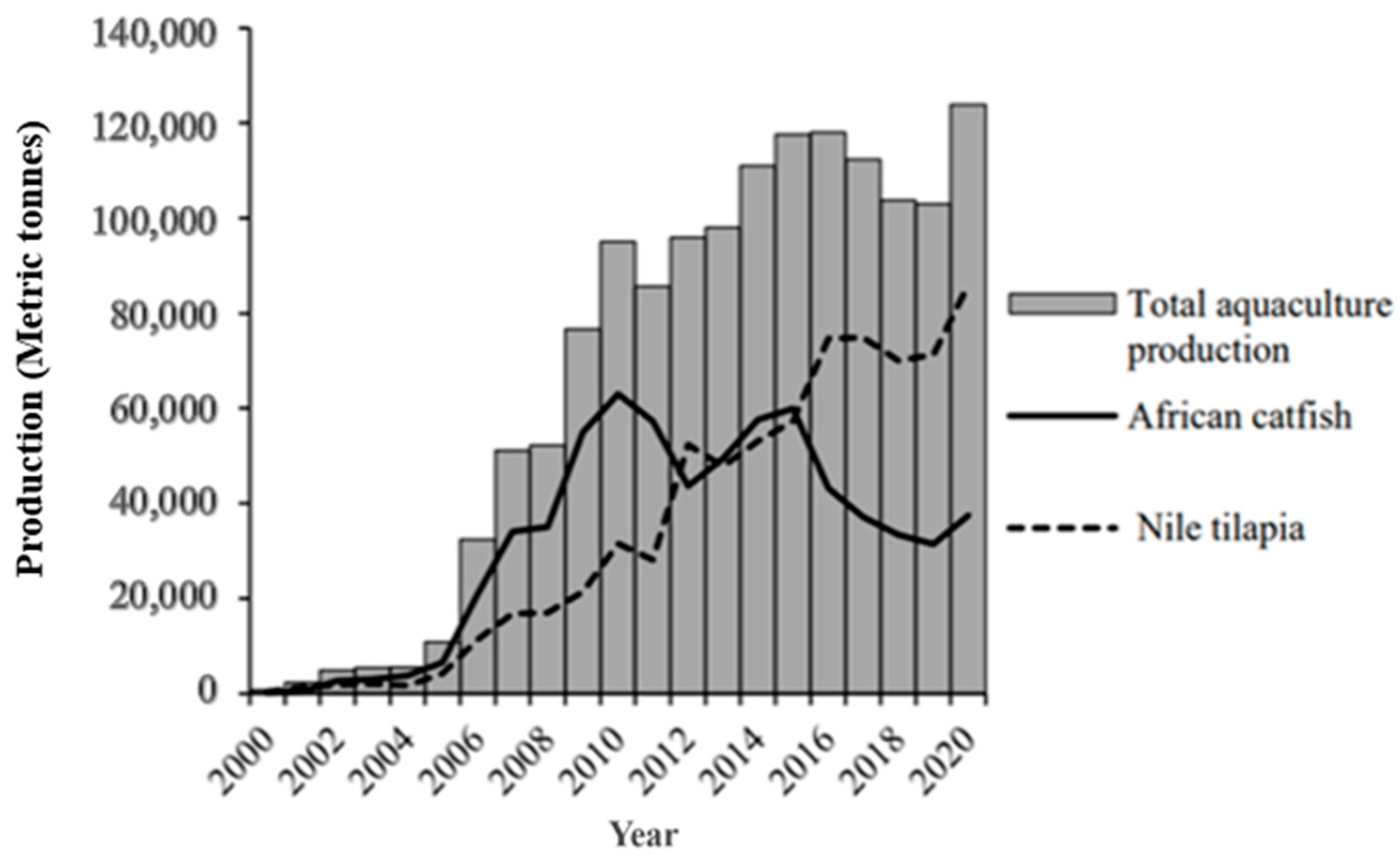
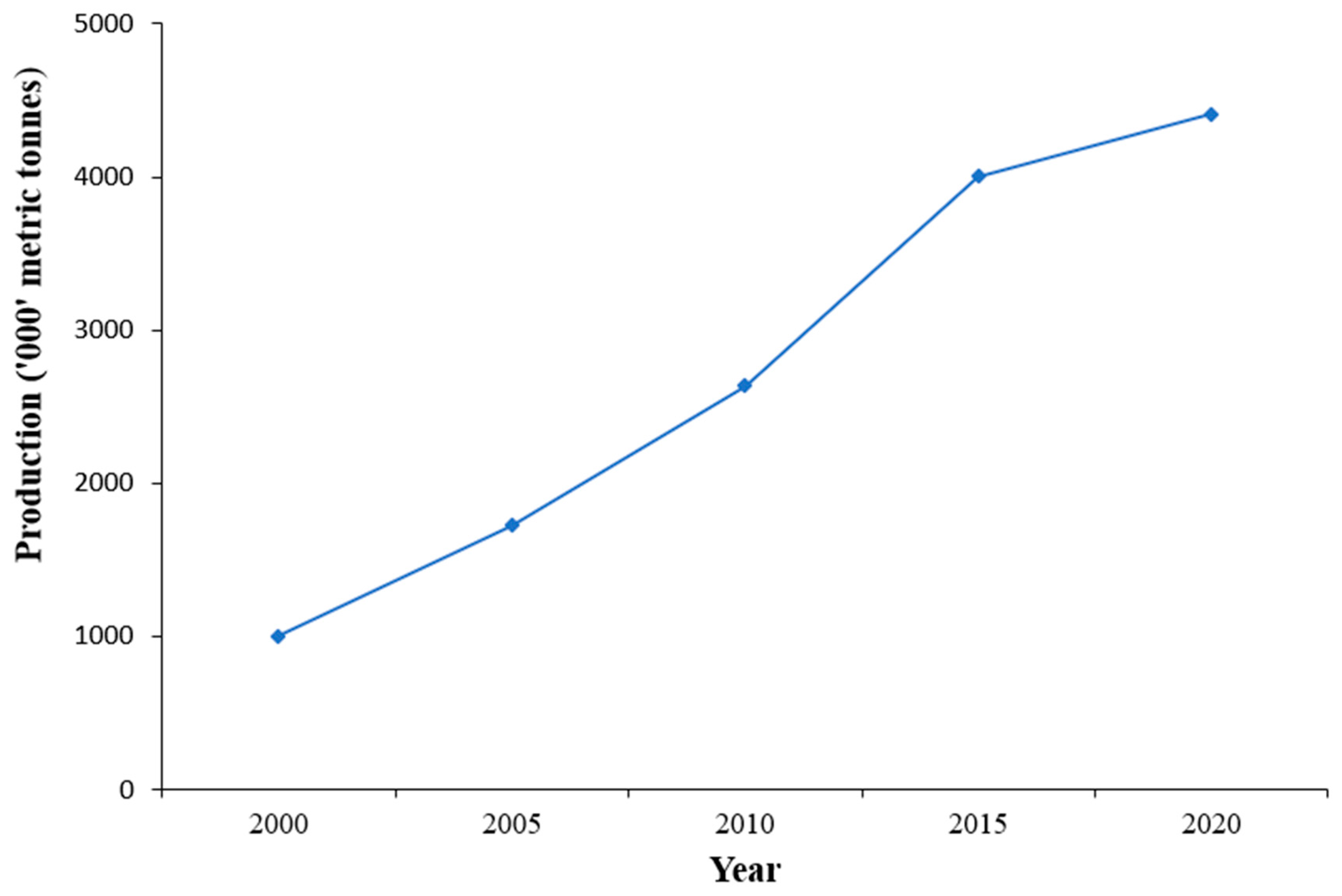
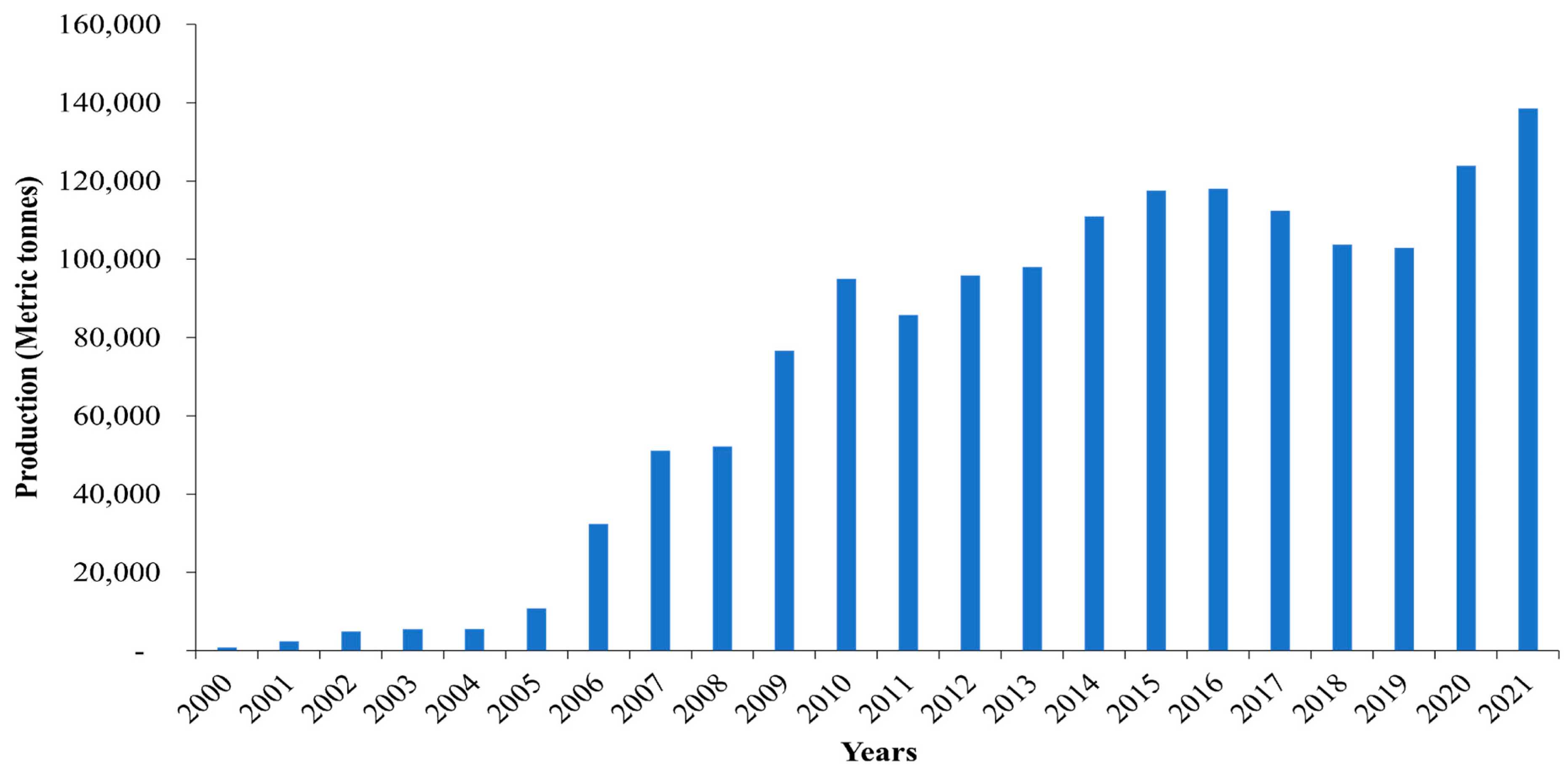
2. Nile tilapia Aquaculture in Uganda
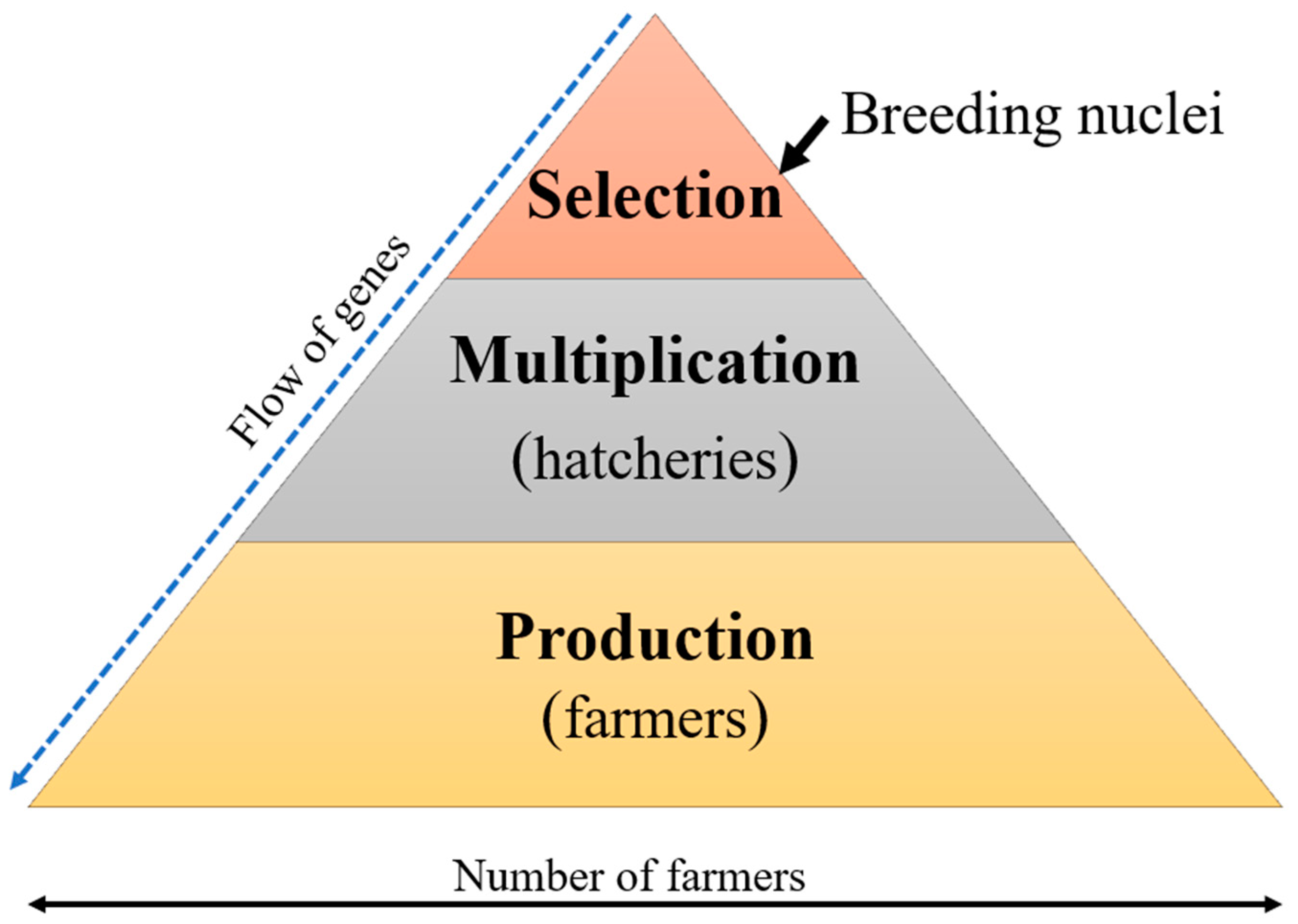
3. Selective Breeding in Aquaculture
Selective Breeding of Nile tilapia
| Country | Strain | Institution | Year Started | Germplasm Source | Target Trait (s) | Generation | Benefits | Reference |
|---|---|---|---|---|---|---|---|---|
| Philippines | GIFT | Bureau of Fisheries and Aquatic Resources and Freshwater Aquaculture Centre (BFAR) of Central Luzon State University | 1988 | Four wild strains from Africa (Egypt, Ghana, Kenya, and Senegal) and four farmed strains in the Philippines | Growth and survival rates | 6th | The 6th generation had 77% faster growth and 60% higher survival rates than locally farmed strains in the Philippines. An average genetic gain of 12–17% per generation across five generations of selection was obtained | [8,65,93,96,97,98,99,100] |
| FaST or FAC-Selected Tilapia | Freshwater Aquaculture Center Central Luzon State University (FAC-CLSU) | 1986 | Nile tilapia strains were collected from Taiwan, Singapore, Thailand, and Israel. These were referred to as the Philippines strain | Growth rate | 12th | A genetic gain in body weight of 12% per generation was observed after 12 generations of selection | [13,97,101,102] | |
| GET EXCEL | National Freshwater Fisheries Technology Center, Bureau of Fisheries and Aquatic Resources | 2002 | Strain crosses and within-family selection using four parent lines: 8th generation GIFT, 13th generation FaST, and Nile tilapia from Egypt and Kenya | Disease resistance, growth, and survival rates | 1st | The strain was more disease resistant, with higher growth and survival rates compared to the 8th generation of GIFT | [13,97,103,104] | |
| GIFT | TGA Farm Incorporated | 2006 | GIFT from WorldFish Center | Growth rate | 2nd | Fast growth performance, coupled with increased farm revenues | [68,92] | |
| Malaysia | GIFT | The WorldFish Center | 2001 | 6th generation from the GIFT project in the Philippines | Growth rate | 10th | An accumulated response of 107% in growth rate, averaging 11.9% per generation | [96] |
| China | NEW GIFT | Shanghai Ocean University | 1994 | 3rd generation of the GIFT project in the Philippines | Growth rate | 8th | Higher growth rate (>30%) than the base population | [105,106,107,108] |
| GIFT | Freshwater Fisheries Research Center (FFRC), Chinese Academy of Fishery Sciences (CAFS) | 2006 | The GIFT project in the Philippines | Growth rate | 3rd | Superior growth performance than that of the existing strains | [106,109] | |
| Hainan Progift | Hainan Progift Aqua-Tech Cooperation Ltd. | 2005 | 5th generation of Nile tilapia from the Vietnam National Breeding Program, earlier obtained from the GIFT of the Philippines | Growth rate | 6th | Genetic growth improvement (60–90%) larger body weight at harvest) after six generations of multi-trait selection | [63] | |
| Egypt | GIANT | The WorldFish Center, Egypt | 2002 | Sourced from the river Nile | Growth and survival rates | 9th | The generation of the GIANT strain grew 28% faster than the commercial strain | [24,25,68,110] |
| Ghana | Akosombo strain | Water Research Institute (WRI) | 2002 | 11th generation of the GIFT strain imported from WorldFish Center in Malaysia | Growth and survival rates | 10th | The 10-year selection resulted in the Aksombo strain with a 30% faster growth rate than other farmed tilapia strains in the West African sub-region | [68,95,111] |
| Kenya | Sagana strain (SAG-F8) | National Aquaculture Research Development and Training Centre, Sagana, Kenya | 2010 | Private and government hatcheries and lakes Victoria and Turkana | Growth and survival rates and FCR | 8th | Faster growth rates, improved feed efficiency, and better survival rates | [20,51] |
| Norway | GenoMar Supreme (GST) Strain | GenoMar, a Norwegian company based in Oslo | 1999 | 10th GIFT generation | Growth and survival rates | 10th–13th | Increased genetic gain (20% per generation). An increase in the survival rate of about 11% per generation was also observed, resulting in the survival of at least 80% in the 13th generation | [13,21,97,103] |
| Bangladesh | GIFT | Bangladesh Fisheries Research Institute (BFRI) | 1998 | The GIFT strain from the Philippines | Growth rate | 6th | F6 generation progeny showed 32.66% higher growth than that of the average group of the GIFT strain (base population) | [112] |
| Vietnam | NOVIT 4 | Research Institute for Aquaculture No. 1 (RIA 1) | 1997 | 5th generation of the GIFT from the Philippines and Thailand tilapia | Growth rate | 8th | Growth improvement, well suited to production systems, with a 52% superior growth rate than the base population | [68,92,113] |
| GIFT | Research Institute for Aquaculture No. 2 (RIA 2) | 2006 | 10th generation of the GIFT strain developed in the Philippines | Harvest weight | 4th–6th | Genetic gains in harvest weight, ranging between 7 and 11% per generation over four to six generations | [67,68] | |
| Sri Lanka | GIFT | National Aquaculture Development Authority (NAQDA) of Sri Lanka | 2007 | GIFT from Malaysia | Growth and survival rates | 4th | Achieved 112% greater growth than the local strain and an 85.4% survival rate compared to 75.5% for the local strain | [68,114,115] |
| Brazil | GIFT | State University of Maringá, Brazil | 2005 | 8th generation of GIFT from Malaysia | Growth rate | 1st–9th | A substantial increase in the daily weight gain of about 3.3% per generation | [116,117] |
| Aquabel strain | Aquabel Pisciculture Station | 1996 | The Chitralada strain from the Asian Institute of Technology (AIT) | Growth and survival rates | N/S | Fast-growing strain with the higher survival rate (94.1%) | [118,119] | |
| AquaAmerica strain | AquaAmerica Company, Brazil | 2012 | GIFT previously crossed with the Chitralada and Bouaké strains | Growth rate | 3rd | Superior growth rates exhibited by the strain | [120] | |
| Thailand | Big Nin | Nam Sai Farms Co. Ltd. | N/S | The GIFT strain from the Philippines | Growth rate | N/S | * A fast growth rate of 2.5 g/day in ponds and 4.4 g/day in cages | [121] |
| GIFT | Manit Farm | 2008 | Thai stocks and from GIFT | Growth and survival rates | 9th | Enhanced harvest weight and survival rate | [68,91] | |
| Pathum Thani Fisheries Test and Research Center (Pathum Thani FTRC | 2000 | 9th generation GIFT from the WorldFish Center, Malaysia | Growth rate | 5th | Superior growth performance | [68,122] |
4. Existing Nile tilapia Genetic Resources, Seed Production, and Genetic Improvement Efforts in Uganda
4.1. Existing Nile tilapia Genetic Resources in Uganda
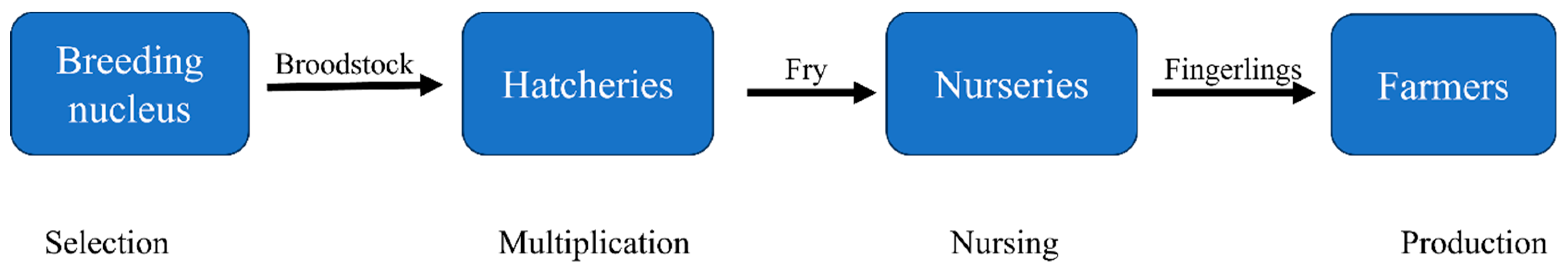
4.2. Nile tilapia Seed Production in Uganda
4.3. Genetic Improvement Efforts of Nile tilapia in Uganda
5. Policy and Legal Framework for Nile tilapia Breeding in Uganda
6. Future Perspectives
7. Conclusions Prioritized
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation; FAO: Rome, Italy, 2022; pp. 1–266. [Google Scholar]
- Verdegem, M.; Buschmann, A.H.; Latt, U.W.; Dalsgaard, A.J.T.; Lovatelli, A. The Contribution of Aquaculture Systems to Global Aquaculture Production. J. World Aquac. Soc. 2023, 54, 206–250. [Google Scholar] [CrossRef]
- Naylor, R.L.; Hardy, R.W.; Buschmann, A.H.; Bush, S.R.; Cao, L.; Klinger, D.H.; Little, D.C.; Lubchenco, J.; Shumway, S.E.; Troell, M. A 20-Year Retrospective Review of Global Aquaculture. Nature 2021, 591, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Mapfumo, B. Regional Review on Status and Trends in Aquaculture Development in Sub-Saharan Africa—2020. In FAO Fisheries and Aquaculture Circular N. 1232/4; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Zengeya, T.A.; Booth, A.J.; Chimimba, C.T. Broad Niche Overlap between Invasive Nile tilapia (Oreochromis niloticus, L. 1758) and Indigenous Congenerics in Southern Africa: Should We Be Concerned? Entropy 2015, 17, 4959–4973. [Google Scholar] [CrossRef]
- El-Sayed, A.F.M.; Fitzsimmons, K. From Africa to the World—The Journey of Nile tilapia. Rev. Aquac. 2023, 15, 6–21. [Google Scholar] [CrossRef]
- Geletu, T.T.; Zhao, J. Genetic Resources of Nile tilapia (Oreochromis niloticus, L. 1758) in Its Native Range and Aquaculture. Hydrobiologia 2023, 850, 2425–2445. [Google Scholar] [CrossRef]
- Eknath, A.E.; Hulata, G. Use and Exchange of Genetic Resources of Nile tilapia (Oreochromis niloticus, L. 1758). Rev. Aquac. 2009, 1, 197–213. [Google Scholar] [CrossRef]
- Trewavas, E. Tilapiine Fishes of the Genera Sarotherodon, Oreochromis, and Danakilia; British Museum (Natural History): London, UK, 1983; ISBN 0565008781. [Google Scholar]
- Fitzsimmons, K. Supply and Demand in Tilapia Markets and Vietnam’s Role. In Proceedings of the Aquaculture 2017 in Can Tho, Can Tho, Vietnam, 27 October 2017. [Google Scholar]
- Munguti, J.M.; Nairuti, R.; Iteba, J.O.; Obiero, K.O.; Kyule, D.; Opiyo, M.A.; Abwao, J.; Kirimi, J.G.; Outa, N.; Muthoka, M.; et al. Nile Tilapia (Oreochromis niloticus, L. 1758) Culture in Kenya: Emerging Production Technologies and Socio-Economic Impacts on Local Livelihoods. Aquac. Fish Fish. 2022, 2, 265–276. [Google Scholar] [CrossRef]
- Abaho, I.; Akoll, P.; Jones, C.L.W.; Masembe, C. Dietary Inclusion of Pine Pollen Alters Sex Ratio and Promotes Growth of Nile tilapia (Oreochromis niloticus, L. 1758). Aquac. Rep. 2022, 27, 101407. [Google Scholar] [CrossRef]
- El-Sayed, A.-F.M. Tilapia Culture, 2nd ed.; CABI Publishing: Wallingford, UK, 2019; pp. 1–293. [Google Scholar]
- Prabu, E.; Rajagopalsamy, C.B.T.; Ahilan, B.; Jeevagan, I.J.M.A.; Renuhadevi, M. Tilapia—An Excellent Candidate Species for World Aquaculture: A Review. Annu. Res. Rev. Biol. 2019, 31, 1–14. [Google Scholar] [CrossRef]
- Anane-Taabeah, G.; Frimpong, E.A.; Hallerman, E. Aquaculture-Mediated Invasion of the Genetically Improved Farmed Tilapia (Gift) into the Lower Volta Basin of Ghana. Diversity 2019, 11, 188. [Google Scholar] [CrossRef]
- Barría, A.; Peñaloza, C.; Papadopoulou, A.; Mahmuddin, M.; Doeschl-Wilson, A.; Benzie, J.A.H.; Houston, R.D.; Wiener, P. Genetic Differentiation Following Recent Domestication Events: A Study of Farmed Nile tilapia (Oreochromis niloticus, L. 1758) Populations. Evol. Appl. 2023, 16, 1220–1235. [Google Scholar] [CrossRef] [PubMed]
- Dey, M.M.; Eknath, A.E.; Sifa, L.; Hussain, M.G.; Thien, T.M.; Van Hao, N.; Aypa, S.; Pongthana, N. Performance and Nature of Genetically Improved Farmed Tilapia: A Bioeconomic Analysis. Aquac. Econ. Manag. 2000, 4, 83–106. [Google Scholar] [CrossRef]
- Abaho, I.; Masembe, C.; Akoll, P.; Jones, C.L.W. The Use of Plant Extracts to Control Tilapia Reproduction: Current Status and Future Perspectives. J. World Aquac. Soc. 2022, 53, 593–619. [Google Scholar] [CrossRef]
- Dey, M.M. The Impact of Genetically Improved Farmed Nile tilapia in Asia. Aquac. Econ. Manag. 2000, 4, 107–124. [Google Scholar] [CrossRef]
- Abwao, J.; Jung’a, J.; Barasa, J.E.; Kyule, D.; Opiyo, M.; Awuor, J.F.; Ogello, E.; Munguti, J.M.; Keya, G.A. Selective Breeding of Nile tilapia (Oreochromis niloticus, L. 1758): A Strategy for Increased Genetic Diversity and Sustainable Development of Aquaculture in Kenya. J. Appl. Aquac. 2021, 35, 237–256. [Google Scholar] [CrossRef]
- Ansah, Y.B.; Frimpong, E.A.; Hallerman, E.M. Genetically-Improved Tilapia Strains in Africa: Potential Benefits and Negative Impacts. Sustainability 2014, 6, 3697–3721. [Google Scholar] [CrossRef]
- Ragasa, C.; Charo-Karisa, H.; Rurangwa, E.; Tran, N.; Shikuku, K.M. Sustainable Aquaculture Development in Sub-Saharan Africa. Nat. Food 2022, 3, 92–94. [Google Scholar] [CrossRef] [PubMed]
- FAO. Lessons from Two Decades of Tilapia Genetic Improvement in Africa—Genetics in Aquaculture. A Case Study; FAO: Rome, Italy, 2023; pp. 1–32. [Google Scholar]
- Rezk, M.A.; Ponzoni, R.W.; Khaw, H.L.; Kamel, E.; Dawood, T.; John, G. Selective Breeding for Increased Body Weight in a Synthetic Breed of Egyptian Nile tilapia (Oreochromis niloticus, L. 1758): Response to Selection and Genetic Parameters. Aquaculture 2009, 293, 187–194. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Mohamed Nasr-Allah, A.; Charo-Karisa, H. Assessment of the Impact of Dissemination of Genetically Improved Abbassa Nile tilapia Strain (GIANT-G9) versus Commercial Strains in Some Egyptian Governorates. Aquac. Res. 2019, 50, 2951–2959. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Zaid, M.Y.A.; Khaw, H.L.; El-Naggar, G.O.; Ponzoni, R.W. Relative Performance of Two Nile tilapia (Oreochromis niloticus, L. 1758) Strains in Egypt: The Abbassa Selection Line and the Kafr El Sheikh Commercial Strain. Aquac. Res. 2013, 44, 508–517. [Google Scholar] [CrossRef]
- Tran, N.; Shikuku, K.M.; Rossignoli, C.M.; Barman, B.K.; Cheong, K.C.; Ali, M.S.; Benzie, J.A.H. Growth, Yield and Profitability of Genetically Improved Farmed Tilapia (GIFT) and Non-GIFT Strains in Bangladesh. Aquaculture 2021, 536, 736486. [Google Scholar] [CrossRef]
- Hamilton, M.G.; Lind, C.E.; Barman, B.K.; Velasco, R.R.; Danting, M.J.C.; Benzie, J.A.H. Distinguishing between Nile tilapia Strains Using a Low-Density Single-Nucleotide Polymorphism Panel. Front. Genet. 2020, 11, 594722. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, A.; Padiyar, A.; Datta, S.; Shikuku, K.M.; Mohan, V.; Trong, T.; Benzie, J.; Phillips, M. Dissemination and Scaling Strategy for Genetically Improved Farmed Tilapia (GIFT) in India, 2020–2030. Strategy; WorldFish Center: Penang, Malaysia, 2021; pp. 1–38. [Google Scholar]
- Hinrichsen, E.; Walakira, J.K.; Langi, S.; Ibrahim, N.A.; Tarus, V.; Badmus, O.; Baumüller, H. Prospects for Aquaculture Development in Africa: A Review of Past Performance to Assess Future Potential. Working Paper 211; ZEF: Bonn, Germany, 2022; pp. 1–45. [Google Scholar]
- Shikuku, K.M.; Ochenje, I.; Muthini, D. A Review of the Performance of Fish Seed Systems in Africa. Program Report; WorldFish Center: Penang, Malaysia, 2021; pp. 1–23. [Google Scholar]
- Adeleke, B.; Robertson-Andersson, D.; Moodley, G.; Taylor, S. Aquaculture in Africa: A Comparative Review of Egypt, Nigeria, and Uganda Vis-À-Vis South Africa. Rev. Fish. Sci. Aquac. 2021, 29, 167–197. [Google Scholar] [CrossRef]
- Kasozi, N.; Rutaisire, J.; Nandi, S.; Sundaray, J.K. A Review of Uganda and Indias Freshwater Aquaculture: Key Practices and Experience from Each Country. J. Ecol. Nat. 2017, 9, 15–29. [Google Scholar] [CrossRef]
- Mwanja, M.T.; Kityo, G.; Achieng, P.; Kasozi, J.M.; Sserwadda, M.; Namulawa, V.T. Growth Performance Evaluation of Four Wild Strains and One Current Farmed Strain of Nile tilapia in Uganda. Int. J. Fish. Aquat. Stud. 2016, 4, 594–598. [Google Scholar]
- Aanyu, M.; Denis, O.; Cassius, A.; Gertrude, A. Potential for Enhancing and Sustaining Commercial Aquaculture in Uganda: Producer Organizations, Contract Farming Schemes and Public-Private Partnerships. Int. J. Fish. Aquat. Stud. 2020, 8, 258–264. [Google Scholar]
- Abaho, I.; Zaabwe, T.; Izaara, A.; Kasigwa, N.H.; Mushabe, N.; Byenkya, S.; Nkambo, M.; Baguma, D.S.; Hafashimana, L.N.D.; Efitre, J. Effect of Stocking Density on Growth and Survival of Nile tilapia (Oreochromis niloticus, L. 1758) under Cage Culture in Lake Albert, Uganda. Int. J. Fish. Aquac. 2020, 12, 26–35. [Google Scholar] [CrossRef]
- FAO. Fisheries and Aquaculture—National Aquaculture Sector Overview—Uganda. Available online: https://www.fao.org/fishery/en/countrysector/ug/en?lang=en (accessed on 17 June 2023).
- MAAIF. National Fisheries and Aquaculture Policy. Ministry of Agriculture, Animal Industry and Fisheries; FAO: Kampala, Uganda, 2017; pp. 1–51.
- Musinguzi, L.; Lugya, J.; Rwezawula, P.; Kamya, A.; Nuwahereza, C.; Halafo, J.; Kamondo, S.; Njaya, F.; Aura, C.; Shoko, A.P.; et al. The Extent of Cage Aquaculture, Adherence to Best Practices and Reflections for Sustainable Aquaculture on African Inland Waters. J. Great Lakes Res. 2019, 45, 1340–1347. [Google Scholar] [CrossRef]
- Kwikiriza, G.; Barekye, A.; Aheisibwe, A.; Byakora, E. Comparative Growth Performance and Proximate Nutrient Composition of Three Local Strains of Nile tilapia (Oreochromis niloticus, L. 1758) Collected from Different Locations in Uganda. Fish. Aqua. J. 2017, 8, 226. [Google Scholar] [CrossRef]
- Atukunda, G.; Atekyereza, P.; Walakira, J.; State, A.E. Increasing Farmers’ Access to Aquaculture Extension Services: Lessons from Central and Northern Uganda. Uganda J. Agric. Sci. 2020, 20, 49–68. [Google Scholar] [CrossRef]
- Ondhoro, C.; Kagolola, I.; Osipa, G.; Odong, R.; Kubiriza, G.; Owere, L. Growth and Economic Evaluation of Different Fish Species for Culture in Uganda’s Mid Altitude Areas Using Local Feeds. J. Appl. Sci. 2023, 23, 71–80. [Google Scholar] [CrossRef]
- Walakira, J.; Akoll, P.; Engole, M.; Sserwadda, M.; Nkambo, M.; Namulawa, V.; Kityo, G.; Musimbi, F.; Abaho, I.; Kasigwa, H.; et al. Common Fish Diseases and Parasites Affecting Wild and Farmed Tilapia and Catfish in Central and Western Uganda. Uganda J. Agric. Sci. 2015, 15, 113–125. [Google Scholar] [CrossRef]
- Abaho, I.; Gabriel, N.N.; Izaara, A.A. Use of Plant Extracts to Control Reproduction in Tilapia Production Systems: An Emerging Eco-Friendly Innovation. In Emerging Sustainable Aquaculture Innovations in Africa. Sustainability Sciences in Asia and Africa; Gabriel, N.N., Omoregie, E., Abasubong, K.P., Eds.; Springer: Singapore, 2023; pp. 167–196. [Google Scholar]
- Kwikiriza, G.; Yegon, M.J.; Byamugisha, N.; Beingana, A.; Atukwatse, F.; Barekye, A.; Nattabi, J.K.; Meimberg, H. Morphometric Variations of Nile tilapia (Oreochromis niloticus, L. 1758) Local Strains Collected from Different Fish Farms in South Western Highland Agro-Ecological Zone (SWHAEZ), Uganda: Screening Strains for Aquaculture. Fishes 2023, 8, 217. [Google Scholar] [CrossRef]
- Mwanja, M.; Ondhoro, C.; Sserwada, M.; Achieng, P.; Ddungu, R.; Mwanja, W. Morphological Variation of Nile tilapia Populations from Major Water Bodies of Uganda. Uganda J. Agric. Sci. 2016, 17, 21–32. [Google Scholar] [CrossRef]
- Mwanja, M.; Rutaisire, J.; Ondhoro, C.; Ddungu, R.; Aruho, C. Current Fish Hatchery Practices in Uganda: The Potential for Future Investment. Int. J. Fish. Aquat. Stud. 2015, 2, 224–232. [Google Scholar]
- Gupta, M.V.; Acosta, B.O. From Drawing Board to Dining Table: The Success Story of the GIFT Project. NAGA WorldFish Center Quarterly; WorldFish Center: Penang, Malaysia, 2004; pp. 1–14. [Google Scholar]
- Khan, S.; Hossain, M.; Hossain, M. Production and Economics of GIFT Strain of Tilapia (Oreochromis niloticus, L. 1758) in Small Seasonal Ponds. Progress. Agric. 2008, 19, 97–104. [Google Scholar] [CrossRef]
- Das, U.N.; Jana, P.; Pahari, T.; Roy, A.; Dhara, K. Comparative Study on Growth Performance and Economics between GIFT and Local Varieties of Oreochromis niloticus (L. 1758) in Pond Culture Systems. Int. J. Pure Appl. Biosci. 2018, 6, 603–610. [Google Scholar] [CrossRef]
- Abwao, J.; Kyule, D.; Junga, J.O.; Barasa, J.E.; Sigana, D.A. On-farm Growth Performance of Different Strains of tilapia (Oreochromis niloticus, L. 1758) Reared in Earthen Ponds. Aquac. Fish Fish. 2023, 3, 247–255. [Google Scholar] [CrossRef]
- Dickson, M.; Nasr-Allah, A.; Kenawy, D.; Kruijssen, F. Increasing Fish Farm Profitability through Aquaculture Best Management Practice Training in Egypt. Aquaculture 2016, 465, 172–178. [Google Scholar] [CrossRef]
- FAO. Fishery and Aquaculture Statistics. Global Aquaculture Production 1950–2021 (FishStatJ). Available online: https://www.fao.org/fishery/en/statistics/software/fishstatj (accessed on 19 April 2024).
- Ambekar, E.; Madan, M.D.; Morten, R.; Bjarne, G.; Abella, T.A.; Ruben, C.S.; Tayamen, M.M.; Reyes, R.A.; Hans, B.B. Selective Breeding of Nile Tilapia for Asia. In Proceedings of the 6th World Congress of Genetics Applied to Livestock Production, Armidale, Australia, 11–16 January 1998; pp. 89–96. [Google Scholar]
- Ponzoni, R.W.; Nguyen, N.H.; Khaw, H.L.; Hamzah, A.; Bakar, K.R.A.; Yee, H.Y. Genetic Improvement of Nile tilapia (Oreochromis niloticus, L. 1758) with Special Reference to the Work Conducted by the World Fish Center with the GIFT Strain. Rev. Aquac. 2011, 3, 27–41. [Google Scholar] [CrossRef]
- Yáñez, J.M.; Joshi, R.; Yoshida, G.M. Genomics to Accelerate Genetic Improvement in Tilapia. Anim. Genet. 2020, 51, 658–674. [Google Scholar] [CrossRef]
- Mwanja, M.T.; Mwanja, W.W. Escape of Farmed Tilapiines into the Wild and Entry of Wild Forms in Fishponds, and the Possible Interactions between Wild and Farmed Tilapiines from a Sample of Smallholder Farms in Central Uganda. Afr. J. Ecol. 2009, 47, 469–475. [Google Scholar] [CrossRef]
- Gjedrem, T.; Robinson, N.; Rye, M. The Importance of Selective Breeding in Aquaculture to Meet Future Demands for Animal Protein: A Review. Aquaculture 2012, 350–353, 117–129. [Google Scholar] [CrossRef]
- Liu, S.; Wang, C.; Li, C. Progress in Aquaculture Genetics and Breeding in China. J. World Aquac. Soc. 2018, 49, 272–276. [Google Scholar] [CrossRef]
- Kang, X.; Wei, D.; Jun, X.; Min, T.; Chun, Z.; Yun, L.; ShaoJun, L. Development and Application of Biological Technologies in Fish Genetic Breeding. Sci. China Life Sci. 2015, 58, 187–201. [Google Scholar] [CrossRef]
- Brummett, R.E.; Ponzoni, R.W. Concepts, Alternatives, and Environmental Considerations in the Development and Use of Improved Strains of Tilapia in African Aquaculture. Rev. Fish. Sci. 2009, 17, 70–77. [Google Scholar] [CrossRef]
- Gjedrem, T.; Robinson, N. Advances by Selective Breeding for Aquatic Species: A Review. Agric. Sci. 2014, 5, 1152–1158. [Google Scholar] [CrossRef]
- Thodesen, J.; Rye, M.; Wang, Y.X.; Yang, K.S.; Bentsen, H.B.; Gjedrem, T. Genetic Improvement of Tilapias in China: Genetic Parameters and Selection Responses in Growth of Nile tilapia (Oreochromis niloticus, L. 1758) after Six Generations of Multi-Trait Selection for Growth and Fillet Yield. Aquaculture 2011, 322–323, 51–64. [Google Scholar] [CrossRef]
- Symonds, J.E.; Clarke, S.M.; King, N.; Walker, S.P.; Blanchard, B.; Sutherland, D.; Roberts, R.; Preece, M.A.; Tate, M.; Buxton, P.; et al. Developing Successful Breeding Programs for New Zealand Aquaculture: A Perspective on Progress and Future Genomic Opportunities. Front. Genet. 2019, 10, 27. [Google Scholar] [CrossRef]
- Gjedrem, T. Genetic Improvement for the Development of Efficient Global Aquaculture: A Personal Opinion Review. Aquaculture 2012, 344–349, 12–22. [Google Scholar] [CrossRef]
- Rye, M. Current Status and Prospects for the Application of Genetic Improvement in Aquaculture Species. In Proceedings of the 9th Biennial Symposium of the Brazilian Society of Animal Breeding, João Pessoa, Brazil, 20–22 June 2012; pp. 1–10. [Google Scholar]
- Komen, H.; Trong, T.Q. Nile Tilapia Genetic Improvement: Achievements and Future. In Proceedings of the 10th International Symposium on Tilapia in Aquaculture—ISTA10, Jerusalem, Israel, 1–10 October 2014; pp. 1–9. [Google Scholar]
- Neira, R. Breeding in Aquaculture Species: Genetic Improvement Programs in Developing Countries. In Proceedings of the 9th World Congress on Genetics Applied to Livestock Production, Leipzig, Germany, 1–6 August 2010; pp. 1–8. [Google Scholar]
- Gjedrem, T. The First Family-Based Breeding Program in Aquaculture. Rev. Aquac. 2010, 2, 2–15. [Google Scholar] [CrossRef]
- Rye, M.; Gjerde, B.; Gjedrem, T. Genetic Improvement Programs for Aquaculture Species in Developed Countries. In Proceedings of the 9th World Congress on Genetics Applied to Livestock Production, Leipzig, Germany, 1–6 August 2010; pp. 1–9. [Google Scholar]
- Ponzoni, R.W.; Nguyen, N.H.; Khaw, H.L. Investment Appraisal of Genetic Improvement Programs in Nile tilapia (Oreochromis niloticus, L. 1758). Aquaculture 2007, 269, 187–199. [Google Scholar] [CrossRef]
- Lind, C.E.; Ponzoni, R.W.; Nguyen, N.H.; Khaw, H.L. Selective Breeding in Fish and Conservation of Genetic Resources for Aquaculture. Reprod. Domest. Anim. 2012, 47, 255–263. [Google Scholar] [CrossRef]
- Ponzoni, R.W.; Nguyen, N.H.; Khaw, H.L.; Ninh, N.H. Accounting for Genotype by Environment Interaction in Economic Appraisal of Genetic Improvement Programs in Common carp (Cyprinus carpio). Aquaculture 2008, 285, 47–55. [Google Scholar] [CrossRef]
- Eze, F. Marker-Assisted Selection in Fish: A Review. Asian J. Fish. Aquat. 2019, 3, 1–11. [Google Scholar] [CrossRef]
- Abdelrahman, H.; ElHady, M.; Alcivar-Warren, A.; Allen, S.; Al-Tobasei, R.; Bao, L.; Beck, B.; Blackburn, H.; Bosworth, B.; Buchanan, J.; et al. Aquaculture Genomics, Genetics and Breeding in the United States: Current Status, Challenges, and Priorities for Future Research. BMC Genom. 2017, 18, 191. [Google Scholar] [CrossRef]
- Song, H.; Dong, T.; Yan, X.; Wang, W.; Tian, Z.; Sun, A.; Dong, Y.; Zhu, H.; Hu, H. Genomic Selection and Its Research Progress in Aquaculture Breeding. Rev. Aquac. 2022, 15, 274–291. [Google Scholar] [CrossRef]
- Houston, R.D.; Bean, T.P.; Macqueen, D.J.; Gundappa, M.K.; Jin, Y.H.; Jenkins, T.L.; Selly, S.L.C.; Martin, S.A.M.; Stevens, J.R.; Santos, E.M.; et al. Harnessing Genomics to Fast-Track Genetic Improvement in Aquaculture. Nat. Rev. Genet. 2020, 21, 389–409. [Google Scholar] [CrossRef]
- Olesen, I.; Gjedrem, T.; Bentsen, H.B.; Gjerde, B.; Rye, M. Breeding Programs for Sustainable Aquaculture. J. Appl. Aquac. 2003, 13, 179–204. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Whatmore, P.; Miller, A.; Knibb, W. Quantitative Genetic Properties of Four Measures of Deformity in Yellowtail Kingfish (Seriola lalandi, V. 1833). J. Fish. Dis. 2016, 39, 217–228. [Google Scholar] [CrossRef]
- Ponzoni, R.W.; Acosta, B.O.; Ponniah, A.G. Development of Aquatic Animal Genetic Improvement and Dissemination Programs: Current Status and Action Plans; WorldFish Center: Penang, Malaysia, 2006; pp. 1–57. [Google Scholar]
- Gjedrem, T.; Rye, M. Selection Response in Fish and Shellfish: A Review. Rev. Aquac. 2018, 10, 168–179. [Google Scholar] [CrossRef]
- Teletchea, F. Fish Domestication in Aquaculture: 10 Unanswered Questions. Anim. Front. 2021, 11, 87–91. [Google Scholar] [CrossRef]
- Kristjánsson, Ó.H.; Gjerde, B.; Ødegård, J.; Lillehammer, M. Quantitative Genetics of Growth Rate and Filet Quality Traits in Atlantic Salmon Inferred from a Longitudinal Bayesian Model for the Left-Censored Gaussian Trait Growth Rate. Front. Genet. 2020, 11, 573265. [Google Scholar] [CrossRef]
- Bentsen, H.B.; Gjerde, B.; Eknath, A.E.; de Vera, M.S.P.; Velasco, R.R.; Danting, J.C.; Dionisio, E.E.; Longalong, F.M.; Reyes, R.A.; Abella, T.A.; et al. Genetic Improvement of Farmed Tilapias: Response to Five Generations of Selection for Increased Body Weight at Harvest in Oreochromis niloticus (L. 1758) and the Further Impact of the Project. Aquaculture 2017, 468, 206–217. [Google Scholar] [CrossRef]
- Ponzoni, R.W.; Nguyen, N.H.; Khaw, H.L.; Rodriguez, B.M., Jr. Considerations about Effective Dissemination of Improved Strains. Working Paper 2012-47; WorldFish Center: Penang, Malaysia, 2012; pp. 1–15. [Google Scholar]
- Tave, D. Inbreeding and Broodstock Management. Fisheries Technical Paper. No. 392; FAO: Rome, Italy, 1999; pp. 1–122. [Google Scholar]
- Kajungiro, R.A.; Mapenzi, L.L.; Nyinondi, C.S.; Haldén, A.N.; Mmochi, A.J.; Chacha, M.; Mtolera, M.S.; Lamtane, H.A.; Jan De Koning, D. The Need of a Structured Tilapia Breeding Program in Tanzania to Enhance Aquaculture Production: A Review. Tanzan J. Sci. 2019, 45, 355–371. [Google Scholar]
- Bentsen, H.B.; Olesen, I. Designing Aquaculture Mass Selection Programs to Avoid High Inbreeding Rates. Aquaculture 2002, 204, 349–359. [Google Scholar] [CrossRef]
- Bolivar, R.B.; Sayco, V.M.R.; Jimenez, T.E.B.; Argueza, B.R.L.; Bolivar, H.L.; Dadag, L.B.; Taduan, A.G.; Borski, R.J. Broodstock Seed Quality and Fingerling Production Systems Rearing for Nile Tilapia in the Philippines. Technical Report; Oregon State University: Corvallis, OR, USA, 2009; pp. 234–254. [Google Scholar]
- Ingthamjitr, S.; Paankhao, N.; Paankhao, S.; Promsri, K. Effect of Maternal Age on Reproductive Reproductive Performance and Growth of Nile tilapia (Oreochromis niloticus, L. 1758) Fry. J. Fish. Environ. 2017, 41, 28–36. [Google Scholar]
- Thodesen, J.; Rye, M.; Lozano, C.; Avitua, V.S.; Johansen, H.; Segovia, H.; Ospina, J. Selective Breeding of Tilapia: Status and Prospects; Spring Genetics: Fortaleza, Brazil, 2016; pp. 1–23. [Google Scholar]
- Ponzoni, R.W.; Khaw, L.; Yee, H.Y. GIFT: The Story Since Leaving ICLARM (Now Known as The WorldFish Center). Socioeconomic, Access and Benefit Sharing and Dissemination Aspects. FNI Report 14/2010; Fridtjof Nansen Institute: Lysaker, Norway, 2010; pp. 1–47. [Google Scholar]
- Eknath, A.E.; Tayamen, M.M.; Palada-de Vera, M.S.; Danting, J.C.; Reyes, R.A.; Dionisio, E.E.; Capili, J.B.; Bolivar, H.L.; Abella, T.A.; Circa, A.V.; et al. Genetic Improvement of Farmed Tilapias: The Growth Performance of Eight Strains of Oreochromis niloticus (L. 1758) Tested in Different Farm Environments. In Genetics in Aquaculture; Elsevier: Amsterdam, The Netherlands, 1993; pp. 171–188. [Google Scholar] [CrossRef]
- Pulin, R.S.V.; Eknath, A.E.; Gjedrem, T.; Tayamen, M.M.; Macaranas, J.E.; Abella, T.A. The Genetic Improvement of Farmed Tilapias (GIFT) Project: The Story So Far. NAGA ICLARM] Quarterly; Contributions No 721; ICLARM: Manila, Philippines, 1991; pp. 3–6. [Google Scholar]
- Sanda, M.K.; Metcalfe, N.B.; Mable, B.K. The Potential Impact of Aquaculture on the Genetic Diversity and Conservation of Wild Fish in Sub-Saharan Africa. Aquat. Conserv. Mar. Freshw. Ecosyst. 2024, 34, e4105. [Google Scholar] [CrossRef]
- Hamzah, A.; Ponzoni, R.W.; Nguyen, N.H.; Khaw, H.L.; Yee, H.Y.; Nor, S.A.M. Performance of the Genetically Improved Farmed Tilapia (GIFT) Strain over Ten Generations of Selection in Malaysia. Pertanika J. Trop Agric. Sci. 2014, 37, 411–429. [Google Scholar]
- Ordoñez, J.F.F.; Santos, M.D.; Tayamen, M.M. Tilapia Genetic R&D in the Philippines: Challenges and Prospects for Future Development; Southeast Asian Fisheries Development Center: Bangkok, Thailand, 2014; pp. 30–44. [Google Scholar]
- Eknath, A.E.; Acosta, B.O. Genetic Improvement of Farmed Tilapias (GIFT) Project: Final Report, March 1988 to December 1997; ICLARM: Manila, Philippines, 1998; pp. 1–372. [Google Scholar]
- Eknath, A.E.; Bentsen, H.B.; Ponzoni, R.W.; Rye, M.; Nguyen, N.H.; Thodesen, J.; Gjerde, B. Genetic Improvement of Farmed Tilapias: Composition and Genetic Parameters of a Synthetic Base Population of Oreochromis niloticus (L. 1758) for Selective Breeding. Aquaculture 2007, 273, 1–14. [Google Scholar] [CrossRef]
- Acosta, B.O.; Sevilleja, R.C.; Gupta, M.V. Public and Private Partnerships in Aquaculture. A Case Study on Tilapia Research and Development; WorldFish Center: Penang, Malaysia, 2006; pp. 1–72. [Google Scholar]
- Bolivar, R.B.; Newkirk, G.F. Response to within Family Selection for Body Weight in Nile tilapia (Oreochromis niloticus, L. 1758) Using a Single-Trait Animal Model. Aquaculture 2002, 204, 371–381. [Google Scholar] [CrossRef]
- Bolivar, R.B. Estimation of Response to Within-Family Selection for Growth in Nile tilapia (Oreochromis niloticus, L. 1758). Ph.D. Thesis, Dalhousie University, Halifax, NS, Canada, 1998; pp. 1–184. [Google Scholar]
- El-Sayed, A.M. Tilapia Culture, 1st ed.; CABI Publishing, CAB International: Oxford, UK, 2006; pp. 1–293. [Google Scholar]
- Tayamen, M.M. Nationwide Dissemination of GET EXCEL Tilapia in the Philippines. In Proceedings of the 6th International Symposium on Tilapia in Aquaculture, Manila, Philippines, 12–16 September 2004; pp. 74–79. [Google Scholar]
- ADB. An Impact Evaluation of the Development of Genetically Improved Farmed Tilapia and Their Dissemination in Selected Countries; Operations Evaluation Department; Asian Development Bank: Mandaluyong, Philippines, 2005; pp. 1–137. [Google Scholar]
- FAO. Aquaculture Development. Development of Aquatic Genetic Resources. A Framework of Essential Criteria. TG5 Suppl. 9; FAO: Rome, Italy, 2018; pp. 1–88. [Google Scholar]
- Li, S.-F.; Cai, W.-Q. Contribution of Genetic Improved Strains to Chinese Tilapia Industry. In Proceedings of the 8th International Symposium on Tilapia in Aquaculture, Cairo, Egypt, 12–14 October 2008; pp. 213–218. [Google Scholar]
- Dey, M.M.; Gupta, M.V. Socioeconomics of Disseminating Genetically Improved Nile tilapia in Asia: An Introduction. Aquac. Econ. Manag. 2000, 4, 5–11. [Google Scholar] [CrossRef]
- Li, L.; Dong, Z.; Su, S.; Xu, P.; Liang, Z.; Ma, L.; Liu, W.; Zhang, J. Morphological Variation and Mathematical Analysis of Effects of Morphological Traits on Body-Weight of GIFT Tilapia after 3 Generations of Breeding. J. Fish. China 2012, 36, 489–496. [Google Scholar] [CrossRef]
- Said, M. Reproductive Performance and Early Growth of Three Strains of Nile tilapia (Oreochromis niloticus, L. 1758) in Egypt: Abbassa, Kafr El Sheikh, and Manzala. J. Anim. Poul. Fish. Prod. 2016, 5, 25–32. [Google Scholar] [CrossRef]
- Trinh, T.Q.; Agyakwah, S.K.; Khaw, H.L.; Benzie, J.A.H.; Attipoe, F.K.Y. Performance Evaluation of Nile tilapia (Oreochromis niloticus, L. 1758) Improved Strains in Ghana. Aquaculture 2021, 530, 735938. [Google Scholar] [CrossRef]
- Hussain, M.G. A Future for the Tilapia in Bangladesh. AQUA Culture Industry Review; AsiaPacific Magazine: Mymensingh, Bangladesh, 2009; pp. 38–40. [Google Scholar]
- Luan, T.D.; Thien, T.M.; Luu, L.T.; Hoa, N.T. Breeding Programme and Nationwide Dissemination of NOVIT 4 Tilapia in Vietnam. In Proceedings of the 25th Anniversary Scientific Conference of NARA on Tropical Aquatic Research towards Sustainable Development, 2007, Research Institute for Aquaculture No.1 (RIA.1), Ninh, Vietnam, 15–16 February; 2007; p. 17. [Google Scholar]
- Nguyen, N.H. Genetic Improvement for Important Farmed Aquaculture Species with a Reference to Carp, Tilapia and Prawns in Asia: Achievements, Lessons, and Challenges. Fish Fish. 2016, 17, 483–506. [Google Scholar] [CrossRef]
- Nguyen, H.N.; Ponzoni, R.W.; Chandrasoma, J.; Herath, H.; Wathurawadu, K. GIFT Tilapia Raise Culture Efficiency in Sri Lanka.Global Aquaculture Advocate; WorldFish Center: Penang, Malaysia, 2011; pp. 32–33. [Google Scholar]
- Yoshida, G.M.; de Oliveira, C.A.L.; Campos, E.C.; Todesco, H.; Araújo, F.C.T.; Karin, H.M.; Zardin, A.M.S.O.; Bezerra Júnior, J.S.; Filho, L.A.; Vargas, L.; et al. A Breeding Program for Nile Tilapia in Brazil: Results from Nine Generations of Selection to Increase the Growth Rate in Cages. J. Anim. Breed. Genet. 2022, 139, 127–135. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, C.A.L.; Ribeiro, R.P.; Yoshida, G.M.; Kunita, N.M.; Rizzato, G.S.; de Oliveira, S.N.; dos Santos, A.I.; Nguyen, N.H. Correlated Changes in Body Shape after Five Generations of Selection to Improve Growth Rate in a Breeding Program for Nile tilapia (Oreochromis niloticus, L. 1758) in Brazil. J. Appl. Genet. 2016, 57, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Aquabel A Premium Tilapia Brand with 25 Years of Product Development in Brazil. Available online: https://genomar.com/products-brands/ (accessed on 22 April 2024).
- Almeida, D.B.; da Costa, M.A.P.; Bassini, L.N.; Calabuig, C.I.P.; Moreira, C.G.A.; Rodrigues, M.D.N.; Pérez, H.J.; Tavares, R.A.; Varela, A.S.; Moreira, H.L.M. Reproductive Performance in Female Strains of Nile tilapia (Oreochromis niloticus, L. 1758). Aquac. Int. 2013, 21, 1291–1300. [Google Scholar] [CrossRef]
- Carvalhoa, J.C.; Filhoa, C.R.A.C.; Oliveirab, C.A.L.; Ribeirob, R.P.; Seraphima, G.N.; Silvaa, A.L.N.; Kinjo Juniora, G.N.; Laicec, L.M.; Fantinid, L.E.; Lopera-Barreroe, N.M.; et al. Growth Curve of Nile tilapia from Different Families of the AquaAmérica Variety. Braz. J. Biol. 2022, 82, e243534. [Google Scholar] [CrossRef]
- Ataguba, G.A.; Ikongbeh, A.O.; Garba, A.A. Reciprocal Crosses of Two Improved Strains of Nile tilapia: Implications on Reproductive and Growth Traits. J. Res. Agric. Anim. Sci. 2020, 7, 68–73. [Google Scholar]
- Sukmanomon, S.; Kamonrat, W.; Poompuang, S.; Nguyen, T.T.T.; Bartley, D.M.; May, B.; Na-Nakorn, U. Genetic Changes, Intra- and Inter-Specific Introgression in Farmed Nile tilapia (Oreochromis niloticus, L. 1758) in Thailand. Aquaculture 2012, 324–325, 44–54. [Google Scholar] [CrossRef]
- Tibihika, P.D.; Meimberg, H.; Curto, M. Understanding the Translocation Dynamics of Nile tilapia (Oreochromis niloticus, L. 1758) and Its Ecological Consequences in East Africa. Afr. Zool. 2022, 57, 171–179. [Google Scholar] [CrossRef]
- Bradbeer, S.J.; Harrington, J.; Watson, H.; Warraich, A.; Shechonge, A.; Smith, A.; Tamatamah, R.; Ngatunga, B.P.; Turner, G.F.; Genner, M.J. Limited Hybridization between Introduced and Critically Endangered Indigenous Tilapia Fishes in Northern Tanzania. Hydrobiologia 2019, 832, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Deines, A.M.; Bbole, I.; Katongo, C.; Feder, J.L.; Lodge, D.M. Hybridisation between Native Oreochromis Species and Introduced Nile tilapia (Oreochromis niloticus, L. 1758) in the Kafue River, Zambia. Afr. J. Aquat. Sci. 2014, 39, 23–34. [Google Scholar] [CrossRef]
- Kariuki, J.; Tibihika, P.D.; Curto, M.; Alemayehu, E.; Winkler, G.; Meimberg, H. Application of Microsatellite Genotyping by Amplicon Sequencing for Delimitation of African Tilapiine Species Relevant for Aquaculture. Aquaculture 2021, 537, 736501. [Google Scholar] [CrossRef]
- Muhlfeld, C.C.; Kalinowski, S.T.; McMahon, T.E.; Taper, M.L.; Painter, S.; Leary, R.F.; Allendorf, F.W. Hybridization Rapidly Reduces Fitness of a Native Trout in the Wild. Biol. Lett. 2009, 5, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Tibihika, P.D.; Curto, M.; Alemayehu, E.; Waidbacher, H.; Masembe, C.; Akoll, P.; Meimberg, H. Molecular Genetic Diversity and Differentiation of Nile tilapia (Oreochromis niloticus, L. 1758) in East African Natural and Stocked Populations. BMC Evol. Biol. 2020, 20, 16. [Google Scholar] [CrossRef]
- Robledo, D.; Ogwang, J.; Byakora, E.; Schulze, J.N.; Benda, K.K.; Fraslin, C.; Salisbury, S.; Solimo, M.; Mayega, J.F.; Peter, B.; et al. Genetic Diversity and Population Structure of Farmed and Wild Nile tilapia (Oreochromis niloticus, L. 1758) in Uganda: The Potential for Aquaculture Selection and Breeding Programs. Genomics 2024, 116, 110781. [Google Scholar] [CrossRef]
- Bolman, B.; Van Duijn, A.P.; Rutaisire, J. Review and Analysis of Small-Scale Aquaculture Production in East Africa. Part 4 Uganda, Report WCDI-18-021; Wageningen University and Research: Wageningen, Netherlands, 2018; pp. 1–53. [Google Scholar]
- Fessehaye, Y.; Komen, H.; Rezk, M.A.; van Arendonk, J.A.M.; Bovenhuis, H. Effects of Inbreeding on Survival, Body Weight and Fluctuating Asymmetry (FA) in Nile tilapia (Oreochromis niloticus, L. 1758). Aquaculture 2007, 264, 27–35. [Google Scholar] [CrossRef]
- Khan, M.G.Q. Marker-Assisted Selection in Enhancing Genetically Male Nile tilapia (Oreochromis niloticus, L. 1758) Production. Ph.D. Thesis, University of Stirling, Scotland, UK, 2011; pp. 1–217. [Google Scholar]
- Tibihika, P.D.; Aruho, C.; Namulawa, V.; Ddungu, R.; Atukunda, G.; Aanyu, M.; Nkambo, M.; Vijayan, T.; Kwikiriza, G.; Curto, M.; et al. Unlocking Nile tilapia (Oreochromis niloticus, L. 1758) Selective Breeding Programmes in Uganda through Geographical Genetic Structure Mapping. Aquac. Fish Fish. 2024, 4, e197. [Google Scholar] [CrossRef]
- MAAIF. Ministry of Agriculture, Animal Industry and Fisheries Performance Report. Financial Year 2017/2018; MAAIF: Entebbe, Uganda, 2018; pp. 1–192.
- Sae-Lim, P.; Kause, A.; Mulder, H.A.; Martin, K.E.; Barfoot, A.J.; Parsons, J.E.; Davidson, J.; Rexroad, C.E.; Van Arendonk, J.A.M.; Komen, H. Genotype-by-Environment Interaction of Growth Traits in Rainbow Trout (Oncorhynchus mykiss): A Continental Scale Study. J. Anim. Sci. 2013, 91, 5572–5581. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Hamzah, A.; Thoa, N.P. Effects of Genotype by Environment Interaction on Genetic Gain and Genetic Parameter Estimates in Red tilapia (Oreochromis Spp.). Front. Genet. 2017, 8, 82. [Google Scholar] [CrossRef]
- MAAIF. Department of Fisheries Resource (DiFR) Annual Report 2010/2011; Ministry of Agriculture Animal Industry and Fisheries (MAAIF): Entebbe, Uganda, 2012; pp. 1–48.
- MAAIF. The Fish (Aquaculture) Rules, 2003. Statutory Instruments 2003 No.81; Parliament of Uganda: Kampala, Uganda, 2003; pp. 1–21.
- MAAIF. The Fish (Aquaculture) Rules, 2022. Statutory Instruments 2022 No.97; Parliament of Uganda: Kampala, Uganda, 2022; pp. 5757–5819.
- MAAIF. The Fisheries and Aquaculture Act, 2022; Ministry of Agriculture, Animal Industry and Fisheries: Kampala, Uganda, 2022; pp. 1–81.
- MAAIF. The Animal Breeding Act, 2001; Food and Agriculture Organization of the United Nations: Kampala, Uganda, 2001; pp. 1–38.
- Janssen, K.; Saatkamp, H.; Komen, H. Cost-Benefit Analysis of Aquaculture Breeding Programs. Genet. Sel. Evol. 2018, 50, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.F.A.; Alvarenga, É.R.; Alves, G.F.O.; Manduca, L.G.; Toral, F.L.B.; Valente, B.D.; Silva, M.A.; Rosa, G.J.M.; Turra, E.M. Genotype by Environment Interaction across Time for Nile tilapia, from Juvenile to Finishing Stages, Reared in Different Production Systems. Aquaculture 2019, 513, 734429. [Google Scholar] [CrossRef]
- De Araújo, F.C.T.; de Oliveira, A.L.C.; Campos, C.E.; Yoshida, M.G.; Lewandowski, V.; Todesco, H.; Nguyen, H.N.; Ribeiro, P.R. Effects of Genotype × Environment Interaction on the Estimation of Genetic Parameters and Gains in Nile tilapia. J. Appl. Genet. 2020, 61, 575–580. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Nader, M.M.; Salem, H.M.; El-Tahan, A.M.; Soliman, M.; Khafaga, A.F. Effect of Environmental Factors on Growth Performance of Nile tilapia (Oreochromis niloticus, L. 1758). Int. J. Biometeorol. 2022, 66, 2183–2194. [Google Scholar] [CrossRef]

| District | Sub County/Division | Name | Ownership |
|---|---|---|---|
| Wakiso | Ssisa | Aquaculture Research and Development Centre-Kajjansi | Public |
| Katabi | Tende Innovation Farm | Private | |
| Wakiso | Aquafarm Fish Farm | Private | |
| Katabi | Victoria Treasures | Private | |
| Mende | Kakunyu Agricultural Farm | Private | |
| Nsangi | Nsangi Fish Farm | Private | |
| Matugga | Matugga Fish Farm Limited | Private | |
| Sissa | Kaama Fish Farm | Private | |
| Kasanje | Nina Fish Farm | Private | |
| Kira | Kireka Fish Farm and Hatchery | Private | |
| Mukono | Mukono municipality | Mukono Zonal Aquaculture Research and Development Institute | Public |
| Goma | Manjorie Fish Farm | Private | |
| Mpaata | Lake Victoria Precious Fish Farm Ltd. | Private | |
| Buikwe | Buikwe Rural | Ferdsult Fish Project | Private |
| Nyenga | Yalelo fish farm | Private | |
| Ngogwe | Agrofish Farm | Private | |
| Luwero | Zirobwe | Sanga Fish Farm | Private |
| Masaka City | Kingo | Ssenya Fish Farm | Private |
| Kyotera | Kirumba | Pokino Multipurpose Fish Project | Private |
| Mbarara | Kakiika Division | Mbarara Zonal Aquaculture Research and Development Institute | Public |
| Rwampara | Bugamba | Dejafa Farm | Private |
| Bugamba | Nyakasana Farm | Private | |
| Bushenyi | Central Division | Ruhandagazi Regional Fry Centre | Public |
| Kyamuhunga | Kabeihura Farmers | Private | |
| Kanungu | Kihihi Town Council | Kihihi Fish Fry Centre | Public |
| Kirima | Waako Fry Centre | Private | |
| Kabale | Ndorwa | Kachwekano Zonal Agricultural Research and Development Institute | Public |
| Rukungiri | Rukungiri Municipality | Rural Aquaculture Development | Private |
| Hoima | Kyabigambire | Bulindi Zonal Aquaculture Research and Development Institute | Public |
| Kibanjwa | AA Fisheries and Aquaculture Farm | Private | |
| Kasese | Maliba | Nehemia Hatchery | Private |
| Kisinga | Blue Valley Fish Farm | Private | |
| Kabarole | Rwengaju Model | Rwebitaba Zonal Agricultural Research and Development Institute | Public |
| Mugusu Town council | Adolf Fish Farm | Private | |
| Fort Portal City | Western Division | GEOB Hatchery | Private |
| Kamwenge | Kahungye Town Council, Rwenkuba | Rubumba Seed Production Research and Training Centre | Private |
| Mbale city | Northern Division | Mbale Regional Fish Fry Centre | Public |
| Bushenyi | Kyamuhunga-Mushunga | Kabehura Farm Limited | Private |
| Busia | Buteba | Salama Integrated Fish Farm Limited | Private |
| Sironko | Bumalimba | Nalugugu Fish Farm | Private |
| Bugiri | Buwuni | Kange Integrated Fish Farm | Private |
| Iganga | Northern Division | Muso4f Enterprises | Private |
| Mayuge | Wairasa | MIG Fish Farm | Private |
| Namutumba | Nsinze | Busoga Farmers Resource | Private |
| Kumi | Atutur | Kumi Wetland Fish Farming Association | Private |
| Tororo | Western Division | Bamukwasi Rock Valley Fish Farm | Private |
| Eastern Division | Rock Springs Fish Farm Limited | Private | |
| Morukatipe | Geossy Fish Farm and Hatchery | Private | |
| Serere | Olio | Kikoota Integrated Fish Farm | Private |
| Arua city | Ayivu East Division | Abi Zonal Agricultural Research and Development Institute | Public |
| Koboko | Nyangilia | Manada Fish Farm | Private |
| Maracha | Kijomoro | Eyofia Memorial Farm Kochi Ltd. | Private |
| Abinyu cell | Wole Mixed Farm | Private | |
| Maracha | Kijomoro | Neville Long Bottom Mixed Farm | Private |
| Amuru | Pabbo Town Council | Lalar Fish Farm | Private |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abaho, I.; Kwikiriza, G.; Atukwatse, F.; Izaara, A.A.; Ekwangu, J.; Baguma, S.D.; Kubiriba, J.; Kasozi, N. Selective Breeding for Genetic Improvement of Nile tilapia (Oreochromis niloticus Linnaeus, 1758) in Uganda: Current Status, Challenges, and Future Perspectives. Animals 2025, 15, 142. https://doi.org/10.3390/ani15020142
Abaho I, Kwikiriza G, Atukwatse F, Izaara AA, Ekwangu J, Baguma SD, Kubiriba J, Kasozi N. Selective Breeding for Genetic Improvement of Nile tilapia (Oreochromis niloticus Linnaeus, 1758) in Uganda: Current Status, Challenges, and Future Perspectives. Animals. 2025; 15(2):142. https://doi.org/10.3390/ani15020142
Chicago/Turabian StyleAbaho, Ivan, Gerald Kwikiriza, Faith Atukwatse, Andrew A. Izaara, Joseph Ekwangu, Sylvester D. Baguma, Jerome Kubiriba, and Nasser Kasozi. 2025. "Selective Breeding for Genetic Improvement of Nile tilapia (Oreochromis niloticus Linnaeus, 1758) in Uganda: Current Status, Challenges, and Future Perspectives" Animals 15, no. 2: 142. https://doi.org/10.3390/ani15020142
APA StyleAbaho, I., Kwikiriza, G., Atukwatse, F., Izaara, A. A., Ekwangu, J., Baguma, S. D., Kubiriba, J., & Kasozi, N. (2025). Selective Breeding for Genetic Improvement of Nile tilapia (Oreochromis niloticus Linnaeus, 1758) in Uganda: Current Status, Challenges, and Future Perspectives. Animals, 15(2), 142. https://doi.org/10.3390/ani15020142







