Welfare Implications of Low-Dose Atipamezole Reversal of Tiletamine/Zolazepam/Xylazine Anaesthesia in Pigs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Management and Acclimation
2.2. Experimental Design
2.3. Anaesthetic and Antagonist Regime
2.4. Assessment of Anaesthesia Recovery
2.5. Determination of Consciousness
2.6. Assessment of Clinical Variables
2.7. Behavioural Assessment
2.8. Statistical Analysis
3. Results
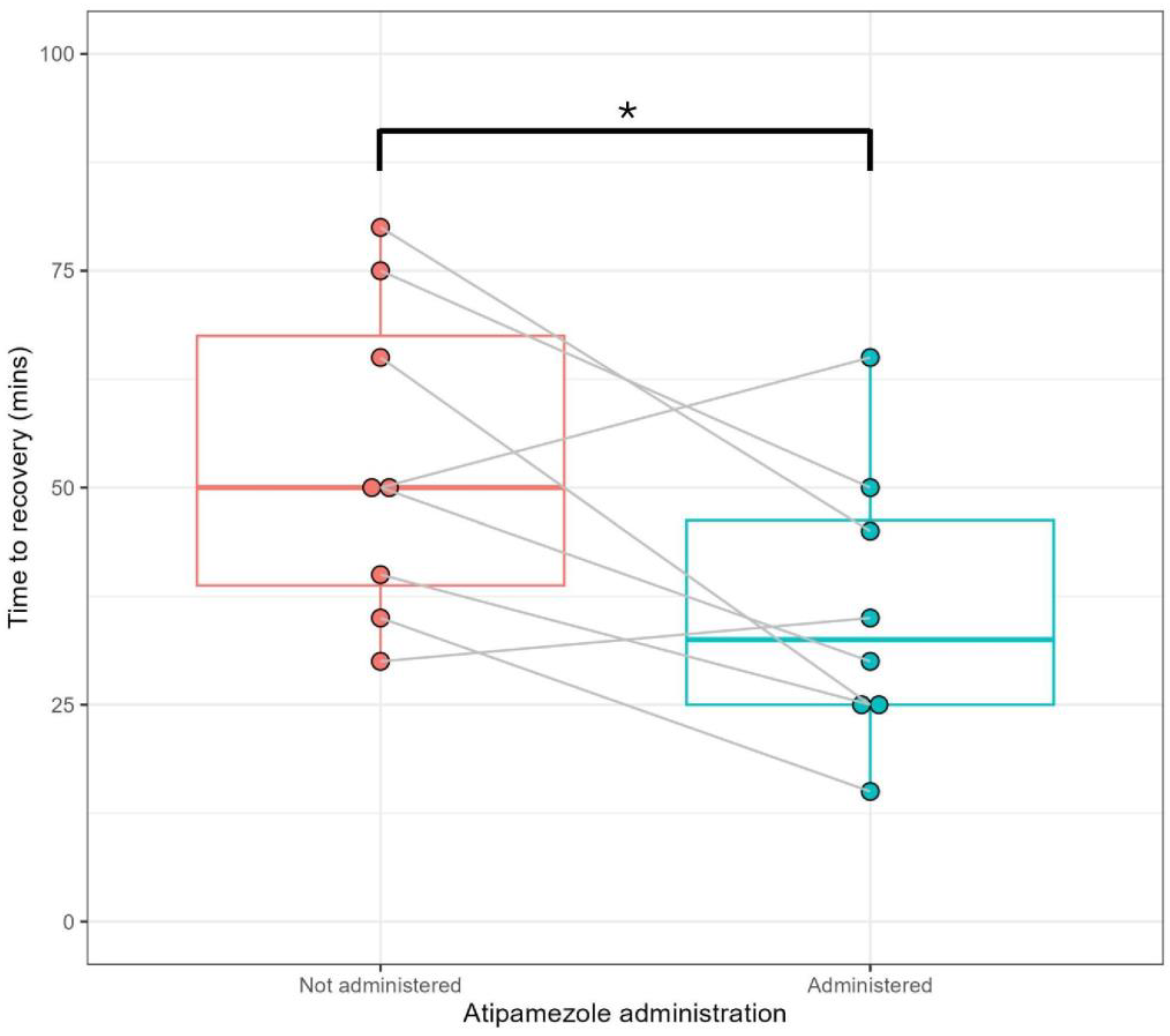
3.1. Low-Dose Atipamezole Leads to More Rapid Recovery from Anaesthesia
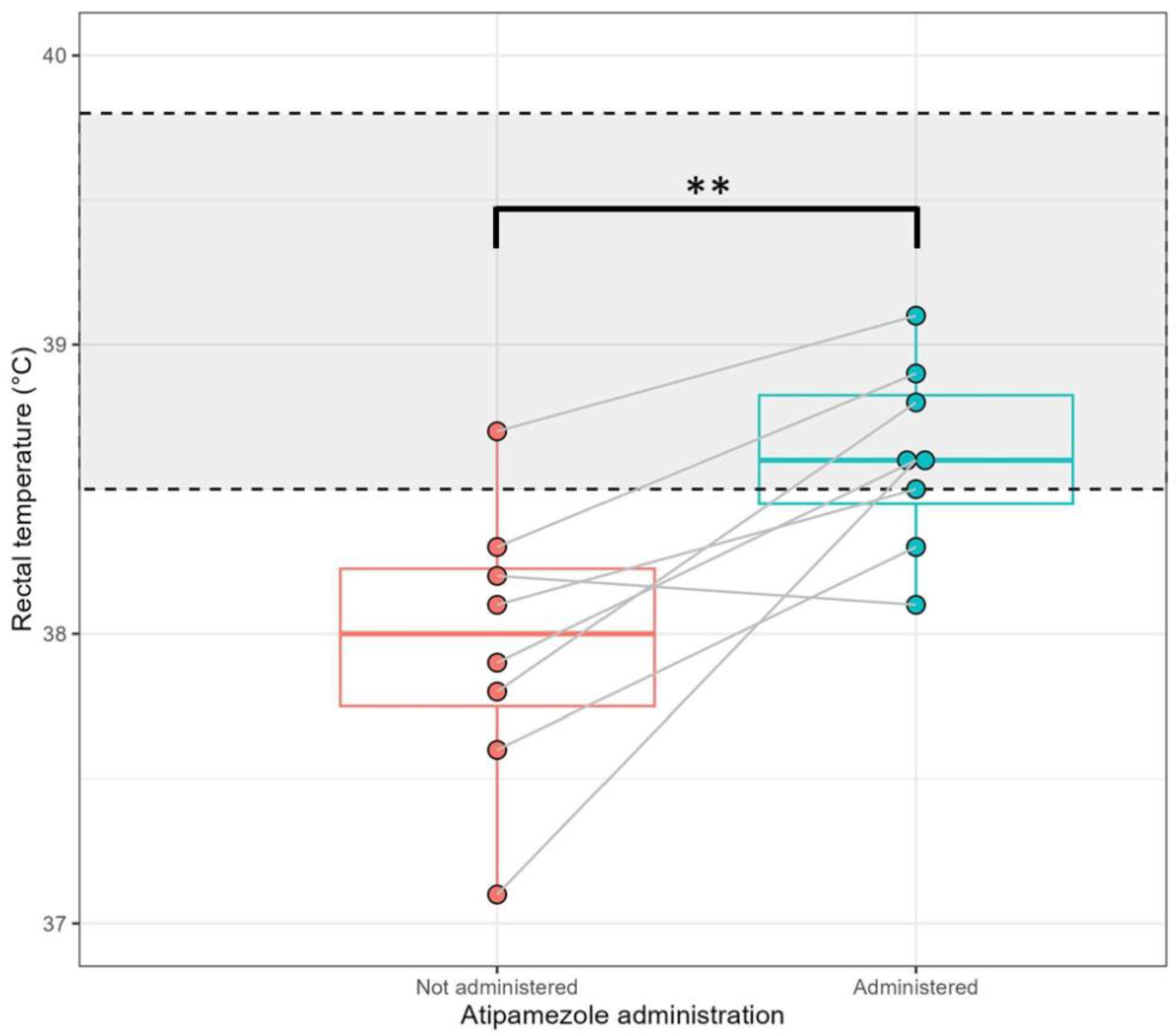
3.2. Low-Dose Atipamezole Improves Thermoregulation During Anaesthetic Recovery
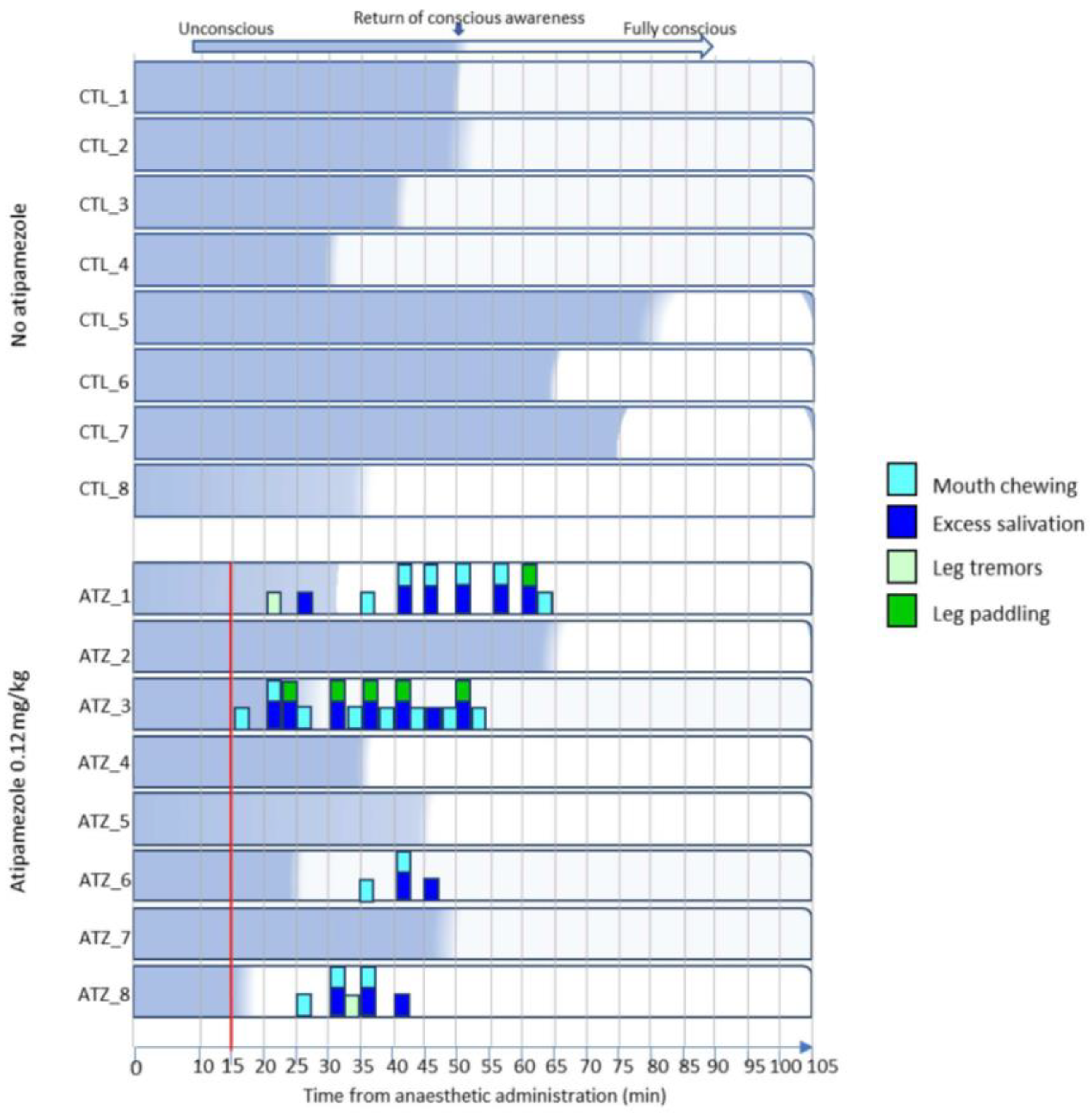
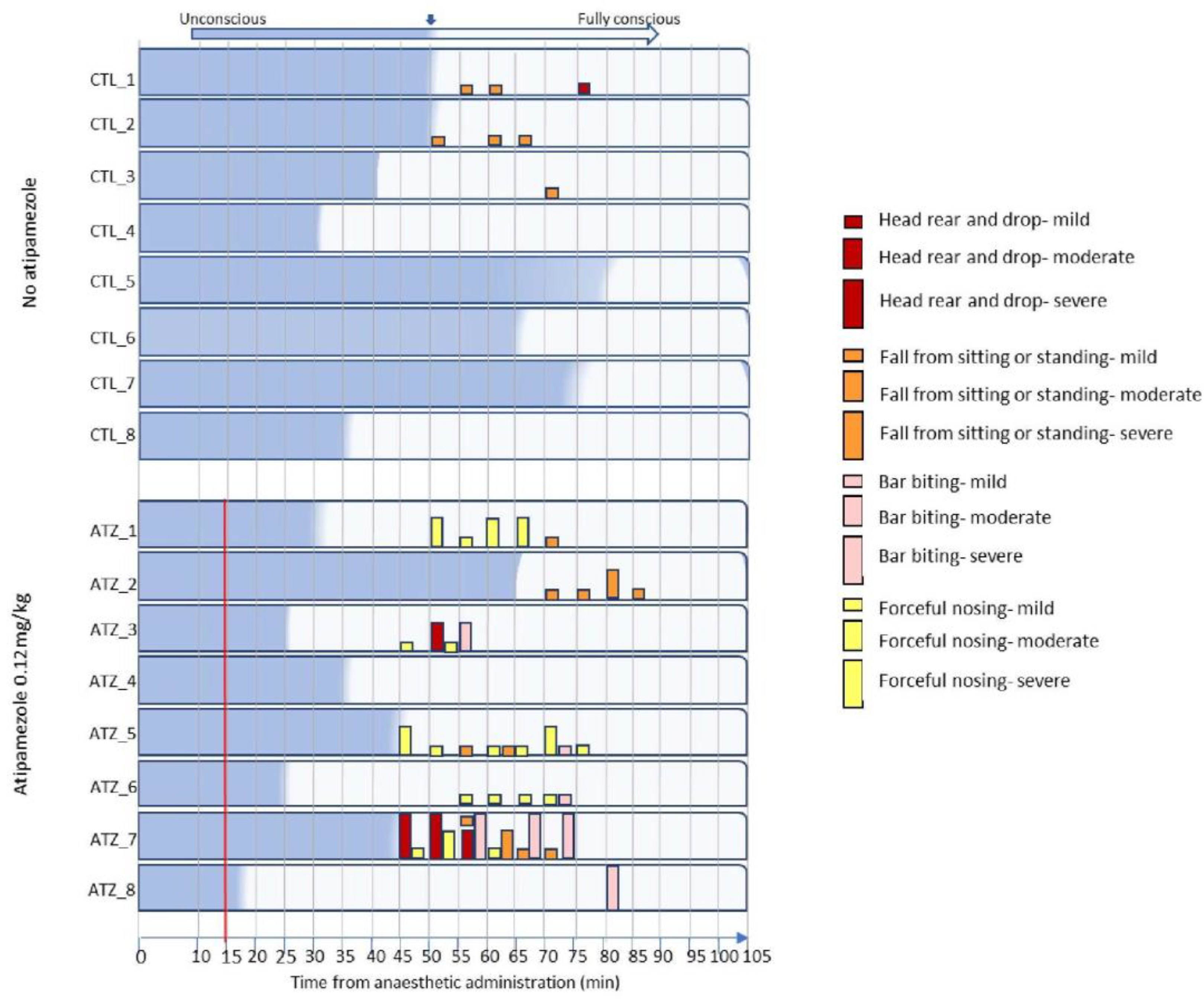
3.3. Low-Dose Atipamezole Increases Adverse Behaviours During Anaesthesia Recovery
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marchant-Forde, J.N.; Herskin, M.S. Pigs as laboratory animals. In Advances in Pig Welfare; Elsevier: Amsterdam, The Netherlands, 2018; pp. 445–475. [Google Scholar] [CrossRef]
- Swindle, M.M.; Makin, A.; Herron, A.J.; Clubb, F.J., Jr.; Frazier, K.S. Swine as models in biomedical research and toxicology testing. Vet. Pathol. 2012, 49, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Klages, C. IACUC and veterinary considerations for review of ABSL3 and ABSL4 research protocols. ILAR J. 2022, 61, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.C.; Swindle, M.M. Preparation of swine for the laboratory. ILAR J. 2006, 47, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Ditchkoff, S.S.; Bodenchuk, M.J. Management of wild pigs. In Invasive Wild Pigs in North America: Ecology, Impacts, and Management, 1st ed.; CRC Press: Boca Raton, FL, USA, 2020; pp. 175–198. [Google Scholar]
- Rushen, J.; Schwarze, N.; Ladewig, J.; Foxcroft, G. Opioid modulation of the effects of repeated stress on ACTH, cortisol, prolactin, and growth hormone in pigs. Physiol. Behav. 1993, 53, 923–928. [Google Scholar] [CrossRef]
- López-Martínez, M.J.; Escribano, D.; Contreras-Aguilar, M.D.; García-Martínez, J.D.; Martínez-Subiela, S.; Cerón, J.J. Salivary D-dimer in pigs: Validation of an automated assay and changes after acute stress. Vet. J. 2020, 259–260, 105472. [Google Scholar] [CrossRef]
- Escribano, D.; Horvatić, A.; Contreras-Aguilar, M.D.; Guillemin, N.; Cerón, J.J.; Tecles, F.; Martinez-Miró, S.; Eckersall, P.D.; Manteca, X.; Mrljak, V. Changes in saliva proteins in two conditions of compromised welfare in pigs: An experimental induced stress by nose snaring and lameness. Res. Vet. Sci. 2019, 125, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Layton, R.; Layton, D.; Beggs, D.; Fisher, A.; Mansell, P.; Stanger, K.J. The impact of stress and anesthesia on animal models of infectious disease. Front. Vet. Sci. 2023, 10, 1086003. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; Galang, K.G.; Gallegos, A.; Ma, B.W.; Isseroff, R.R. Sling Training with Positive Reinforcement to Facilitate Porcine Wound Studies. JID Innov. 2021, 1, 100016. [Google Scholar] [CrossRef]
- O’Malley, C.I.; Hubley, R.; Tambadou, H.; Turner, P.V. Refining restraint techniques for research pigs through habituation. Front. Vet. Sci. 2022, 9, 1016414. [Google Scholar] [CrossRef] [PubMed]
- Copps, J. Issues related to the use of animals in biocontainment research facilities. ILAR J. 2005, 46, 34–43. [Google Scholar] [CrossRef]
- Swindle, M.M.; Nolan, T.; Jacobson, A.; Wolf, P.; Dalton, M.J.; Smith, A.C. Vascular access port (VAP) usage in large animal species. Contemp. Top. Lab. Anim. Sci. 2005, 44, 7–17. [Google Scholar] [PubMed]
- Lee, J.Y.; Kim, M.C. Anesthesia of growing pigs with tiletamine-zolazepam and reversal with flumazenil. J. Vet. Med. Sci. 2012, 74, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jee, H.; Jeong, S.; Park, C.; Kim, M. Comparison of anaesthetic and cardiorespiratory effects of xylazine or medetomidine in combination with tiletamine/zolazepam in pigs. Vet. Rec. 2010, 167, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Bai, H.; Zhang, B.; Shen, M.; Gao, L. Comparison of cardiorespiratory and anesthetic effects of ketamine-midazolam-xylazine-sufentanil and tiletamine-zolazepam-xylazine in miniature pigs. PLoS ONE 2022, 17, e0271325. [Google Scholar] [CrossRef] [PubMed]
- Nash, P.B. Susceptibility to low dose influenza in mice is increased by administration of ketamine/xylazine. J. Immunol. 2021, 206 (Suppl. 1), 20.25. [Google Scholar] [CrossRef]
- Guo, L.Y.; Kaustov, L.; Brenna, C.T.A.; Patel, V.; Zhang, C.; Choi, S.; Halpern, S.; Wang, D.S.; Orser, B.A. Cognitive deficits after general anaesthesia in animal models: A scoping review. Br. J. Anaesth. 2023, 130, e351–e360. [Google Scholar] [CrossRef]
- Herling, A. Anesthesia of Experimental Animals. In Drug Discovery and Evaluation: Pharmacological Assays; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2016; pp. 4269–4275. [Google Scholar] [CrossRef]
- Swindle, M.M. Swine in the Laboratory: Surgery, Anesthesia, Imaging, and Experimental Techniques; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar] [CrossRef]
- Ellis, C.K.; Wehtje, M.E.; Wolfe, L.L.; Wolff, P.L.; Hilton, C.D.; Fisher, M.C.; Green, S.; Glow, M.P.; Halseth, J.M.; Lavelle, M.J. Comparison of the efficacy of four drug combinations for immobilization of wild pigs. Eur. J. Wildl. Res. 2019, 65, 78. [Google Scholar] [CrossRef]
- Haapalinna, A.; Viitamaa, T.; MacDonald, E.; Savola, J.M.; Tuomisto, L.; Virtanen, R.; Heinonen, E. Evaluation of the effects of a specific α2-adrenoceptor antagonist, atipamezole, on α1-and α2-adrenoceptor subtype binding, brain neurochemistry and behaviour in comparison with yohimbine. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1997, 356, 570–582. [Google Scholar] [CrossRef]
- Nishimura, R.; Kim, H.; Matsunaga, S.; Sakaguchi, M.; Sasaki, N.; Tamura, H.; Takeuchi, A. Antagonism of medetomidine sedation by atipamezole in pigs. J. Vet. Med. Sci. 1992, 54, 1237–1240. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.Z.; Fan, H.G.; Kun, M.; Song, Z.L.; Ming, Y.S.; Sheng, J.; Wang, H.B. Antagonistic effect of atipamezole, flumazenil and naloxone following anaesthesia with xylazine, tramadol and tiletamine/zolazepam combinations in pigs. Vet. Anaesth. Analg. 2011, 38, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Morelli, J.; Rossi, S.; Fuchs, B.; Richard, E.; Barros, D.S.B.; Küker, S.; Arnemo, J.M.; Evans, A.L. Evaluation of Three Medetomidine-Based Anesthetic Protocols in Free-Ranging Wild Boars (Sus scrofa). Front. Vet. Sci. 2021, 8, 655345. [Google Scholar] [CrossRef] [PubMed]
- Sipos, W.; Wiener, S.; Entenfellner, F.; Sipos, S. Physiological changes of rectal temperature, pulse rate and respiratory rate of pigs at different ages including the critical peripartal period. Vet. Med. Austria 2013, 100, 96. [Google Scholar]
- Bunnag, N.; Akaraphutiporn, E.; Durongphongtorn, S.; Soontornvipart, K.; Sharp, P.; Pacharinsak, C.; Wangdee, C. Assessment of a Combination of Tiletamine/Zolazepam, Ketamine, and Dexmedetomidine for Anesthesia of Swine (Sus domesticus). J. Am. Assoc. Lab. Anim. Sci. 2023, 62, 423–429. [Google Scholar] [CrossRef]
- Ruetzler, K.; Kurz, A. Consequences of perioperative hypothermia. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 157, pp. 687–697. [Google Scholar] [CrossRef]
- Kim, M.J.; Park, C.S.; Jun, M.H.; Kim, M.C. Antagonistic effects of yohimbine in pigs anaesthetised with tiletamine/zolazepam and xylazine. Vet. Rec. 2007, 161, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Appenheimer, M.M.; Evans, S.S. Temperature and adaptive immunity. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 156, pp. 397–415. [Google Scholar] [CrossRef]
- Dent, B.T.; Stevens, K.A.; Clymer, J.W. Forced-air warming provides better control of body temperature in porcine surgical patients. Vet. Sci. 2016, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Bonhomme, V.; Vanhaudenhuyse, A.; Demertzi, A.; Bruno, M.-A.; Jaquet, O.; Bahri, M.A.; Plenevaux, A.; Boly, M.; Boveroux, P.; Soddu, A. Resting-state network-specific breakdown of functional connectivity during ketamine alteration of consciousness in volunteers. Anesthesiology 2016, 125, 873–888. [Google Scholar] [CrossRef] [PubMed]
- Haskins, S.C. General guidelines for judging anesthetic depth. Vet. Clin. N. Am. Small Anim. Pract. 1992, 22, 432–434. [Google Scholar] [CrossRef]
- Lin, H.C.; Thurmon, J.C.; Benson, G.J.; Tranquilli, W.J. Telazol—A review of its pharmacology and use in veterinary medicine. J. Vet. Pharmacol. Ther. 1993, 16, 383–418. [Google Scholar] [CrossRef]
- Gross, M.E.; Pablo, L.S. Ophthalmic patients. In Veterinary Anesthesia and Analgesia: The Fifth Edition of Lumb and Jones; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 961–982. [Google Scholar] [CrossRef]
- Sarasso, S.; Boly, M.; Napolitani, M.; Gosseries, O.; Charland-Verville, V.; Casarotto, S.; Rosanova, M.; Casali, A.G.; Brichant, J.F.; Boveroux, P.; et al. Consciousness and Complexity during Unresponsiveness Induced by Propofol, Xenon, and Ketamine. Curr. Biol. 2015, 25, 3099–3105. [Google Scholar] [CrossRef]
- Mirra, A.; Gamez Maidanskaia, E.; Carmo, L.P.; Levionnois, O.; Spadavecchia, C. How is depth of anaesthesia assessed in experimental pigs? A scoping review. PLoS ONE 2023, 18, e0283511. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, O. Practical sedation and anaesthesia in pigs. In Practice 2007, 29, 34–39. [Google Scholar] [CrossRef]
- National Health and Medical Research Council. Australian Code for the Care and Use of Animals for Scientific Purposes; National Health and Medical Research Council: Canberra, Australia, 2013; ISBN 1864965975. [Google Scholar]
| Study Day | Study Event | Anaesthesia Administration | Atipamezole Administration | Anaesthesia Recovery Assessment |
|---|---|---|---|---|
| 0 |
| No | No | N/A |
| 0–13 |
| No | No | N/A |
| 14 |
| Yes | No | No |
| 63 |
| Yes | No | No |
| 70 |
| Yes | No | Yes |
| 77 |
| Yes | Yes | Yes |
| 85 |
| Yes | No | No |
| Recovery Score | Definition |
|---|---|
| 0 | Standing for 5 s or longer |
| 1 | Sitting on pelvic limbs and/or standing for less than 5 s |
| 2 | Keeping the position of ventral recumbency for 5 s or longer |
| 3 | Lateral recumbency with apparent spontaneous movement and response to stimulation (head lifting or purposeful limb movement) |
| 4 | Lateral recumbency with subtle, spontaneous movement (ear and nose twitching or blinking) |
| 5 | Lateral recumbency with absence of spontaneous movement (with the exception of breathing) |
| Recovery Characteristic or Behaviour | Definition |
|---|---|
| Eye twitch | Partial contraction of eyelid with eyes open or closed |
| Eye blink | Top and bottom eyelids meet then separate |
| Ear twitch | Ear muscle contraction and release causing visible muscle movement |
| Tail twitch | Any tail movement in a back/forth motion |
| Leg paddle | Any leg movement in a single back/forth motion |
| Forceful nosing | An upward nose thrust whilst nose is under the pen bar |
| Head rear and drop | Lifting of the head followed by hard contact with the floor |
| Fall from sitting or standing | Uncontrolled hard contact with the floor from a sitting or standing position |
| Excess salivation | Any observation of foam or pooled saliva coming from the mouth |
| Mouth chewing | Open or closed mouth moving in a single up/down chewing motion |
| Bar biting | Open mouth around a pen bar with a biting down motion |
| Behaviour | Mild | Moderate | Severe |
|---|---|---|---|
| Head rear and drop | ≤2 | >2 to ≤15 | >15 |
| Fall from sitting or standing | 1 | ≥2 to ≤5 | >5 |
| Bar biting | ≤10 | >10 to ≤20 | >20 |
| Forceful nosing | ≤10 | >10 to ≤20 | >20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Layton, R.; Beggs, D.S.; Fisher, A.; Mansell, P.; Layton, D.; Durr, P.A.; Allen, T.; Taylor, G.; Kelly, M.L.; Williams, D.T.; et al. Welfare Implications of Low-Dose Atipamezole Reversal of Tiletamine/Zolazepam/Xylazine Anaesthesia in Pigs. Animals 2025, 15, 258. https://doi.org/10.3390/ani15020258
Layton R, Beggs DS, Fisher A, Mansell P, Layton D, Durr PA, Allen T, Taylor G, Kelly ML, Williams DT, et al. Welfare Implications of Low-Dose Atipamezole Reversal of Tiletamine/Zolazepam/Xylazine Anaesthesia in Pigs. Animals. 2025; 15(2):258. https://doi.org/10.3390/ani15020258
Chicago/Turabian StyleLayton, Rachel, David S. Beggs, Andrew Fisher, Peter Mansell, Daniel Layton, Peter A. Durr, Teegan Allen, Grace Taylor, Michael L. Kelly, David T. Williams, and et al. 2025. "Welfare Implications of Low-Dose Atipamezole Reversal of Tiletamine/Zolazepam/Xylazine Anaesthesia in Pigs" Animals 15, no. 2: 258. https://doi.org/10.3390/ani15020258
APA StyleLayton, R., Beggs, D. S., Fisher, A., Mansell, P., Layton, D., Durr, P. A., Allen, T., Taylor, G., Kelly, M. L., Williams, D. T., & Stanger, K. J. (2025). Welfare Implications of Low-Dose Atipamezole Reversal of Tiletamine/Zolazepam/Xylazine Anaesthesia in Pigs. Animals, 15(2), 258. https://doi.org/10.3390/ani15020258






