Retrospective Analysis of Suspensory Ligament Branch Injuries in 70 Dressage Horses
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Acquisition
2.2. Clinical Examination
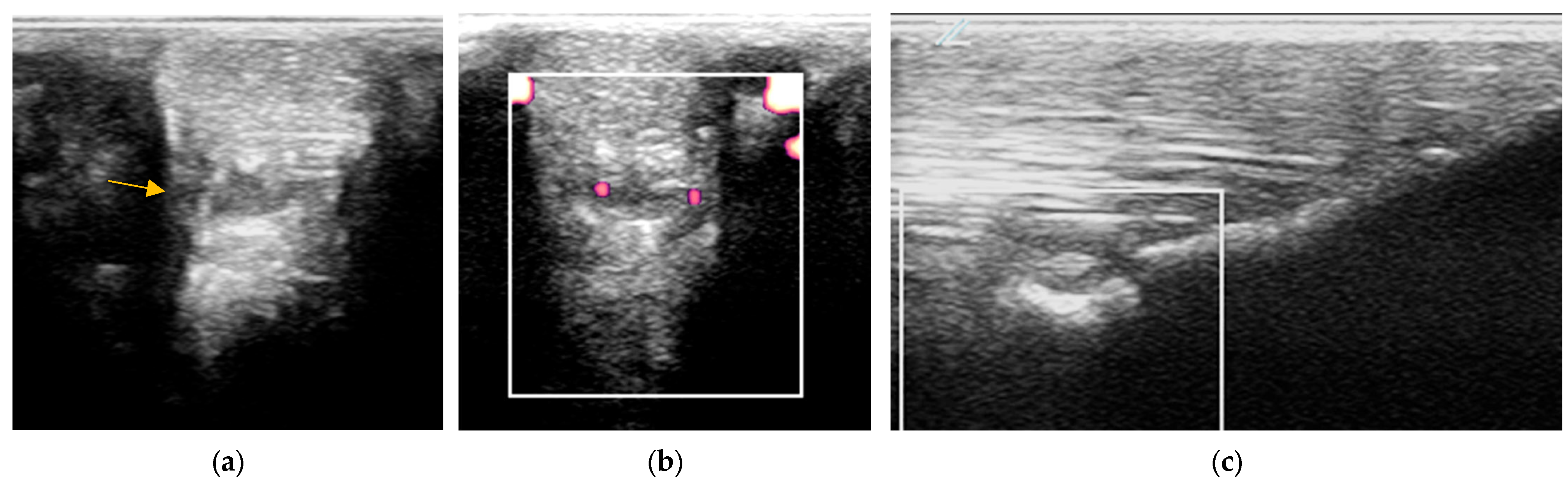
2.3. Diagnostic Imaging
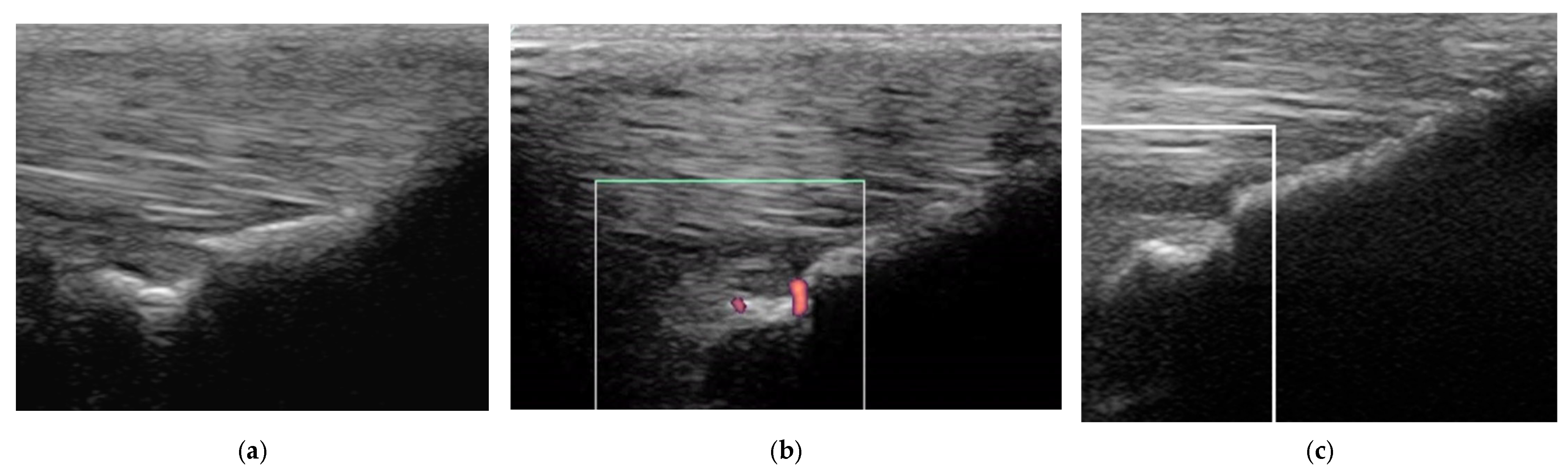
2.3.1. B-Mode Ultrasonography
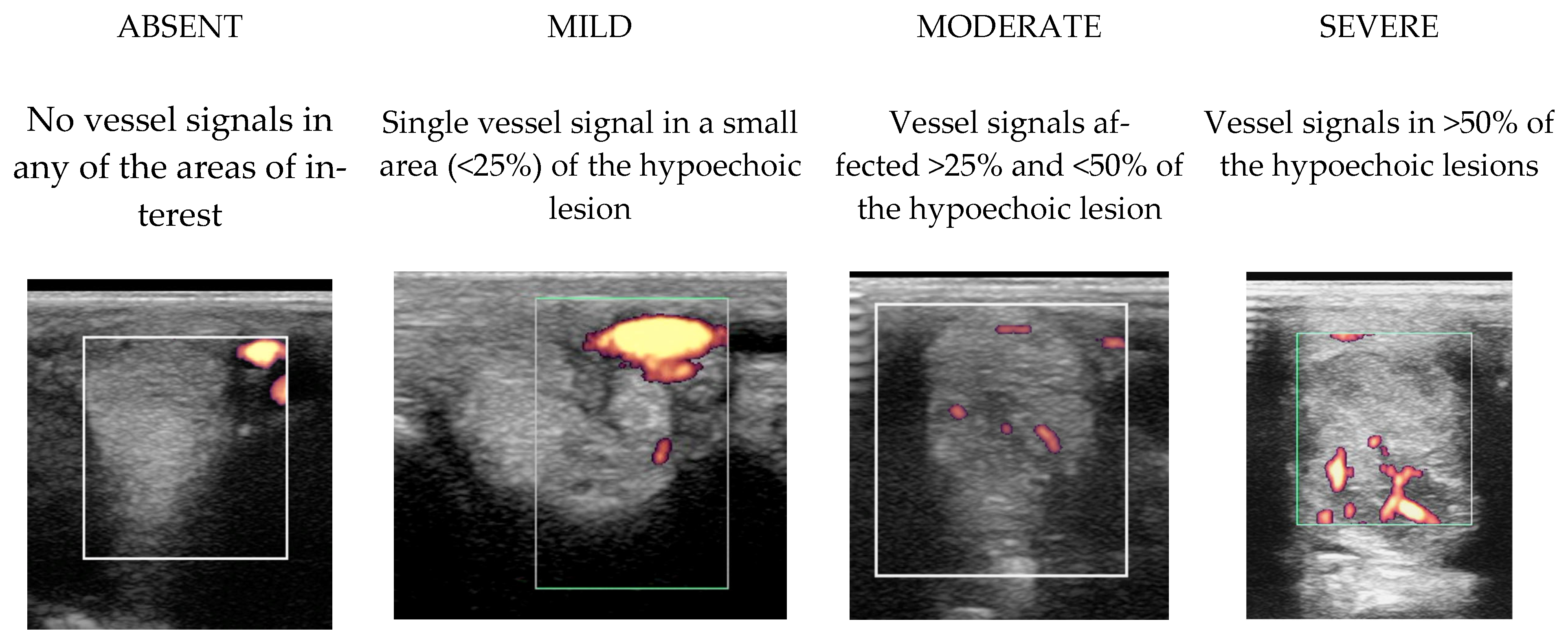
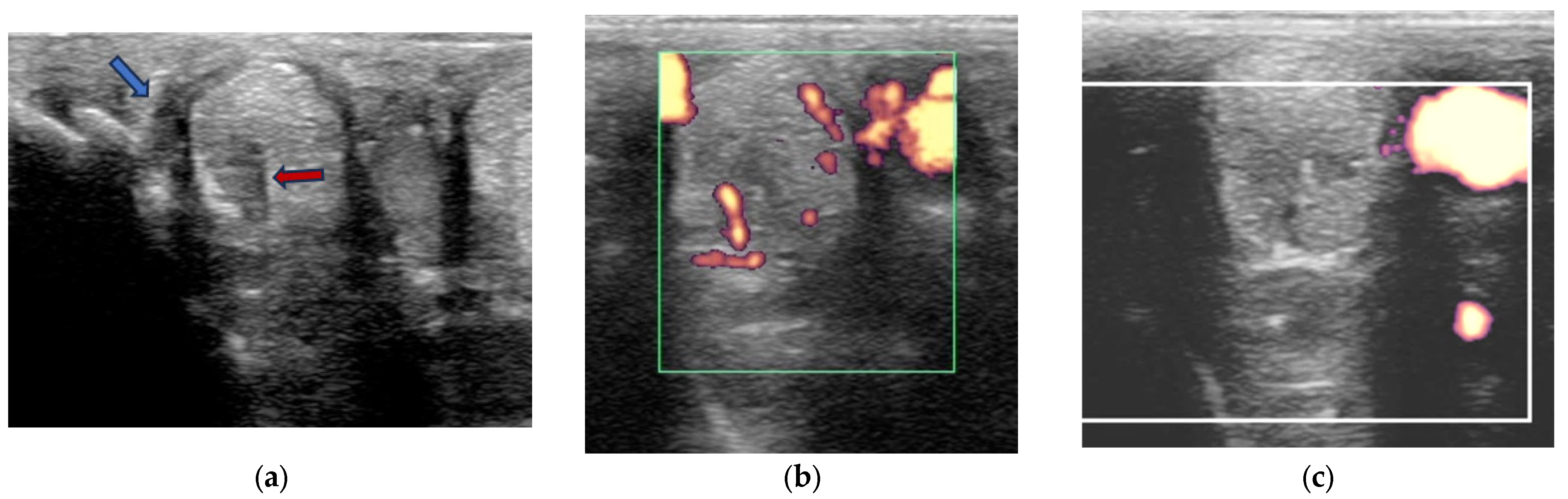
2.3.2. Power Doppler Examination
2.3.3. Radiography
2.4. Image Interpretation
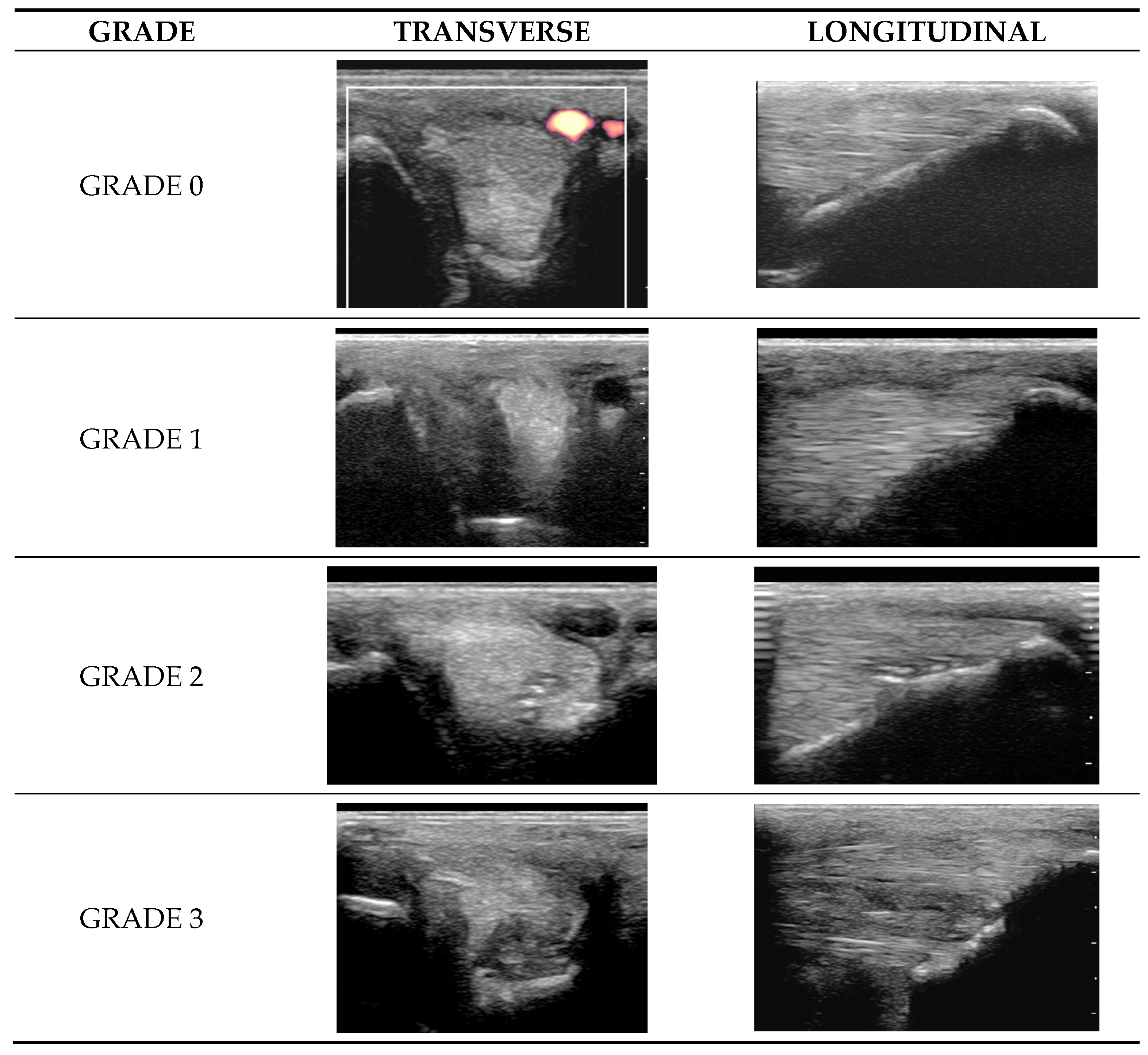
2.4.1. Classification of Ultrasonographic Lesions
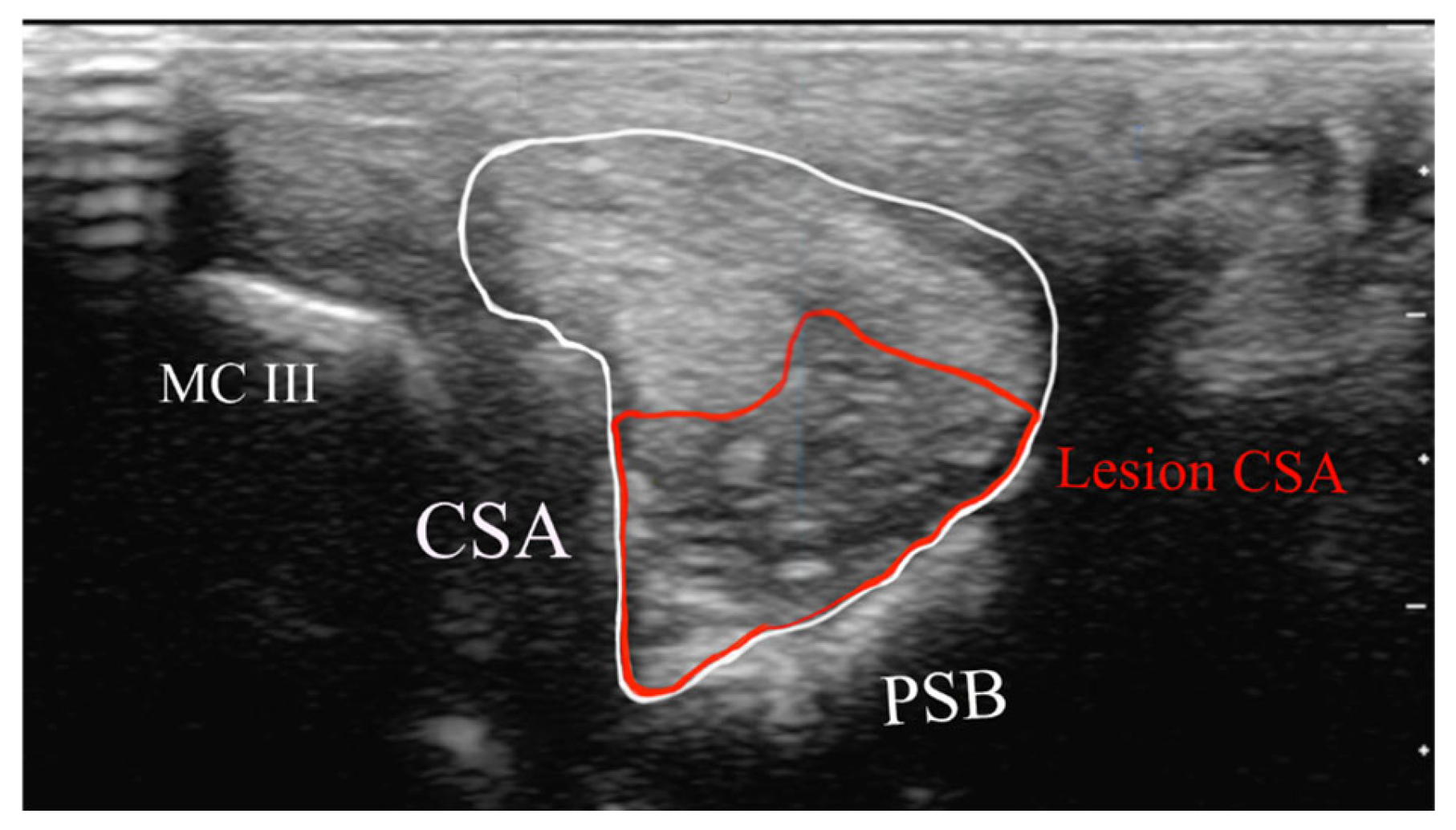
- Cross-sectional area of the SL branch (cm2);
- Lesion CSA (cm2).
- Grade 0 = normal: uniform normal echogenicity; long linear parallel echoes in longitudinal images.
- Grade 1 = mild: less than 25% of the CSA of the ligament was hypoechoic with localised hypoechoic or anechoic lesions. Loss of linear echoes, reduced echogenicity in demarcated areas in longitudinal images.
- Grade 2 = moderate: Hypoechoic or anechoic regions occupying 25–50% of the CSA of the ligament. Loss of long linear parallel echoes in longitudinal images and mild to moderate changes at the enthesis such as irregularity of the bone surface or hyperechoic regions in the SL.
- Grade 3 = severe: Large anechoic and hypoechoic areas occupying >50% of the CSA of the ligament in transverse images. Hypoechoic or anechoic regions in longitudinal images and/or large hyperechoic regions (an avulsion or dystrophic mineralisation) in the SL and/or considerable irregularity of the bone surface at the enthesis.
2.4.2. Radiological Interpretation
- Linear radiolucencies originating from the abaxial surface (widened vascular channels [27]) subdivided into one linear radiolucent line or more than one linear radiolucent line.
- Focal approximately oval or circular radiolucencies.
2.5. Follow-Up Ultrasonographic Examinations
2.6. Treatment and Follow-Up
2.6.1. Modification of Exercise
2.6.2. Additional Treatments
- First-choice treatment was intra-articular administration of corticosteroids (triamcinolone, [6–12 mg/joint varying according to lesion severity and concomitant treatments], bethamethasone [6–18 mg/joint] or dexamethasone [6–18 mg/joint]) (variable manufacturers’ products for each corticosteroid were used). If more than one joint was treated, the dose was adjusted to a safe maximum dose [28].
- For more advanced osteoarthritis, polyacrylamide gel was administered (Arthramid Gel, Contura 301 Mallory Lane, Suite100, Fanklin, TN, USA).
2.6.3. Management After Return to Full Work
2.6.4. Acquisition of Follow-Up Data
2.7. Data Analysis
3. Results
3.1. Demographic Data and Clinical Examination
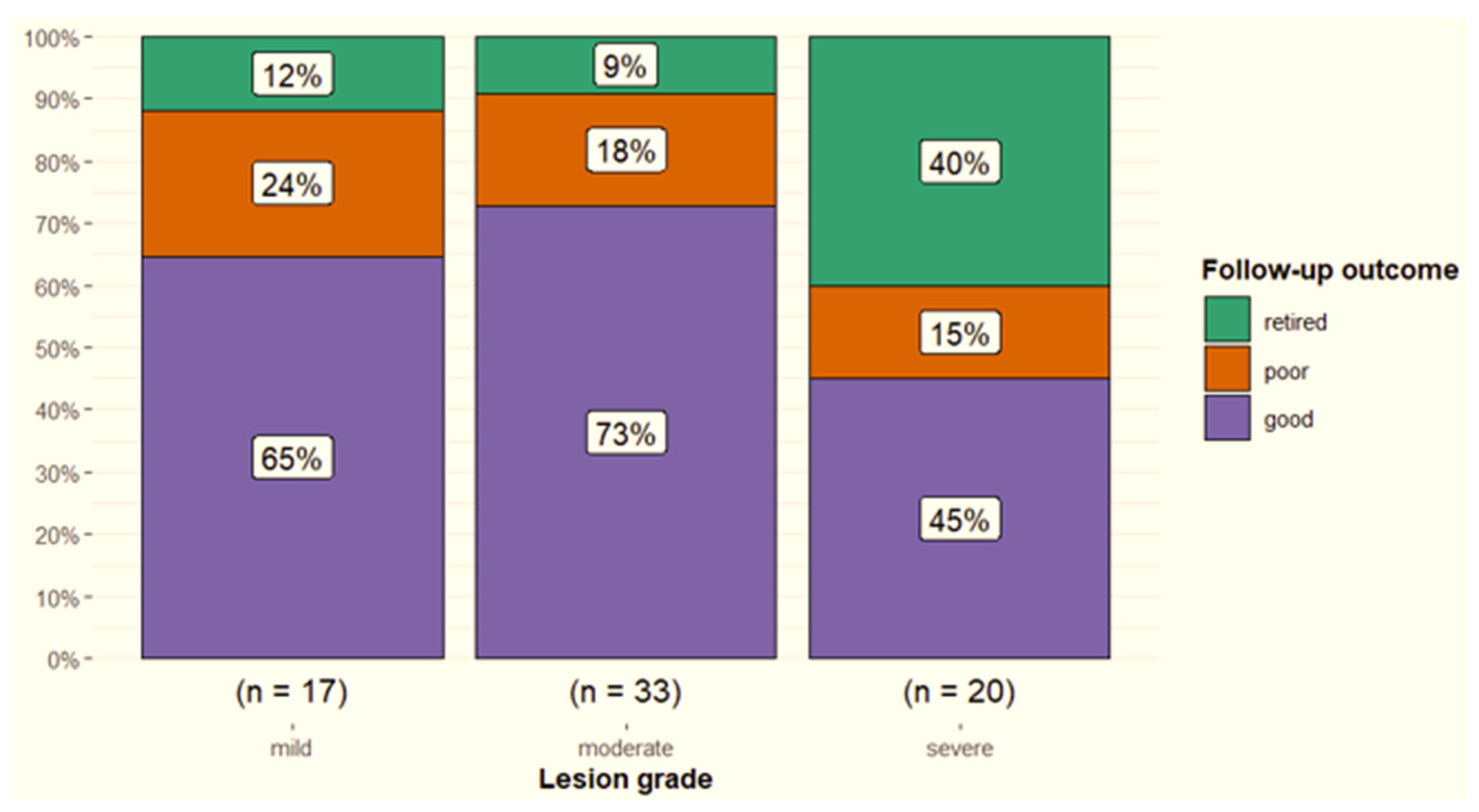
3.2. Diagnostic Ultrasonography
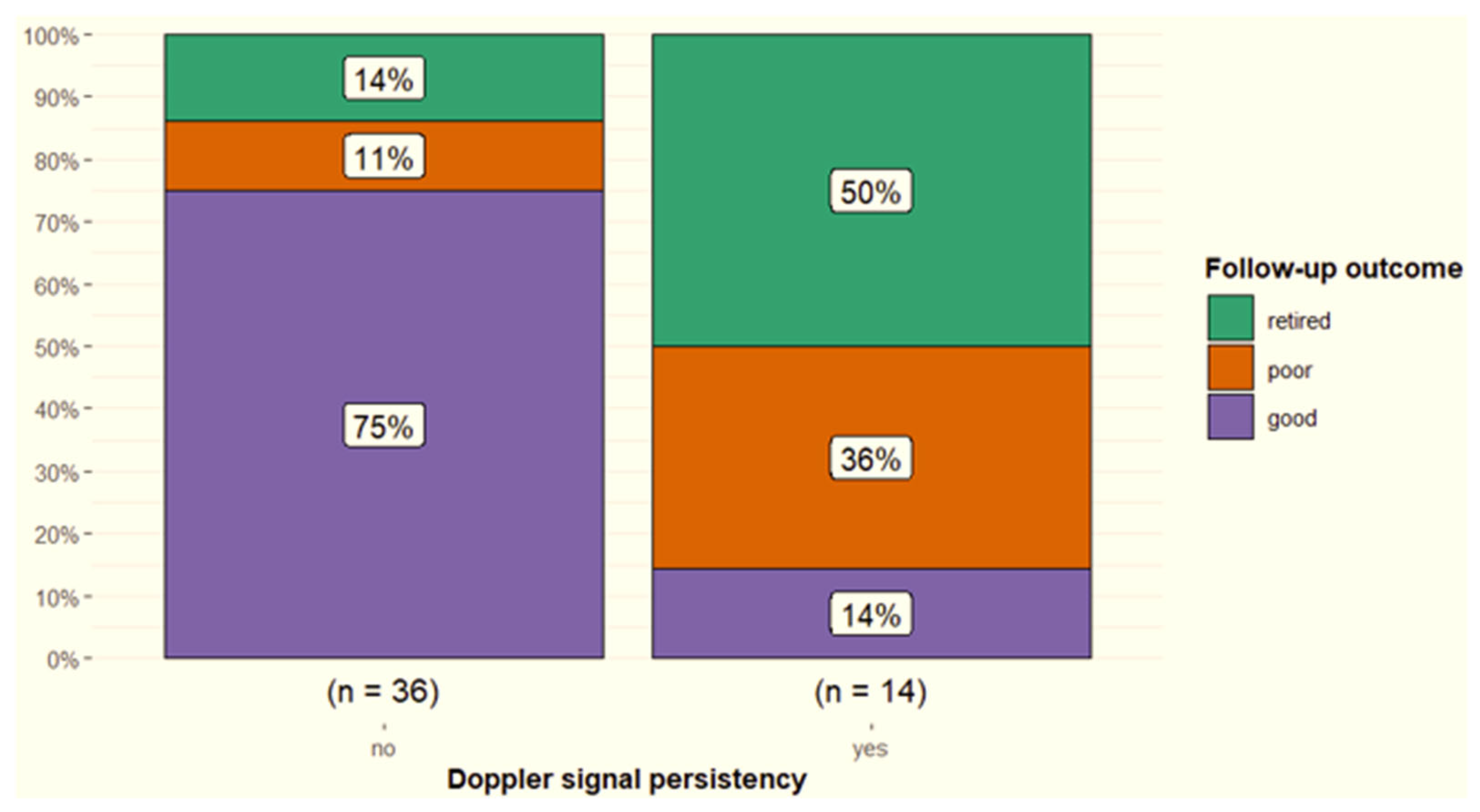
3.3. Power Doppler Examination
3.4. Radiographic Examination
3.5. Other Concurrent Injuries in the Limb with a Branch Injury
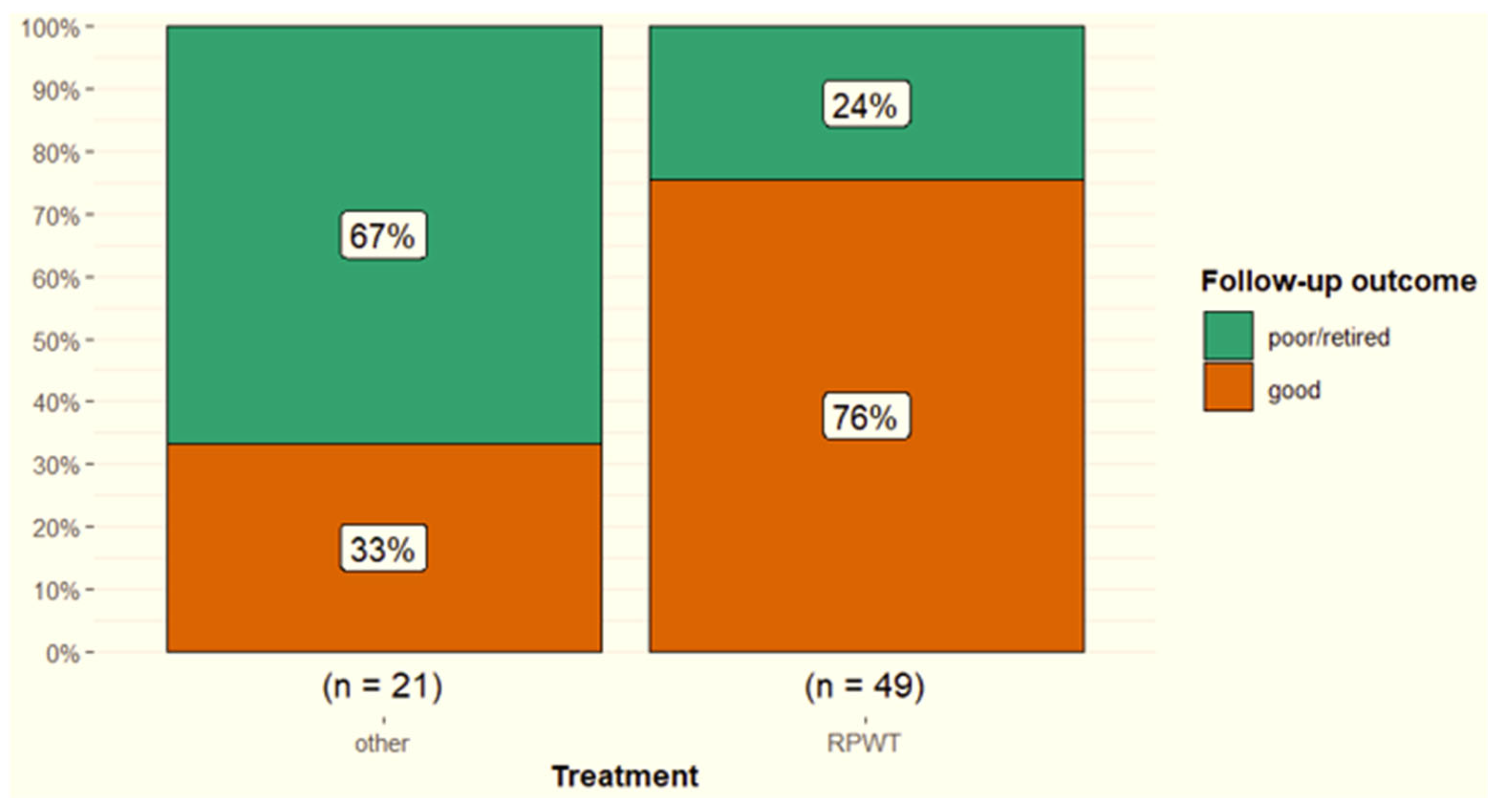
3.6. Treatment and Outcome
3.7. Recurrent Injuries
3.8. Second Injuries
4. Discussion
4.1. Results Related to Hypotheses
4.1.1. Lateral Versus Medial Branches and Forelimbs Versus Hindlimbs
4.1.2. Distal Lesions: The Enthesis
4.1.3. Ultrasonographic Parameters and Outcome
4.2. Power Doppler
4.3. Monitoring: The Use of Power Doppler in Non-Healed Cases; Uncontrolled Inflammatory Response
4.4. Periligamentous Fibrosis
4.5. Primary Fetlock Osteoarthritis, Secondary Branch Desmitis; Compensatory Overload?
4.6. Treatment, Rehabilitation and Outcome of SL Branch Desmitis
4.7. Is Prevention of Injury Possible?
4.8. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marneris, D.; Dyson, S.J. Clinical Features, Diagnostic Imaging Findings and Concurrent Injuries in 71 Sports Horses with Suspensory Branch Injuries. Equine Vet. Educ. 2014, 26, 312–321. [Google Scholar] [CrossRef]
- Minshall, G.J.; Wright, I.M. Arthroscopic Diagnosis and Treatment of Intra-Articular Insertional Injuries of the Suspensory Ligament Branches in 18 Horses. Equine Vet. J. 2006, 38, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, A.; Iagnocco, A.; Perrotta, F.M.; Modesti, M.; Scarno, A.; Valesini, G. Clinical and Ultrasonography Assessment of Peripheral Enthesitis in Ankylosing Spondylitis. Rheumatology 2011, 50, 2080–2086. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, M.; Toumi, H.; Ralphs, J.R.; Bydder, G.; Best, T.M.; Milz, S. Where Tendons and Ligaments Meet Bone: Attachment Sites (‘entheses’) in Relation to Exercise and/or Mechanical Load. J. Anat. 2006, 208, 471–490. [Google Scholar] [CrossRef]
- Denoix, J.M. Functional Anatomy of Tendons and Ligaments in the Distal Limbs (Manus and Pes). Vet. Clin. N. Am. Equine Pract. 1994, 10, 273–322. [Google Scholar] [CrossRef]
- Denoix, J.-M. Bioemechanical analysis of lateral movements: The forelimbs. In Biomechanics and Physical Training of the Horse, 1st ed.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2013; pp. 72–80. [Google Scholar]
- Plevin, S.; McLellan, J. The Effect of Insertional Suspensory Branch Desmitis on Racing Performance in Juvenile Thoroughbred Racehorses. Equine Vet. J. 2014, 46, 451–457. [Google Scholar] [CrossRef]
- McLellan, J.; Plevin, S. Do Radiographic Signs of Sesamoiditis in Yearling Thoroughbreds Predispose the Development of Suspensory Ligament Branch Injury? Equine Vet. J. 2014, 46, 446–450. [Google Scholar] [CrossRef]
- Peat, F.J.; Kawcak, C.E.; McIlwraith, C.W.; Berk, J.T.; Keenan, D.P.; Selberg, K.T.; Ojeda, A. Ultrasonography of the Suspensory Ligament Branches in Yearling and 2-Year-Old Thoroughbred Sales Horses: Prevalence, Progression of Findings and Associations with Racing Performance. Equine Vet. J. 2025, 57, 384–397. [Google Scholar] [CrossRef]
- Ramzan, P.H.L.; Palmer, L.; Dallas, R.S.; Shepherd, M.C. Subclinical Ultrasonographic Abnormalities of the Suspensory Ligament Branch of the Athletic Horse: A Survey of 60 Thoroughbred Racehorses. Equine Vet. J. 2013, 45, 159–163. [Google Scholar] [CrossRef]
- Fairburn, A.J.; Busschers, E.; Barr, A.R.S. Subclinical Ultrasonographic Abnormalities of the Suspensory Ligament Branches in National Hunt Racehorses. Equine Vet. J. 2017, 49, 475–479. [Google Scholar] [CrossRef]
- Read, R.M.; Boys-Smith, S.; Bathe, A.P. Subclinical Ultrasonographic Abnormalities of the Suspensory Ligament Branches Are Common in Elite Showjumping Warmblood Horses. Front. Vet. Sci. 2020, 7, 117. [Google Scholar] [CrossRef]
- Rabba, S.; Grulke, S.; Verwilghen, D.; Evrard, L.; Busoni, V. B-Mode and Power Doppler Ultrasonography of the Equine Suspensory Ligament Branches: A Descriptive Study on 13 Horses. Vet. Radiol. Ultrasound 2018, 59, 453–460. [Google Scholar] [CrossRef]
- International Equestrian Federation. FEI Dressage Handbook Guidelines for Judging; Féderation Equestre Internationale: Laussane, Switzerland, 2007. [Google Scholar]
- Féderation Equestre Internationale Dressage Rules 26th Edition, Effective 1 January 2024. Available online: www.fei.org/fei/regulations/dressage (accessed on 23 July 2025).
- Clayton, H.M.; Hobbs, S.J. A Review of Biomechanical Gait Classification with Reference to Collected Trot, Passage and Piaffe in Dressage Horses. Animals 2019, 9, 763. [Google Scholar] [CrossRef]
- Walker, V.A.; Tranquille, C.A.; Newton, J.R.; Dyson, S.J.; Brandham, J.; Northrop, A.J.; Murray, R.C. Comparison of Limb Kinematics between Collected and Lengthened (Medium/Extended) Trot in Two Groups of Dressage Horses on Two Different Surfaces. Equine Vet. J. 2017, 49, 673–680. [Google Scholar] [CrossRef]
- Dyson, S.; Murray, R. Management of Hindlimb Proximal Suspensory Desmopathy by Neurectomy of the Deep Branch of the Lateral Plantar Nerve and Plantar Fasciotomy: 155 Horses (2003–2008). Equine Vet. J. 2012, 44, 361–367. [Google Scholar] [CrossRef]
- Gruyaert, M.; Pollard, D.; Dyson, S.J. An Investigation into the Occurrence of, and Risk Factors for Concurrent Suspensory Ligament Injuries in Horses with Hindlimb Proximal Suspensory Desmopathy. Equine Vet. Educ. 2020, 32, 173–182. [Google Scholar] [CrossRef]
- Murray, R.C.; Walters, J.M.; Snart, H.; Dyson, S.J.; Parkin, T.D.H. Identification of Risk Factors for Lameness in Dressage Horses. Vet. J. 2010, 184, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Boado, A.; Pollard, D.; Dyson, S. A Retrospective Study of the Evolution of Orthopaedic Injuries in 70 Dressage Horses. Animals 2025, 15, 1740. [Google Scholar] [CrossRef] [PubMed]
- Boado, A.; Pollard, D.; Lopez-Sanroman, J.; Dyson, S. Orthopaedic injuries in 272 dressage horses: A retrospective study. Animals 2025, 15, 2972. [Google Scholar] [CrossRef]
- Bassage, L.H.; Ross, M.W. Diagnostic Analgesia. In Diagnosis and Management of Lameness in the Horse, 2nd ed.; Ross, M.W., Dyson, S.J., Eds.; W.B. Saunders: Saint Louis, MO, USA, 2011; pp. 100–135. ISBN 978-1-4160-6069-7. [Google Scholar]
- Szkudlarek, M.; Court-Payen, M.; Jacobsen, S.; Klarlund, M.; Thomsen, H.S.; Østergaard, M. Interobserver Agreement in Ultrasonography of the Finger and Toe Joints in Rheumatoid Arthritis. Arthritis Rheum. 2003, 48, 955–962. [Google Scholar] [CrossRef]
- Butler, J.A.; Colles, C.M.; Dyson, S.J.; Kold, S.E.; Poulos, P.W. Metacarpophalangeal and metatarsophalangeal (fetlock) joints. In Clinical Radiology of the Horse, 4th ed.; Wiley-Blackwell: Oxford, UK, 2016; pp. 175–213. ISBN 9781118912263. [Google Scholar]
- Butler, J.A.; Colles, C.M.; Dyson, S.J.; Kold, S.E.; Poulos, P.W. General Principles. In Clinical Radiology of the Horse, 4th ed.; Wiley-Blackwell: Oxford, UK, 2016; pp. 1–39. ISBN 9781118912263. [Google Scholar]
- Lloyd, K.A.; Ayodele, B.A.; Hitchens, P.L.; Beck, C.; Mackie, E.J.; Whitton, R.C. Associations between the Radiographic Appearance of Vascular Channels in Proximal Sesamoid Bones, Their Microstructural Characteristics and Past Racing Performance in Thoroughbreds. Equine Vet. J. 2020, 52, 670–677. [Google Scholar] [CrossRef]
- Boorman, S.; McMaster, M.A.; Groover, E.; Caldwell, F. Review of Glucocorticoid Therapy in Horses: Intra-Articular Corticosteroids. Equine Vet. Educ. 2023, 35, 327–336. [Google Scholar] [CrossRef]
- Perneger, T.V. Adjusting for Multiple Testing in Studies Is Less Important than Other Concerns. Brit Med. J. 1999, 318, 1288. [Google Scholar] [CrossRef] [PubMed]
- Rabba, S.; Petrucci, V.; Petrizzi, L.; Giommi, D.W.; Busoni, V. B-Mode Ultrasonographic Abnormalities and Power Doppler Signal in Suspensory Ligament Branches of Nonlame Working Quarter Horses. J. Equine Vet. Sci. 2020, 94, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.; Kleyer, A.; Bayat, S.; Knitza, J.; Valor-Mendez, L.; Schweiger, M.; Schett, G.; Tascilar, K.; Hueber, A.J. Biomechanical Stress in the Context of Competitive Sports Training Triggers Enthesitis. Arthritis Res. Ther. 2021, 23, 172. [Google Scholar] [CrossRef]
- Joostens, Z.; Audigié, F.; de la Rebière de Pouyade, G.; Garigliany, M.M.; Busoni, V. Histological Study of the Normal Proximal Third Interosseus Muscle Enthesis in the Equine Pelvic Limb. J. Vet. Med. Series C Anat. Histol. Embryol. 2025, 54, e70059. [Google Scholar] [CrossRef]
- Peat, F.J.; Kawcak, C.E.; McIlwraith, C.W.; Keenan, D.P.; Berk, J.T.; Mork, D.S. Radiological Findings in the Proximal Sesamoid Bones of Yearling and 2-Year-Old Thoroughbred Sales Horses: Prevalence, Progression and Associations with Racing Performance. Equine Vet. J. 2025, 57, 87–100. [Google Scholar] [CrossRef]
- Le Goff, B.; Berthelot, J.M.; Guillot, P.; Glémarec, J.; Maugars, Y. Assessment of Calcific Tendonitis of Rotator Cuff by Ultrasonography: Comparison between Symptomatic and Asymptomatic Shoulders. Jt. Bone Spine 2010, 77, 258–263. [Google Scholar] [CrossRef]
- Boesen, M.I.; Boesen, M.; Langberg, H.; Koenig, M.J.; Boesen, A.; Bliddal, H.; Torp-Pedersen, S. Musculoskeletal Colour/Power Doppler in Sports Medicine: Image Parameters, Artefacts, Image Interpretation and Therapy. Clin. Exp. Rheumatol. 2010, 28, 103–113. [Google Scholar]
- Rubin, J.M. Musculoskeletal Power Doppler. Eur. Radiol. 1999, 9, 403–406. [Google Scholar] [CrossRef]
- Zanetti, M.; Metzdorf, A.; Kundert, H.P.; Zollinger, H.; Vienne, P.; Seifert, B.; Hodler, J. Achilles Tendons: Clinical Relevance of Neovascularization Diagnosed with Power Doppler US. Radiology 2003, 227, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.S.; Adler, R.S.; Bude, R.O.; Rubin, J.M. Detection of Soft-Tissue Hyperemia: Value of Power Doppler Sonography. Am. J. Roent 1994, 163, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Wakefield, R.J.; Brown, A.K.; O’Connor, P.J.; Emery, P. Power Doppler Sonography: Improving Disease Activity Assessment in Inflammatory Musculoskeletal Disease. Arthritis Rheum. 2003, 48, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, C.; Stieler, M.; Saunders, R.; Bisset, L.; Vicenzino, B. Diagnostic Accuracy of Power Doppler Ultrasound in Patients with Chronic Tennis Elbow. Br. J. Sports Med. 2008, 42, 572–576. [Google Scholar] [CrossRef]
- Bhasin, S.; Cheung, P.P. The Role of Power Doppler Ultrasonography as Disease Activity Marker in Rheumatoid Arthritis. Dis. Markers 2015, 2015, 325909. [Google Scholar] [CrossRef]
- Carotti, M.; Galeazzi, V.; Catucci, F.; Zappia, M.; Arrigoni, F.; Barile, A.; Giovagnoni, A. Clinical Utility of Eco-Color-Power Doppler Ultrasonography and Contrast Enhanced Magnetic Resonance Imaging for Interpretation and Quantification of Joint Synovitis: A Review. Acta Biomed. 2018, 89, 48–77. [Google Scholar] [CrossRef]
- Murata, D.; Misumi, K.; Fujiki, M. A Preliminary Study of Diagnostic Color Doppler Ultrasonography in Equine Superficial Digital Flexor Tendonitis. J. Vet. Med. Sci. 2012, 74, 1639–1642. [Google Scholar] [CrossRef]
- Eliasson, P.; Couppé, C.; Lonsdale, M.; Svensson, R.B.; Neergaard, C.; Kjær, M.; Friberg, L.; Magnusson, S.P. Ruptured Human Achilles Tendon Has Elevated Metabolic Activity up to 1 Year after Repair. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1868–1877. [Google Scholar] [CrossRef]
- Daniel, A.J.; Judy, C.E.; Saveraid, T. Magnetic Resonance Imaging of the Metacarpo(tarso)phalangeal Region in Clinically Lame Horses Responding to Diagnostic Analgesia of the Palmar Nerves at the Base of the Proximal Sesamoid Bones: Five Cases. Equine Vet. Educ. 2013, 25, 222–228. [Google Scholar] [CrossRef]
- Brommer, H.; Van Weeren, P.R.; Brama, P.A.J.; Barneveld, A. Quantification and Age-Related Distribution of Articular Cartilage Degeneration in the Equine Fetlock Joint. Equine Vet. J. 2003, 35, 697–701. [Google Scholar] [CrossRef]
- Sherlock, C.E.; Mair, T.S.; Ter Braake, F. Osseous Lesions in the Metacarpo(Tarso)Phalangeal Joint Diagnosed Using Low-Field Magnetic Resonance Imaging in Standing Horses. Vet. Radiol. Ultrasound 2009, 50, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Vandersmissen, M.; Evrard, L.; Charles, A.; Audigié, F.; Busoni, V. Diagnostic Imaging Findings in Lame Warmblood Horses with Bone Injuries of the Medial Proximal Phalanx Glenoid Cavity. Vet. Radiol. Ultrasound 2025, 66, e13449. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, J.E.; Joostens, Z.; Broeckx, B.J.G.; Hauspie, S.; Mariën, T.; Vanderperren, K. Low-Field Magnetic Resonance Imaging of Sagittal Groove Disease of the Proximal Phalanx in Non-Racing Sport Horses. Equine Vet. J. 2025, 57, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Dyson, S.J. Combined Standing Low-Field Magnetic Resonance Imaging and Fan-Beam Computed Tomographic Diagnosis of Fetlock Region Pain in 27 Sports Horses. Equine Vet. J. 2025, 57, 1313–1327. [Google Scholar] [CrossRef]
- Dyson, S. (Independent Researcher, Diss, UK). Unpublished Data, 2012–2019.
- Hansen, S.H.; Bramlage, L.R.; Moore, G.E. Racing Performance of Thoroughbred Racehorses with Suspensory Ligament Branch Desmitis Treated with Mesenchymal Stem Cells (2010–2019). Equine Vet. J. 2024, 56, 503–513. [Google Scholar] [CrossRef]
- Pluim, M.; Martens, A.; Vanderperren, K.; van Weeren, R.; Oosterlinck, M.; Dewulf, J.; Kichouh, M.; Van Thielen, B.; Koene, M.H.W.; Luciani, A.; et al. High-Power Laser Therapy Improves Healing of the Equine Suspensory Branch in a Standardized Lesion Model. Front. Vet. Sci. 2020, 7, 600. [Google Scholar] [CrossRef]
- Pluim, M.; Martens, A.; Vanderperren, K.; Sarazin, S.; Koene, M.; Luciani, A.; van Weeren, P.; Delesalle, C. Short- and long term follow-up of 150 sports horses diagnosed with tendinopathy or desmopathy by ultrasonographic examination and treated with high-power laser therapy. Res. Vet. Sci. 2018, 119, 232–238. [Google Scholar] [CrossRef]
- Kadic, D.T.N.; Minshall, G.J.; Wright, I.M. Surgical Management of Marginal Tears/Avulsions of the Suspensory Ligament Branches in 29 Thoroughbred Racehorses. Equine Vet. J. 2019, 51, 310–315. [Google Scholar] [CrossRef]
- Lőffeld, S.; Boening, K.J.; Weitkamp, K.; Stadler, P. Radial extracorporeal shock wave therapy for horses with chronic insertion desmopathy of the proximal suspensory ligament–a controlled study. Pferdeheilkunde 2002, 18 (Suppl. S2), 147–154. [Google Scholar] [CrossRef]
- Crowe, O.M.; Dyson, S.J.; Wright, I.M.; Schramme, M.C.; Smith, R. Treatment of chronic or recurrent proximal suspensory desmitis using radial pressure wave therapy in the horse. Equine Vet. J. 2004, 36, 313–316. [Google Scholar] [CrossRef]
- Osborne, C. Does extracorporeal shockwave therapy or radial pressure wave therapy improve return to function over conservative and/or surgical management in horses with proximal suspensory desmitis? Equine Vet. Educ. 2021, 33, 278–280. [Google Scholar] [CrossRef]
- Johnson, S.; Richards, R.; Frisbie, D.; Esselman, A.; McClure, S. Equine shock wave therapy-where are we now? Equine Vet. J. 2023, 55, 593–606. [Google Scholar] [CrossRef]
- Boström, A.; Bergh, A.; Hyytiäinen, H.; Asplund, K. Systematic Review of Complementary and Alternative Veterinary Medicine in Sport and Companion Animals: Extracorporeal Shockwave Therapy. Animals 2022, 12, 3124. [Google Scholar] [CrossRef]
- Pezzanite, L.; Bass, L.; Kawcak, C.; Goodrich, L.; Moorman, V. The Relationship between Sagittal Hoof Conformation and Hindlimb Lameness in the Horse. Equine Vet. J. 2019, 51, 464–469. [Google Scholar] [CrossRef]
- Hodgson, D.; McGowan, C. An Overview of Performance and Sports Medicine. In The Athletic Horse: Principles and Practice of Equine Sports Medicine, 2nd ed.; Hodgson, D., McGowan, C., McKeever, C., Eds.; Elsevier, Saunders: Saint Louis, MO, USA, 2013; pp. 1–8. [Google Scholar]
- Murray, R.C.; Walters, J.; Snart, H.; Dyson, S.; Parkin, T. How Do Features of Dressage Arenas Influence Training Surface Properties Which Are Potentially Associated with Lameness? Vet. J. 2010, 186, 172–179. [Google Scholar] [CrossRef]
- Smyth, E.A.; Newman, P.; Waddington, G.; Weissensteiner, J.R.; Drew, M.K. Injury Prevention Strategies Specific to Pre-Elite Athletes Competing in Olympic and Professional Sports-A Systematic Review. J. Sci. Med. Sport 2019, 22, 887–901. [Google Scholar] [CrossRef]



| Factors Potentially Associated with Outcome | p-Value | |
|---|---|---|
| Horse factors | Age | 0.85 |
| Breed | 0.24 | |
| Training level | 0.53 | |
| Number of injured branches | 0.27 | |
| Number of limbs affected | 0.62 | |
| Ultrasonography | Localisation of lesion | 0.65 |
| Lesion grade | 0.07 | |
| CSA of injured branch | 0.96 | |
| CSA of lesion | 0.28 | |
| CSA of lesion as a percentage of SL CSA | 0.40 | |
| Acoustic shadowing | 0.16 | |
| Periligamentous fibrosis | 0.60 | |
| Severity of power Doppler signal | 0.20 | |
| Persistence of power Doppler signal over time | <0.001 | |
| Radiography | Osteoarthritis of MCP/MTP joint | 0.73 |
| Radiological abnormalities of PSBs | 0.69 | |
| Treatment | Radial pressure wave treatment versus other | 0.003 |
| Horse | Initial Injury | Injury Grade | Outcome | Second Injury | Outcome | Third Injury | Outcome |
|---|---|---|---|---|---|---|---|
| 2 | LH Lateral | 3 | Good | RH Lateral | Good | ||
| 4 | LF Medial | 2 | Good | RH Lateral | Good | Recurrent RH Lateral | Good |
| 6 | RF Lateral | 3 | Good | LH Lateral | Retired | ||
| 43 | LH Medial | 3 | Good | RF Lateral | Good | ||
| 47 | LF Lateral | 3 | Good | LH Lateral | Good | ||
| 58 | LF Lateral | 2 | Good | RF Lateral and PSD | Retired |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boado, A.; Pollard, D.; Dyson, S. Retrospective Analysis of Suspensory Ligament Branch Injuries in 70 Dressage Horses. Animals 2025, 15, 3079. https://doi.org/10.3390/ani15213079
Boado A, Pollard D, Dyson S. Retrospective Analysis of Suspensory Ligament Branch Injuries in 70 Dressage Horses. Animals. 2025; 15(21):3079. https://doi.org/10.3390/ani15213079
Chicago/Turabian StyleBoado, Ana, Danica Pollard, and Sue Dyson. 2025. "Retrospective Analysis of Suspensory Ligament Branch Injuries in 70 Dressage Horses" Animals 15, no. 21: 3079. https://doi.org/10.3390/ani15213079
APA StyleBoado, A., Pollard, D., & Dyson, S. (2025). Retrospective Analysis of Suspensory Ligament Branch Injuries in 70 Dressage Horses. Animals, 15(21), 3079. https://doi.org/10.3390/ani15213079






