Fasciola gigantica Recombinant Abelson Tyrosine Protein Kinase (rFgAbl) Regulates Various Functions of Buffalo Peripheral Blood Mononuclear Cells
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Source of Parasite
2.3. Cell Isolation and Culture
2.4. Cloning and Expression of Fasciola gigantica (Fg) Abelson Tyrosine Protein Kinase (Abl)
2.5. Expression of Recombinant Fasciola gigantica (Fg) Abelson Tyrosine Protein Kinase (Abl) Protein
2.6. Purification and Identification of Recombinant Fasciola gigantica (Fg) Abelson Tyrosine Protein Kinase (Abl) Protein
2.7. Preparation of Polyclonal Antibodies and Western Blotting
2.8. Tissue Localization of Abelson Tyrosine Protein Kinase (Abl) Protein in F. gigantica
2.9. Immunofluorescence Detection of Recombinant Fasciola gigantica (Fg) Abelson Tyrosine Protein Kinase (Abl) Protein Binding to Buffaloe Peripheral Blood Mononuclear Cells (PBMCs)
2.10. The Effect of Recombinant Fasciola gigantica (Fg) Abelson Tyrosine Protein Kinase (Abl) Protein on Cell Viability
2.11. Determination of Nitric Oxide
2.12. The Effect of Recombinant Fasciola gigantica (Fg) Abelson Tyrosine Protein Kinase (Abl) Protein on Cell Migration
2.13. Effect of Recombinant Fasciola gigantica (Fg) Abelson Tyrosine Protein Kinase (Abl) Protein on Phagocytic Activity
2.14. Cytokine Analysis
2.15. Statistical Analysis
3. Results
3.1. RFgAbl Expression, Purification, and Identification
3.2. Polyclonal Antibody Specificity
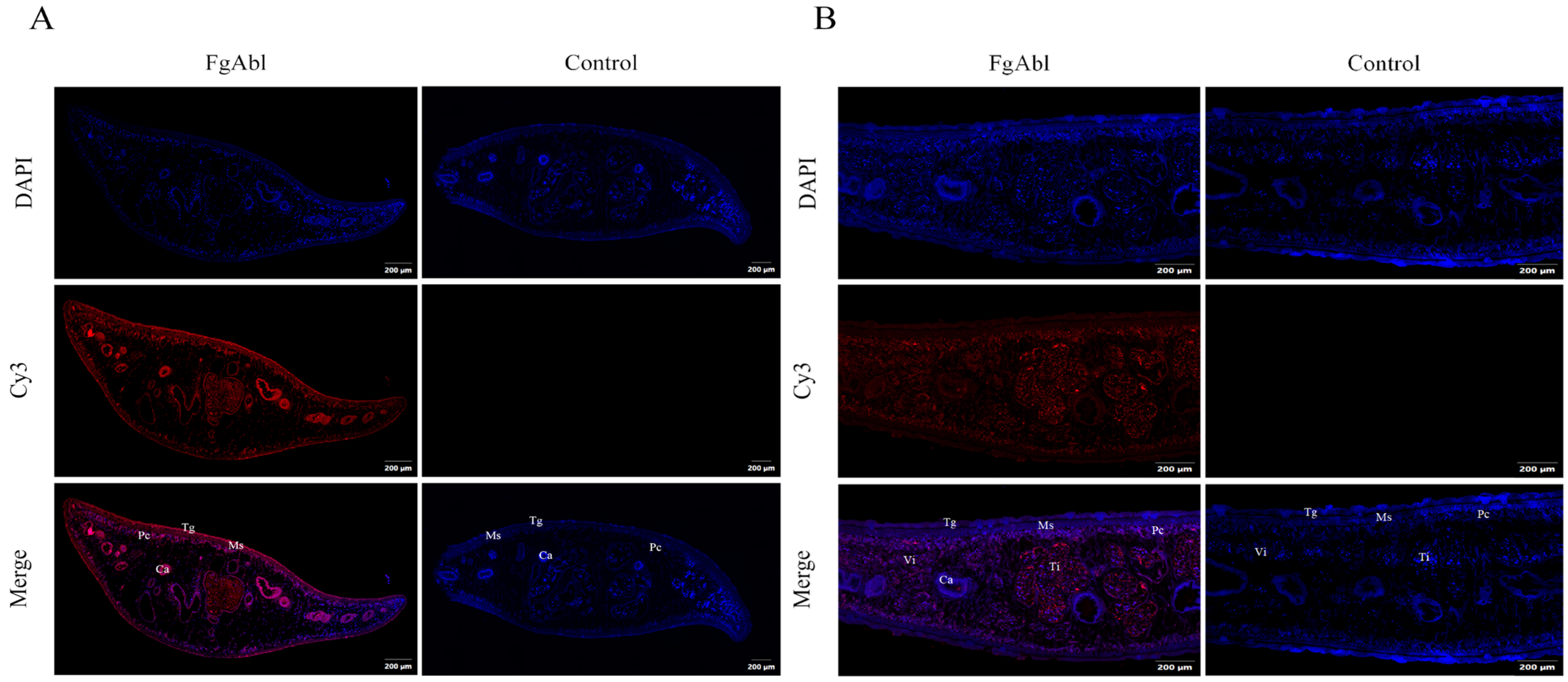
3.3. Abl Immunofluorescence Localization in F. gigantica
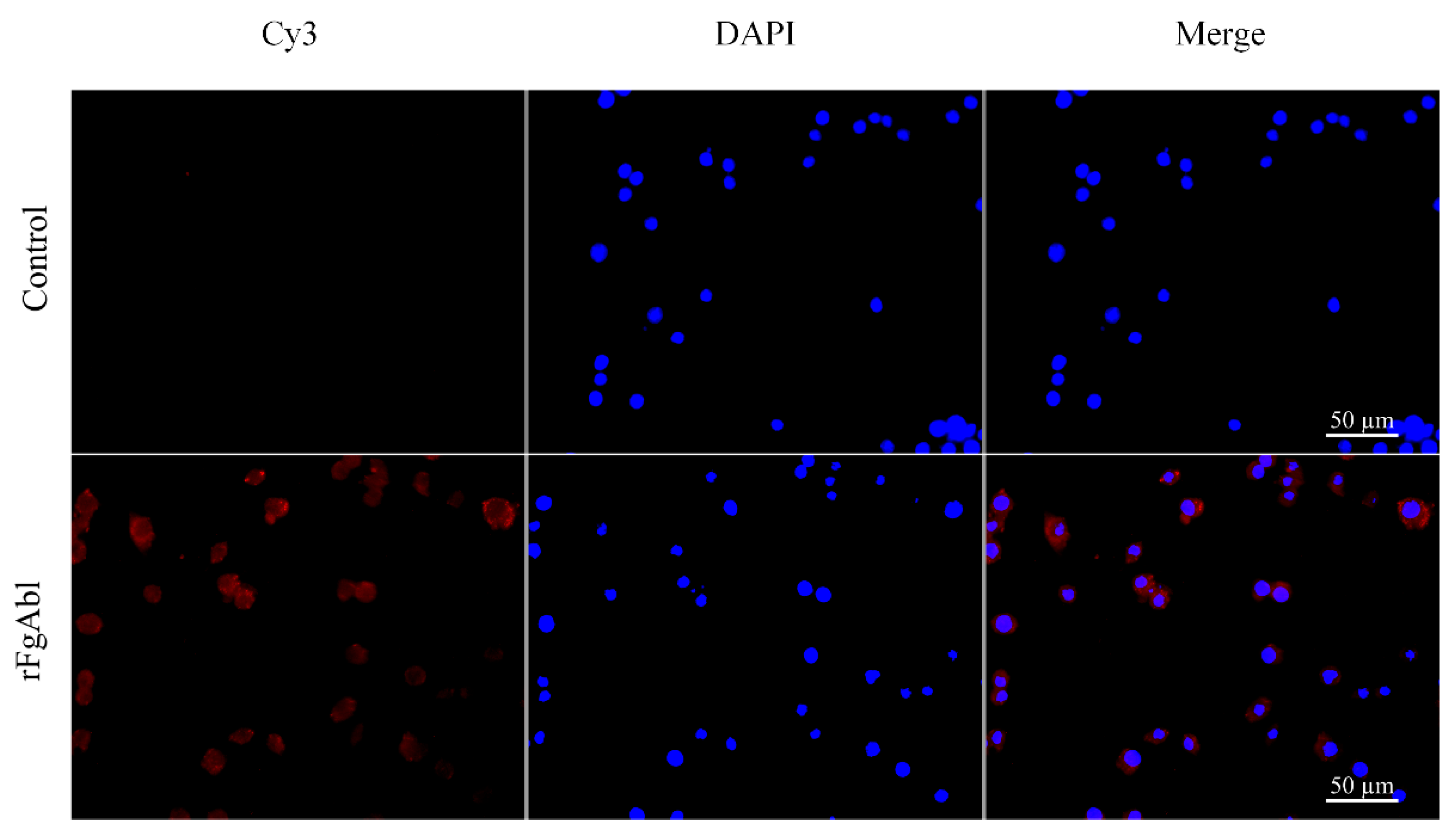
3.4. Binding Affinity of rFgAbl Protein to Buffaloe Peripheral Blood Mononuclear Cells (PBMCs)
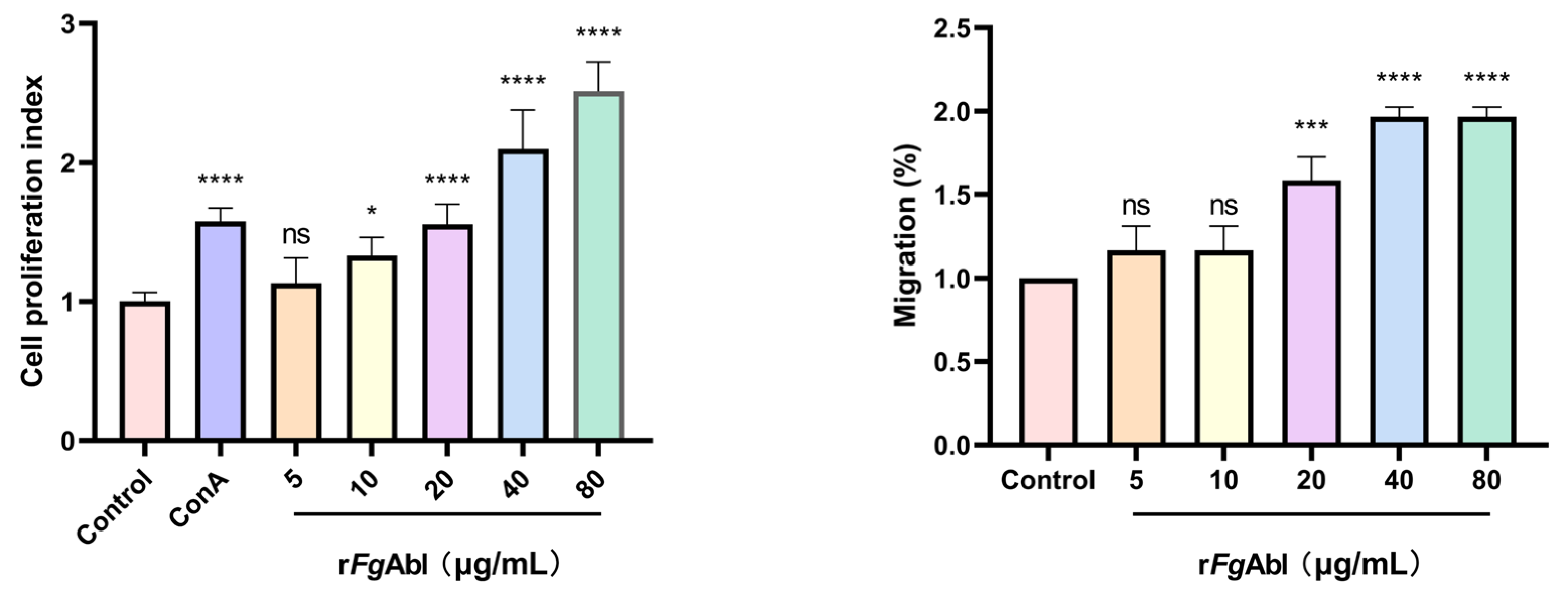
3.5. RFgAbl Promotes the Proliferation and Migration of Buffalo Peripheral Blood Mononuclear Cells (PBMCs)
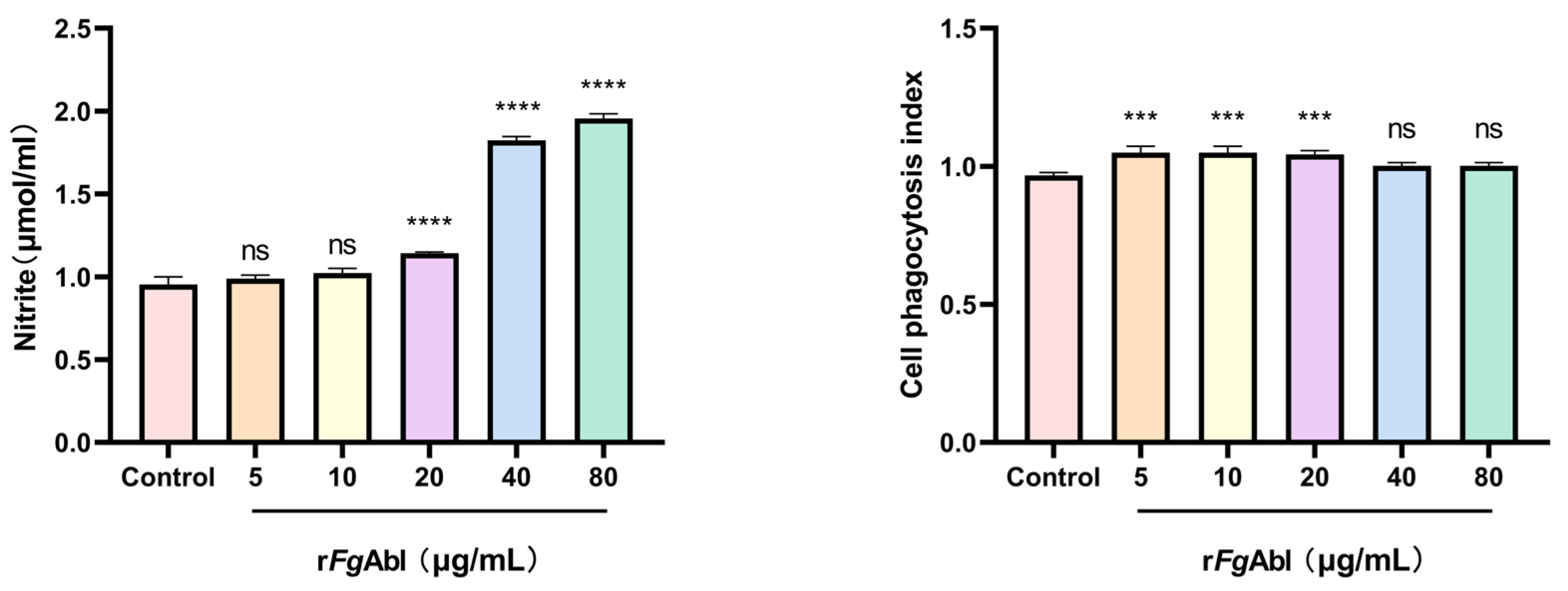
3.6. RFgAbl Promotes NO Production and Cellular Phagocytosis of Buffaloe Peripheral Blood Mononuclear Cells (PBMCs)
3.7. RFgAbl Modulation of Buffaloe Peripheral Blood Mononuclear Cells (PBMCs) Cytokines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanna, R.E.; Moffett, D.; Forster, F.I.; Trudgett, A.G.; Brennan, G.P.; Fairweather, I. Fasciola hepatica: A light and electron microscope study of the ovary and of the development of oocytes within eggs in the uterus provides an insight into reproductive strategy. Vet. Parasitol. 2016, 221, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Zerna, G.; Spithill, T.W.; Beddoe, T. Current Status for Controlling the Overlooked Caprine Fasciolosis. Animals 2021, 11, 1819. [Google Scholar] [CrossRef]
- Mas-Coma, S.; Bargues, M.D.; Valero, M.A. Human fascioliasis infection sources, their diversity, incidence factors, analytical methods and prevention measures—CORRIGENDUM. Parasitology 2020, 147, 601. [Google Scholar] [CrossRef] [PubMed]
- Siles-Lucas, M.; Becerro-Recio, D.; Serrat, J.; González-Miguel, J. Fascioliasis and fasciolopsiasis: Current knowledge and future trends. Res. Vet. Sci. 2021, 134, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Villa-Mancera, A.; Reynoso-Palomar, A. Bulk tank milk ELISA to detect IgG1 prevalence and clustering to determine spatial distribution and risk factors of Fasciola hepatica-infected herds in Mexico. J. Helminthol. 2019, 93, 704–710. [Google Scholar] [CrossRef]
- Fairweather, I.; Brennan, G.P.; Hanna, R.E.B.; Robinson, M.W.; Skuce, P.J. Drug resistance in liver flukes. Int. J. Parasitol. Drugs Drug Resist. 2020, 12, 39–59. [Google Scholar] [CrossRef]
- Hanna, R.E. Fasciola hepatica: Glycocalyx replacement in the juvenile as a possible mechanism for protection against host immunity. Exp. Parasitol. 1980, 50, 103–114. [Google Scholar] [CrossRef]
- Rodríguez, E.; Noya, V.; Cervi, L.; Chiribao, M.L.; Brossard, N.; Chiale, C.; Carmona, C.; Giacomini, C.; Freire, T. Glycans from Fasciola hepatica Modulate the Host Immune Response and TLR-Induced Maturation of Dendritic Cells. PLoS Negl. Trop. Dis. 2015, 9, e0004234. [Google Scholar] [CrossRef]
- Robinson, M.W.; Dalton, J.P.; O’Brien, B.A.; Donnelly, S. Fasciola hepatica: The therapeutic potential of a worm secretome. Int. J. Parasitol. 2013, 43, 283–291. [Google Scholar] [CrossRef]
- Cervi, L.; Rossi, G.; Masih, D.T. Potential role for excretory-secretory forms of glutathione-S-transferase (GST) in Fasciola hepatica. Parasitology 1999, 119 Pt 6, 627–633. [Google Scholar] [CrossRef]
- Buffoni, L.; Zafra, R.; Pérez-Ecija, A.; Martínez-Moreno, F.J.; Martínez-Galisteo, E.; Moreno, T.; Pérez, J.; Martínez-Moreno, A. Immune response of goats immunised with glutathione S-transferase and experimentally challenged with Fasciola hepatica. Parasitol. Int. 2010, 59, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, M.F.; González-Miguel, J.; Carmona, C.; Dalton, J.P.; Cwiklinski, K.; Tort, J.; Siles-Lucas, M. Low allelic diversity in vaccine candidates genes from different locations sustain hope for Fasciola hepatica immunization. Vet. Parasitol. 2018, 258, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, M.H.; Ruelas, D.S.; Wolff, B.; Snedecor, J.; Lim, K.C.; Xu, F.; Renslo, A.R.; Williams, J.; McKerrow, J.H.; Caffrey, C.R. Drug discovery for schistosomiasis: Hit and lead compounds identified in a library of known drugs by medium-throughput phenotypic screening. PLoS Negl. Trop. Dis. 2009, 3, e478. [Google Scholar] [CrossRef]
- Gocek, E.; Moulas, A.N.; Studzinski, G.P. Non-receptor protein tyrosine kinases signaling pathways in normal and cancer cells. Crit. Rev. Clin. Lab. Sci. 2014, 51, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Haeberlein, S.; Zhao, L.; Mughal, M.N.; Zhu, T.; Liu, L.; Fang, R.; Zhou, Y.; Zhao, J.; Grevelding, C.G.; et al. The ABL kinase inhibitor imatinib causes phenotypic changes and lethality in adult Schistosoma japonicum. Parasitol. Res. 2019, 118, 881–890. [Google Scholar] [CrossRef]
- Morawietz, C.M.; Houhou, H.; Puckelwaldt, O.; Hehr, L.; Dreisbach, D.; Mokosch, A.; Roeb, E.; Roderfeld, M.; Spengler, B.; Haeberlein, S. Targeting Kinases in Fasciola hepatica: Anthelminthic Effects and Tissue Distribution of Selected Kinase Inhibitors. Front. Vet. Sci. 2020, 7, 611270. [Google Scholar] [CrossRef]
- Beckmann, S.; Grevelding, C.G. Imatinib has a fatal impact on morphology, pairing stability and survival of adult Schistosoma mansoni in vitro. Int. J. Parasitol. 2010, 40, 521–526. [Google Scholar] [CrossRef]
- Montano, H.; Anandkrishnan, R.; Carruthers, V.B.; Gaji, R.Y. TgTKL4 Is a Novel Kinase That Plays an Important Role in Toxoplasma Morphology and Fitness. mSphere 2023, 8, e0064922. [Google Scholar] [CrossRef]
- Zhang, X.X.; Cong, W.; Elsheikha, H.M.; Liu, G.H.; Ma, J.G.; Huang, W.Y.; Zhao, Q.; Zhu, X.Q. De novo transcriptome sequencing and analysis of the juvenile and adult stages of Fasciola gigantica. Infect. Genet. Evol. 2017, 51, 33–40. [Google Scholar] [CrossRef]
- Tian, A.L.; Lu, M.; Zhang, F.K.; Calderón-Mantilla, G.; Petsalaki, E.; Tian, X.; Wang, W.; Huang, S.Y.; Li, X.; Elsheikha, H.M.; et al. The pervasive effects of recombinant Fasciola gigantica Ras-related protein Rab10 on the functions of goat peripheral blood mononuclear cells. Parasit. Vectors 2018, 11, 579. [Google Scholar] [CrossRef]
- Ehsan, M.; Hu, R.S.; Hou, J.L.; Elsheikha, H.M.; Li, X.D.; Liang, P.H.; Zhu, X.Q. Fasciola gigantica tegumental calcium-binding EF-hand protein 4 exerts immunomodulatory effects on goat monocytes. Parasit. Vectors 2021, 14, 276. [Google Scholar]
- Ghasemi, A.; Zahediasl, S. Normality tests for statistical analysis: A guide for non-statisticians. Int. J. Endocrinol. Metab. 2012, 10, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Campillo, M.T.; Barrero-Torres, D.M.; Abril, N.; Pérez, J.; Zafra, R.; Buffoni, L.; Martínez-Moreno, Á.; Martínez-Moreno, F.J.; Molina-Hernández, V. Fasciola hepatica primoinfections and reinfections in sheep drive distinct Th1/Th2/Treg immune responses in liver and hepatic lymph node at early and late stages. Vet. Res. 2023, 54, 2. [Google Scholar] [CrossRef]
- Pacheco, I.L.; Abril, N.; Morales-Prieto, N.; Bautista, M.J.; Zafra, R.; Escamilla, A.; Ruiz, M.T.; Martínez-Moreno, A.; Pérez, J. Th1/Th2 balance in the liver and hepatic lymph nodes of vaccinated and unvaccinated sheep during acute stages of infection with Fasciola hepatica. Vet. Parasitol. 2017, 238, 61–65. [Google Scholar] [CrossRef]
- Chen, D.; Tian, A.L.; Hou, J.L.; Li, J.X.; Tian, X.; Yuan, X.D.; Li, X.; Elsheikha, H.M.; Zhu, X.Q. The Multitasking Fasciola gigantica Cathepsin B Interferes With Various Functions of Goat Peripheral Blood Mononuclear Cells In Vitro. Front. Immunol. 2019, 10, 1707. [Google Scholar] [CrossRef]
- Bando, H.; Lee, Y.; Sakaguchi, N.; Pradipta, A.; Ma, J.S.; Tanaka, S.; Cai, Y.; Liu, J.; Shen, J.; Nishikawa, Y.; et al. Inducible Nitric Oxide Synthase Is a Key Host Factor for Toxoplasma GRA15-Dependent Disruption of the Gamma Interferon-Induced Antiparasitic Human Response. mBio 2018, 9, e01738-18. [Google Scholar] [CrossRef]
- Gazzinelli, R.T.; Oswald, I.P.; James, S.L.; Sher, A. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-gamma-activated macrophages. J. Immunol. 1992, 148, 1792–1796. [Google Scholar] [CrossRef]
- Colasanti, M.; Gradoni, L.; Mattu, M.; Persichini, T.; Salvati, L.; Venturini, G.; Ascenzi, P. Molecular bases for the anti-parasitic effect of NO. Int. J. Mol. Med. 2002, 9, 131–134. [Google Scholar] [CrossRef]
- Park, A.Y.; Hondowicz, B.D.; Scott, P. IL-12 is required to maintain a Th1 response during Leishmania major infection. J. Immunol. 2000, 165, 896–902. [Google Scholar] [CrossRef]
- Kumar, S.; Jeong, Y.; Ashraf, M.U.; Bae, Y.S. Dendritic Cell-Mediated Th2 Immunity and Immune Disorders. Int. J. Mol. Sci. 2019, 20, 2159. [Google Scholar] [CrossRef]
- Di Benedetto, P.; Ruscitti, P.; Vadasz, Z.; Toubi, E.; Giacomelli, R. Macrophages with regulatory functions, a possible new therapeutic perspective in autoimmune diseases. Autoimmun. Rev. 2019, 18, 102369. [Google Scholar] [CrossRef] [PubMed]
- Tian, A.L.; Lu, M.; Calderón-Mantilla, G.; Petsalaki, E.; Dottorini, T.; Tian, X.; Wang, Y.; Huang, S.Y.; Hou, J.L.; Li, X.; et al. A recombinant Fasciola gigantica 14-3-3 epsilon protein (rFg14-3-3e) modulates various functions of goat peripheral blood mononuclear cells. Parasit. Vectors 2018, 11, 152. [Google Scholar] [CrossRef] [PubMed]
- Moura, V.B.; Lima, S.B.; Matos-Silva, H.; Vinaud, M.C.; Loyola, P.R.; Lino, R.S. Cellular immune response in intraventricular experimental neurocysticercosis. Parasitology 2016, 143, 334–342. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Zou, Y.; Chen, W.; Wu, D.; Xian, C.; Yang, H.; Tan, J.; Di, W.; Wu, W.; Wang, D. Fasciola gigantica Recombinant Abelson Tyrosine Protein Kinase (rFgAbl) Regulates Various Functions of Buffalo Peripheral Blood Mononuclear Cells. Animals 2025, 15, 179. https://doi.org/10.3390/ani15020179
Zhao M, Zou Y, Chen W, Wu D, Xian C, Yang H, Tan J, Di W, Wu W, Wang D. Fasciola gigantica Recombinant Abelson Tyrosine Protein Kinase (rFgAbl) Regulates Various Functions of Buffalo Peripheral Blood Mononuclear Cells. Animals. 2025; 15(2):179. https://doi.org/10.3390/ani15020179
Chicago/Turabian StyleZhao, Min, Yu Zou, Wanting Chen, Dongqi Wu, Chengjun Xian, Haoqing Yang, Jiacheng Tan, Wenda Di, Wende Wu, and Dongying Wang. 2025. "Fasciola gigantica Recombinant Abelson Tyrosine Protein Kinase (rFgAbl) Regulates Various Functions of Buffalo Peripheral Blood Mononuclear Cells" Animals 15, no. 2: 179. https://doi.org/10.3390/ani15020179
APA StyleZhao, M., Zou, Y., Chen, W., Wu, D., Xian, C., Yang, H., Tan, J., Di, W., Wu, W., & Wang, D. (2025). Fasciola gigantica Recombinant Abelson Tyrosine Protein Kinase (rFgAbl) Regulates Various Functions of Buffalo Peripheral Blood Mononuclear Cells. Animals, 15(2), 179. https://doi.org/10.3390/ani15020179





