Association of Environmental Temperature and Relative Humidity with Ocular and Flank Temperatures in Dromedary Camels
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Study Location
2.3. Thermal Imaging
2.4. Statistical Analysis
- y = dependent variable
- intercept
- is slope/rate of change () and independent variable (x)
- correlation between x and y
- sum of deviation of x from its mean
- Ssum of deviation of y from its mean
- sum of squared deviation of x from its mean
- Ssum of squared deviation of y from its mean
3. Results
3.1. Association of Eye Temperature (ET) with Age, Sex, and Physiological State
3.2. Association of Flank Temperature (FT) with Age and Sex
3.3. Association of Camels’ Superficial Skin Temperature and Environment
3.4. Correlation Between Temperature and Environment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vieira, R.A.; Dias, E.A.; Stumpf, M.T.; Pereira, G.R.; Barcellos, J.O.J.; Kolling, G.J.; McManus, C. Use of Thermography and Physiological Rate to Assess Heat Tolerance in Cattle Breeds. Trop. Anim. Health Prod. 2023, 55, 223. [Google Scholar] [CrossRef] [PubMed]
- Giro, A.; de Campos Bernardi, A.C.; Barioni Junior, W.; Lemes, A.P.; Botta, D.; Romanello, N.; do Nascimento Barreto, A.; Garcia, A.R. Application of microchip and infrared thermography for monitoring body temperature of beef cattle kept on pasture. J. Therm. Biol. 2019, 84, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Q.; Chu, M.; Kang, X.; Liu, G. Application of infrared thermography and machine learning techniques in cattle health assessments: A review. Biosyst. Engg. 2023, 230, 361–387. [Google Scholar] [CrossRef]
- Lovarelli, D.; Bacenetti, J.; Guarino, M. A review on dairy cattle farming: Is precision livestock farming the compromise for an environmental, economic and social sustainable production? J. Clean. Prod. 2020, 262, 121409. [Google Scholar] [CrossRef]
- Rosa, G.J.M. Grand challenge in precision livestock farming. Front. Anim. Sci. 2021, 2, 650324. [Google Scholar] [CrossRef]
- Lavers, C.; Franks, K.; Floyd, M.; Plowman, A. Application of remote thermal imaging and night vision technology to improve endangered wildlife resource management with minimal animal distress and hazard to humans. J. Phys. Conf. Ser. 2005, 15, 2007–2012. [Google Scholar] [CrossRef]
- Ghezzi, M.D.; Napolitano, F.; Casas-Alvarado, A.; Hernández-Ávalos, I.; Domínguez-Oliva, A.; Olmos-Hernández, A.; Pereira, A.M.F. Utilization of Infrared Thermography in Assessing Thermal Responses of Farm Animals under Heat Stress. Animals 2024, 14, 616. [Google Scholar] [CrossRef]
- Wijffels, G.; Sullivan, M.; Gaughan, J. Methods to Quantify Heat Stress in Ruminants: Current Status and Future Prospects. Methods 2021, 186, 3–13. [Google Scholar] [CrossRef]
- Lees, A.M.; Salvin, H.E.; Colditz, I.G.; Lee, C. The influence of temperament on body temperature response to handling in Angus cattle. Animals 2020, 10, 172. [Google Scholar] [CrossRef]
- Padalino, B.; Menchetti, L. The first protocol for assessing the welfare of dromedary camels (Camelus dromedarius) kept under nomadic pastoralism. Front. Vet. Sci. 2024, 11, 1416714. [Google Scholar] [CrossRef]
- Mullakkalparambil-Velayudhan, S.; Sejian, V.; Devaraj, C.; Ruban, W.; Kadam, V.; König, S. Novel Insights to Assess Climate Resilience in Goats Using a Holistic Approach of Skin-Based Advanced NGS Technologies. Int. J. Mol. Sci. 2023, 24, 10319. [Google Scholar] [CrossRef] [PubMed]
- Morgado, J.N.; Lamonaca, E.; Santeramo, F.G.; Caroprese, M.; Albenzio, M.; Ciliberti, M.G. Effects of management strategies on animal welfare and productivity under heat stress: A synthesis. Front. Vet. Sci. 2023, 10, 1145610. [Google Scholar] [CrossRef] [PubMed]
- Sejian, V.; Bhatta, R.; Gaughan, J.B.; Dunshea, F.R.; Lacetera, N. Review: Adaptation of Animals to Heat Stress. Animals 2018, 12, s431–s444. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Pereira, M.F.A.; Wang, D.; Martínez-Burnes, J.; Ghezzi, M.; Hernández-Ávalos, I.; Lendez, P.; Mora-Medina, P.; Casas, A.; Olmos-Hernández, A. Clinical Applications and Factors Involved in Validating Thermal Windows in Large Ruminants to Assess Health and Productivity. Animals 2021, 11, 2247. [Google Scholar] [CrossRef]
- Travain, T.; Valsecchi, P. Infrared thermography in the study of animals’ emotional responses: A critical review. Animals 2021, 11, 2510. [Google Scholar] [CrossRef]
- Knízková, İ.; Kunc, P.; Gürdil, G.; Pınar, Y.; Selvi, K.Ç. Applications of infrared thermography in animal production. Anadolu J. Agri. Sci. 2007, 22, 329–336. [Google Scholar]
- Bowers, S.; Gandy, S.; Anderson, B.; Ryan, P.; Willard, S. Assessment of Pregnancy in the Late-Gestation Mare Using Digital Infrared Thermography. Theriogenology 2009, 72, 372–377. [Google Scholar] [CrossRef]
- Da Silva, L.G.; Siqueira, N.M.C.; Gama, D.B.F.; de Souza Martins, J.V.; de Oliveira Lima, R.; de Oliveira, L.V.; Mateus, R.G.; Rita, P.H.S. Termografia de Capivaras (Hydrochoerus hydrochaeris) Do Perímetro Urbano de Campo Grande-MS. Pubvet 2019, 13, 148. [Google Scholar] [CrossRef]
- McCafferty, D.; Pandraud, G.; Gilles, J.; Fabra-Puchol, M.; Henry, P.-Y. Animal thermoregulation: A review of insulation, physiology and behaviour relevant to temperature control in buildings. Bioinspirat. Biomim. 2017, 13, 011001. [Google Scholar] [CrossRef]
- Gebreyohanes, M.G.; Awol, M.A. A Review: Adaptation Mechanisms of Camels (Camelus dromedarius) for Desert Environment. Vet. Sci. Technol. 2017, 8, 6. [Google Scholar] [CrossRef]
- Ouajd, O.; Kamel, B. Physiological particularities of Dromedary (Camelus dromedarius) and experimental implications. Scand. J. Lab. Anim. Sci. 2009, 36, 19–29.6. [Google Scholar]
- Wu, H.; Guang, X.; Al-Fageeh, M.B.; Cao, J.; Pan, S.; Zhou, H.; Zhang, L.; Abutarboush, M.H.; Xing, Y.; Xie, Z.; et al. Camelid genomes reveal evolution and adaptation to desert environments. Nat. Commun. 2014, 5, 5188. [Google Scholar] [CrossRef] [PubMed]
- Abdoun, K.A.; Samara, E.M.; Okab, A.B.; Al-Haidary, A.A. Assessment of Changes in Body Surface Temperature Associated with Ambient Temperature Using Infrared Thermography in Camels (Camelus dromedarius). In Proceedings of the 3rd ISOCARD International Conference, Muscat, Oman, 29 January–2 February 2012; pp. 275–276. [Google Scholar]
- Ostrowski, S.; Williams, J.B.; Ismael, K. Heterothermy and the water economy of free-living Arabian oryx (Oryx leucoryx). J. Exp. Biol. 2003, 206, 1471–1478. [Google Scholar] [CrossRef]
- Schmidt-Nielsen, K. Physiological adaptations of camels. J. Appl. Physiol. 1964, 19, 245–252. [Google Scholar]
- Bouâouda, H.; Achâaban, M.R.; Ouassat, M.; Oukassou, M.; Piro, M.; Challet, E.; El Allali, K.; Pévet, P. Daily regulation of body temperature rhythm in the camel (Camelus dromedarius) exposed to experimental desert conditions. Physiol. Rep. 2014, 2, e12151. [Google Scholar] [CrossRef]
- Schmidt-Nielson, B.; Schmidt-Nielson, K.; Houpt, T.R.; Jarnium, S.A. Body temperature of the camel and its relation to water economy. Am. J. Phys. 1957, 188, 103–112. [Google Scholar] [CrossRef]
- El Allali, K.; Achaâban, M.R.; Bothorel, B.; Piro, M.; Bouâouda, H.; El Allouchi, M.; Ouassat, M.; Malan, A.; Pévet, P. Entrainment of the circadian clock by daily ambient temperature cycles in the camel (Camelus dromedarius). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R1044–R1052. [Google Scholar] [CrossRef]
- Robertshaw, D. Mechanisms for the control of respiratory evaporative heat loss in panting animals. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 2006, 101, 664–668. [Google Scholar] [CrossRef]
- Bengoumi, M.; Faye, B. Adaptation du dromadaire à la déshydratation. Sécheresse 2002, 13, 121–129. [Google Scholar]
- Lee, D.G.; Schmidt-Nielsen, K. The skin, sweat glands and hair follicles of the camel. Anat. Res. 1962, 143, 71–94. [Google Scholar] [CrossRef]
- Faye, B. Le Guide de L’élevage du Dromadaire; Sanofi: Libourne, France, 1997; 126p. [Google Scholar]
- Grigg, G.; Beard, L.; Dorges, B.; Heucke, J.; Coventry, J.; Coppock, A.; Blomberg, S. Strategic (adaptive) hypothermia in bull dromedary camels during rut; could it increase reproductive success? Biol. Lett. 2009, 5, 853–856. [Google Scholar] [CrossRef] [PubMed]
- El-Tookhy, O.; Al-Sobayil, F.A.; Ahmed, A.F. Normal ocular Ecobiometry of the Dromedary camels. J. Camel Pract. Res. 2012, 19, 13–17. [Google Scholar]
- Kowalczyk, K.; Zdun, M.; Frąckowiak, H. Arterial Patterns of the Face in Camelidamorpha. Anat. Rec. 2018, 301, 2122–2127. [Google Scholar] [CrossRef] [PubMed]
- Gaughan, J.B.; Bonner, S.; Loxton, I.; Mader, T.L.; Lisle, A.; Lawrence, R. Effect of shade on body temperature and performance of feedlot steers. J. Anim. Sci. 2010, 88, 4056–4067. [Google Scholar] [CrossRef] [PubMed]
- Fuller, A.; Maloney, S.K.; Kamerman, P.R.; Mitchell, G.; Mitchell, D. Absence of selective brain cooling in free-ranging zebras in their natural habitat. Exp. Physiol. 2000, 85, 209–217. [Google Scholar]
- Hoter, A.; Rizk, S.; Naim, H.Y. Cellular and molecular adaptation of Arabian camel to heat stress. Front. Genet. 2019, 19, 588. [Google Scholar] [CrossRef]
- Strauss, W.M.; Hetem, R.S.; Mitchell, D.; Maloney, S.K.; O’Brien, H.D.; Meyer, L.C.R.; Fuller, A. Body water conservation through selective brain cooling by the carotid rete: A physiological feature for surviving climate change? Conserv. Physiol. 2017, 5, cow078. [Google Scholar] [CrossRef]
- Maloney, S.K.; Fuller, A.; Meyer, L.C.R.; Kamerman, P.R.; Mitchell, G.; Mitchell, D. Brain thermal inertia, but no evidence for selective brain cooling, in free-ranging western grey kangaroos (Macropus fuliginosus). J. Comp. Physiol. 2009, 179, 241–251. [Google Scholar] [CrossRef]
- Samara, E.M.; Ayadi, M.; Al-Haidary, A.A.; Aljumaah, R.S. Thermophysiological study in lactating and dry camels (Camelus dromedarius) under summer conditions. Emir. J. Food Agric. 2013, 25, 308–313. [Google Scholar] [CrossRef]
- Colak, A.; Yilmaz, O.; Kucuk, O. Correlation of body surface temperatures with environmental conditions and implications for thermal stress in various species. Vet. J. 2020, 246, 105–112. [Google Scholar] [CrossRef]
- Akin, J.A. Homeostatic Processes for Thermoregulation. Nat. Educ. Knowl. 2011, 3, 7. [Google Scholar]
- Lewis, G.F.; Gatto, R.G.; Porges, S.W. A novel method for extracting respiration rate and relative tidal volume from infrared thermography. Psychophysiology 2011, 48, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Pereira, A.M.F.; Martínez-Burnes, J.; Domínguez-Oliva, A.; Mora-Medina, P.; Casas-Alvarado, A.; Rios-Sandoval, J.; de Mira Geraldo, A.; Wang, D. Thermal Imaging to Assess the Health Status in Wildlife Animals under Human Care: Limitations and Perspectives. Animals 2022, 12, 3558. [Google Scholar] [CrossRef]
- Verdegaal, E.-L.J.M.M.; Howarth, G.S.; McWhorter, T.J.; Delesalle, C.J.G. Thermoregulation during Field Exercise in Horses Using Skin Temperature Monitoring. Animals 2024, 14, 136. [Google Scholar] [CrossRef]
- Lisboa, B.R.F.; Silva, J.A.R.; Silva, W.C.; Barbosa, A.V.C.; Silva, L.K.X.; Lourenço-Júnior, J.d.B. Evaluation of thermoregulation of horses (Equus caballus) submitted to two methods of post-exercise cooling, in hot and humid climate conditions, in the Eastern Amazon. Front. Vet. Sci. 2023, 10, 1150763. [Google Scholar] [CrossRef]
- Yarnell, K.; Fleming, J.; Stratton, T.D.; Brassington, R. Monitoring changes in skin temperature associated with exercise in horses on a water treadmill by use of infrared thermography. J. Therm. Biol. 2014, 45, 110–116. [Google Scholar] [CrossRef]
- Schroter, R.C.; Robertshaw, D.; Baker, M.A.; Shoemaker, V.H.; Holmes, R.; Schmidt-Nielsen, K. Respiration in heat stressed camels. Respir. Physiol. 1987, 70, 97–112. [Google Scholar] [CrossRef]
- Hussen, J.; Al-Sukruwah, M.A. The Impact of the Animal Housing System on Immune Cell Composition and Function in the Blood of Dromedary Camels. Animals 2022, 12, 317. [Google Scholar] [CrossRef]
- Gauthier-Pilters, H.; Dagg, A.I. The Camel: Its Evolution, Ecology and Behavior; University of Chicago Press: Chicago, IL, USA, 1989. [Google Scholar]
- Al-Salihi, K.A.; Iman, M.K.; Abass, O.A.; Abdullah, H.J. Macroscopic features of arabian camels (Dromedaries) eyes. Mirror Res. Vet. Sci. Anim. 2023, 12, 45–52. [Google Scholar]
- Abedellaah, B.; Sharshar, A.; Shoghy, K.; Rashed, R. Normal ocular structure of dromedary camel (Camelus dromedaries): Gross, ultrasonographic and computed tomographic study. Assiut Vet. Med. J. 2017, 63, 231–236. [Google Scholar] [CrossRef]
- Samuelson, D. Ophthalmic anatomy. In Veterinary Ophthalmology, 4th ed.; Gelatt, K.N., Ed.; Wiley Black Well: Ames, IA, USA, 2007; pp. 37–148. [Google Scholar]
- Mota-Rojas, D.; Titto, C.G.; de Mira Geraldo, A.; Martínez-Burnes, J.; Gómez, J.; Hernández-Ávalos, I.; Casas, A.; Domínguez, A.; José, N.; Bertoni, A. Efficacy and Function of Feathers, Hair, and Glabrous Skin in the Thermoregulation Strategies of Domestic Animals. Animals 2021, 11, 3472. [Google Scholar] [CrossRef] [PubMed]
- Ijichi, C.; Evans, L.; Woods, H.; Yarnell, K. The Right Angle: Validating a Standardised Protocol for the Use of Infra-Red Thermography of Eye Temperature as a Welfare Indicator. Anim. Welf. 2020, 29, 123–131. [Google Scholar] [CrossRef]
- Pastrana, I.C.; Navas González, F.J.; Ciani, E.; Marín Navas, C.; Delgado Bermejo, J.V. Thermographic ranges of dromedary camels during physical exercise: Applications for physical health/welfare monitoring and phenotypic selection. Front. Vet. Sci. 2023, 10, 1297412. [Google Scholar] [CrossRef]
- Yarmolenko, P.S.; Moon, E.J.; Landon, C.; Manzoor, A.; Hochman, D.W.; Viglianti, B.L.; Dewhirst, M.W. Thresholds for thermal damage to normal tissues: An update. Int. J. Hyperth. 2011, 27, 320–343. [Google Scholar] [CrossRef] [PubMed]
- Hussen, J. Changes in Cell Vitality, Phenotype, and Function of Dromedary Camel Leukocytes After Whole Blood Exposure to Heat Stress in vitro. Front. Vet. Sci. 2021, 8, 647609. [Google Scholar] [CrossRef]
- Maśko, M.; Witkowska-Piłaszewicz, O.; Jasiński, T.; Domino, M. Thermal Features, Ambient Temperature and Hair Coat Lengths: Limitations of Infrared Imaging in Pregnant Primitive Breed Mares within a Year. Reprod. Domest. Anim. 2021, 56, 1315–1328. [Google Scholar] [CrossRef]
- Hamdi, I.; Benaissa, A.; Babelhadj, B.; Bedda, H.; Aboub, S.; Loubaki, R. Composition and structure of the skin of dromedary (Camelus dromedarius) in relation to thermal adaptation. J. Anim. Behav. Biometeorol. 2022, 10, 2217. [Google Scholar] [CrossRef]
- Faye, B.; Ratovonanahary, R. The camel: An animal for the future. Anim. Res. 1995, 44, 1–20. [Google Scholar]
- Fath El-Bab, M.R.; Abou-Elhamd, A.S.; Abd-Elkareem, M. How the structure of the sweat glands of camel symphonizes their reliable function. J. Anim. Health Prod. 2017, 5, 19–23. [Google Scholar] [CrossRef]
- Abdoun, K.A.; Samara, E.M.; Okab, A.B.; Al-haidary, A.A. Regional and Circadian Variations of Sweating Rate and Body Surface Temperature in Camels (Camelus dromedarius). Anim. Sci. J. 2012, 83, 556–561. [Google Scholar] [CrossRef]
- Hamad, B.; Aggad, H.; Hadef, L.; Adaika, A. Effect of cold and hot seasons on thermoregulation and hemogram blood parameters of dromedary camel (Camelus dromedarius) in Algeria. Livest. Res. Rural. Dev. 2017, 29, 140. [Google Scholar]
- Al-Umeri, S.K.; Al-Mamoori, N.A. Comparative histological and histochemical study of flank region skin, in camel, cow and buffalo. Al-Qadisiyah J. Vet. Med. Sci. 2016, 15, 102. [Google Scholar]
- Abdoun, K.A.; Samara, E.M.; Okab, A.B.; Al-Haidary, A.A. The Relationship between coat color and thermoregulation in dromedary camels (Camelus dromedarius). J. Camel Pract. Res. 2014, 20, 251–255. [Google Scholar]
- Khaleel, I.M.; Khalid, H.K.; Mahdi, A.K.A.; Sarah, A.K. Histological and histochemical comparative study of the skin of three different locations between gazelle and camel. J. Camelid Sci. 2022, 16, 1–12. [Google Scholar]
- Sanusi, A.O. Effects of Coat Colour Genes on Heat Stress and Tolerance to Haemonchus Contortus Among West African Dwarf Sheep. Master’s Thesis, University of Agriculture, Abeokuta, Nigeria, 2008. [Google Scholar]
- Gerken, M. Relationships between integument characteristics and thermoregulation in South American camelids. Animal 2010, 4, 1451–1459. [Google Scholar] [CrossRef]
- McManus, C.; Tanure, C.B.; Peripolli, V.; Seixas, L.; Fischer, V.; Gabbi, A.M.; Menegassi, S.R.O.; Stumpf, M.T.; Kolling, G.J.; Dias, E.; et al. Infrared thermography in animal production: An overview. Comput. Electron. Agric. 2016, 123, 10–16. [Google Scholar] [CrossRef]
- Otoikhian, C.S.O.; Orheruata, J.A.; Imasuen, J.A.; Akporhuarho, O.P. Physiological response of local (West African Dwarf) and adapted switndzerla (White Bornu) goat breed to varied climatic conditions in South-South Nigeria. Afr. J. Gen. Agric. 2009, 5, 1–6. [Google Scholar]
- Castanheira, M.; Paiva, S.R.; Louvandini, H.; Landim, A.; Fiorvanti, M.C.S.; Dallago, B.S.; Correa, P.S.; McManus, C. Use of heat tolerance traits in discriminating between groups of sheep in central Brazil. Trop. Anim. Health Prod. 2010, 42, 1821–1828. [Google Scholar] [CrossRef]
- Nomura, R.H.C.; de Freitas, I.B.; Guedes, R.L.; Araújo, F.F.; Mafra, A.C.D.N.; Ibañez, J.F.; Dornbusch, P.T. Thermographic Images from Healthy Knees between Dogs with Long and Short Hair. Ciência Rural. 2018, 48, e20170040. [Google Scholar] [CrossRef]
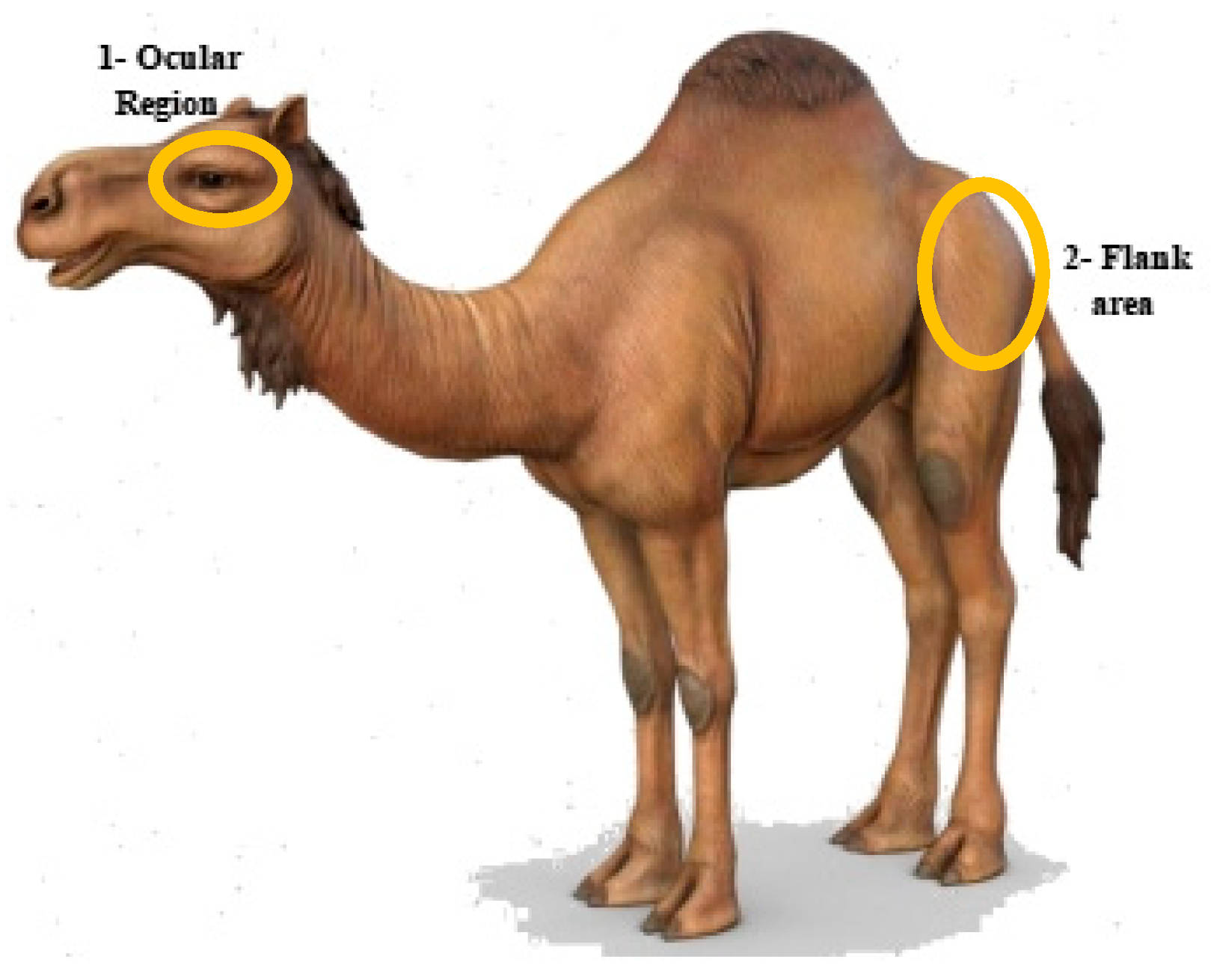
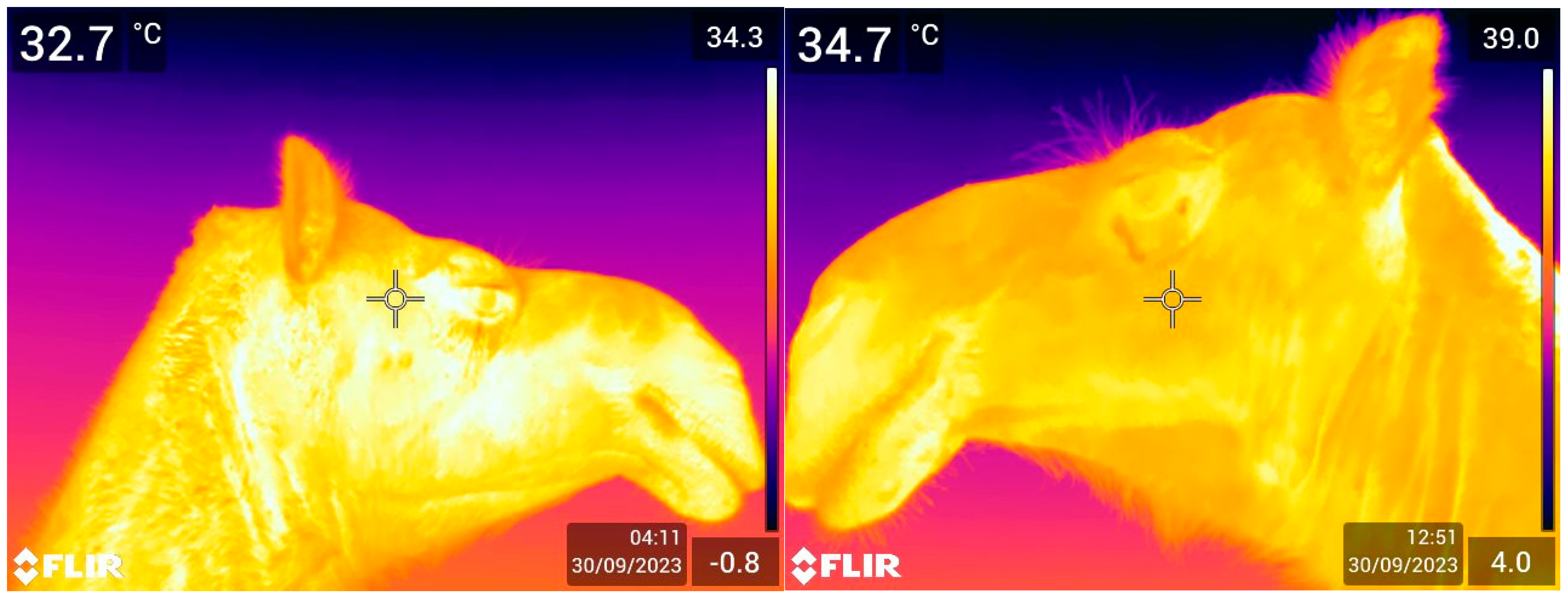
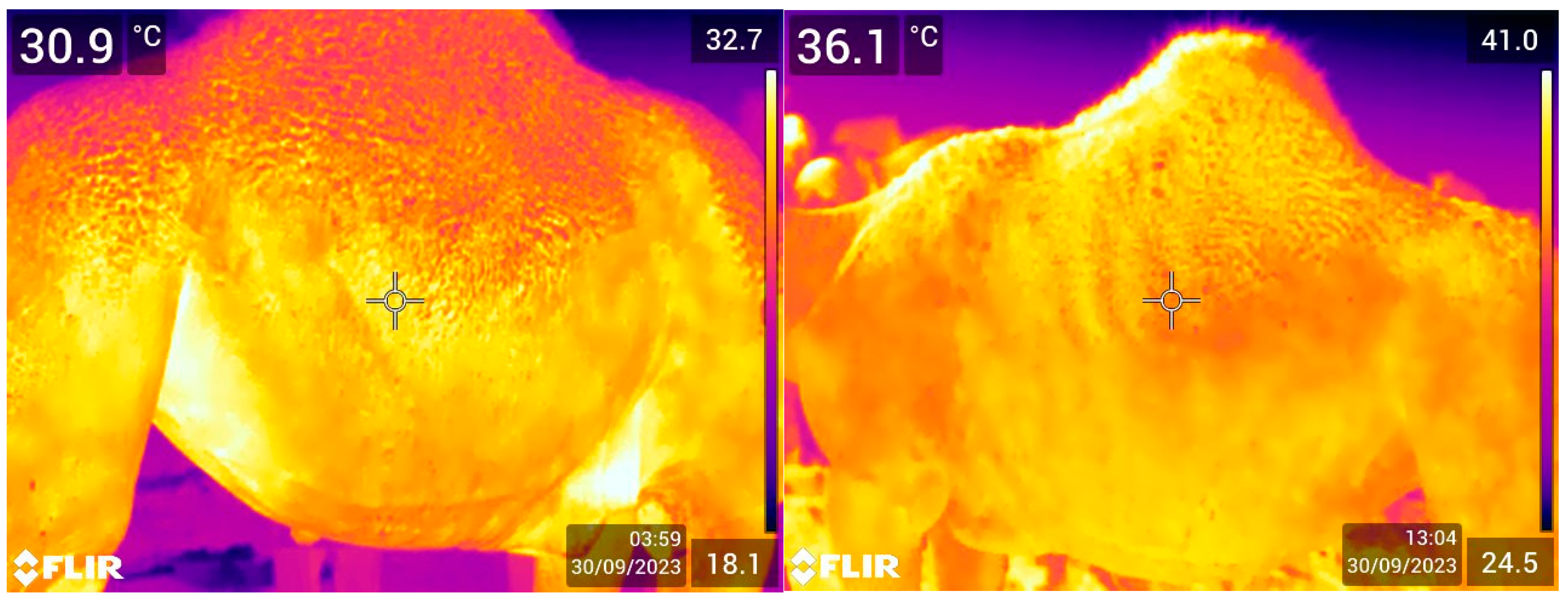
| Category | N | Average Eye Temp (°C) ± SE * | p Value * | |
|---|---|---|---|---|
| Age | Adult | 245 | 34.98 ± 0.11 | 0.3530 |
| Pubertal | 229 | 35.14 ± 0.11 | ||
| Young | 25 | 35.41 ± 0.33 | ||
| Sex | Male | 15 | 35.04 ± 0.43 | 0.9296 |
| Female | 484 | 35.08 ± 0.07 | ||
| Physiological Status | Breeding male | 5 | 34.78 ± 0.74 ab | 0.0458 |
| Immature female | 10 | 35.17 ± 0.52 ab | ||
| Lactating | 227 | 35.20 ± 0.11 a | ||
| Non-lactating | 97 | 35.32 ± 0.17 a | ||
| Pregnant | 160 | 34.75 ± 0.13 b | ||
| Category | N | Average Flank Temp (°C) ± SE | p Value | |
|---|---|---|---|---|
| Age | Adult | 241 | 36.62 ± 0.30 | 0.5867 |
| Pubertal | 226 | 37.03 ± 0.31 | ||
| Young | 27 | 37.12 ± 0.88 | ||
| Sex | Male | 15 | 37.18 ± 1.19 | 0.7713 |
| Female | 479 | 36.83 ± 0.21 | ||
| Physiological Status | Breeding | 5 | 37.42 ± 2.05 | 0.1163 |
| Immature | 10 | 37.06 ± 1.45 | ||
| Lactating | 226 | 36.73 ± 0.30 | ||
| Non-lactating | 97 | 37.89 ± 0.46 | ||
| Pregnant | 156 | 36.31 ± 0.37 | ||
| Independent Variables | Regression | R2 Adjusted | Tolerance I-R2 | VIF * (1/Tolerance) | |
|---|---|---|---|---|---|
| Eye | Temperature | ETA = 24.7476 + 0.3092T | 0.6399 | 0.3594 | 4.4 |
| Humidity | ETA = 38.5857 − 0.0727H | 0.4938 | 0.5052 | 4.4 | |
| Lux | ETA = 35.20 + 0.000001678Lux | 0.0024 | 0.9948 | 1.0 | |
| Flank | Temperature | FTA = 13.4236 + 0.7012T | 0.4299 | 0.5689 | 4.4 |
| Humidity | FTA = 44.3975 − 0.1564H | 0.2999 | 0.6987 | 4.4 | |
| Lux | FTA = 36.71 + 0.0000426Lux | 0.0445 | 0.9529 | 1.0 | |
| Sr. No. | Category | r | Remarks |
|---|---|---|---|
| 1 | ET and FT | 0.6643 *** | If eye temperature ↑ then flank temperature also ↑ |
| 2 | T and H | −0.8785 *** | If temperature ↑ then humidity ↓ |
| 3 | H and ET | −0.7444 *** | If humidity ↑ then eye temperature ↓ |
| 4 | H and FT | −0.5519 *** | If humidity ↑ then flank temperature ↓ |
| 5 | T and ET | 0.7887 *** | If temperature ↑ then eye temperature also ↑ |
| 6 | T and FT | 0.6280 *** | If temperature ↑ then flank temperature also ↑ |
| 7 | Lux and ET | 0.1019 * | If lux ↑ then eye temperature also ↑ |
| 8 | Lux and FT | 0.2650 *** | If lux ↑ then flank temperature also ↑ |
| 9 | Lux and H | −0.0579 NS | If lux ↑ then humidity ↓ |
| 10 | Lux and T | 0.0674 NS | If lux ↑ then temperature also ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faraz, A.; Masebo, N.T.; Hussain, S.M.; Waheed, A.; Ishaq, H.M.; Tauqir, N.A.; Abbasi, A.R.; Saleem, F.; Padalino, B. Association of Environmental Temperature and Relative Humidity with Ocular and Flank Temperatures in Dromedary Camels. Animals 2025, 15, 309. https://doi.org/10.3390/ani15030309
Faraz A, Masebo NT, Hussain SM, Waheed A, Ishaq HM, Tauqir NA, Abbasi AR, Saleem F, Padalino B. Association of Environmental Temperature and Relative Humidity with Ocular and Flank Temperatures in Dromedary Camels. Animals. 2025; 15(3):309. https://doi.org/10.3390/ani15030309
Chicago/Turabian StyleFaraz, Asim, Naod Thomas Masebo, Syeda Maryam Hussain, Abdul Waheed, Hafiz Muhammad Ishaq, Nasir Ali Tauqir, Ali Raza Abbasi, Faizan Saleem, and Barbara Padalino. 2025. "Association of Environmental Temperature and Relative Humidity with Ocular and Flank Temperatures in Dromedary Camels" Animals 15, no. 3: 309. https://doi.org/10.3390/ani15030309
APA StyleFaraz, A., Masebo, N. T., Hussain, S. M., Waheed, A., Ishaq, H. M., Tauqir, N. A., Abbasi, A. R., Saleem, F., & Padalino, B. (2025). Association of Environmental Temperature and Relative Humidity with Ocular and Flank Temperatures in Dromedary Camels. Animals, 15(3), 309. https://doi.org/10.3390/ani15030309






