The Use of Antimicrobials in Animal Husbandry as a Potential Factor for the Increased Incidence of Colorectal Cancer: Food Safety and Kinetics in a Murine Model
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of the Molecules of Interest
2.2. Evaluation of Exposure Level
- A.
- Selections of foods for which there is a regulatory limit for sulfonamides and tetracyclines (Table 1);
- B.
- Calculation of daily requirements in terms of major nutrients (carbohydrates, fats, and proteins) expressed in g/day for a 30-year-old adult individual;
- C.
- Identification of the amount of food of animal origin corresponding to the daily requirement;
- D.
- Definition of a standard meal in order to calculate the intake of sulfonamides and tetracyclines.
- A.
- Regarding all food matrices of animal origin in EU Regulation 37/2010, we decided to exclude liver, fat, and kidney, as these are rarely used in human nutrition [21]; we therefore used the following matrices:
- Muscle (from beef, pork, poultry, and fish);
- Cow’s milk;
- Hen eggs (for chlortetracycline only).
- B.
- To calculate the daily requirements in terms of major nutrients (carbohydrates, fat, and protein) for a 60 kg adult, we used the interactive tool that the EFSA makes available online (DRV Finder) [22] and applied the dietary reference values (DRVs) established by the EFSA.In this study, the following parameters were adopted:
- Thirty-year-old adults of both sexes;
- Nutrients: protein;
- Population Recommended Intake (PRI) values.
- C.
- To trace the EFSA DRV Finder values for foods commonly used in the Italian diet, the Food Composition Tables Database available online from the CREA (Center for Research on Food and Nutrition) website was used; the standard reference quantity for a portion of food was set to 100 g and the following matrices were selected:
- Adult beef or veal loin [muscle tissue stripped of visible fat];
- Pork loin, raw;
- Chicken breast, raw;
- Cod or hake, raw;
- Pasteurized cow’s milk, semi-skimmed;
- Hen eggs, whole.
- D.
- On the basis of these data, a typical day’s diet was drawn up for an adult weighing 60 kg; from these amounts of food, the intake of tetracyclines and sulfonamides was calculated, assuming that the foods contained concentrations of these antimicrobials equal to the regulatory limit (EU Regulation 37/2010).
- E.
- For the mouse dosage calculation, the conversion from human to mouse was determined using the following equation [23]:
2.3. In Vitro Study
- Cell viability assay;
- Wound healing assay (48 h);
- Colony-forming assay (15 days).
2.4. In Vivo Study
2.5. Sampling for Microbiota Analysis
2.6. DNA Extraction and Sequencing
2.7. Histopathological Examination and Immunohistochemistry
3. Results
3.1. Calculation of Dosages
- Adult bovine loin: 21.8 g of protein per 100 g of edible part;
- Pork loin: 20.7 g of protein per 100 g of edible part;
- Chicken breast: 23.3 g protein per 100 g edible part;
- Cod: 17 g protein per 100 g edible part;
- Milk: 3.5 g of protein per 100 g of edible part;
- Eggs: 12.4 g protein per 100 g edible part.
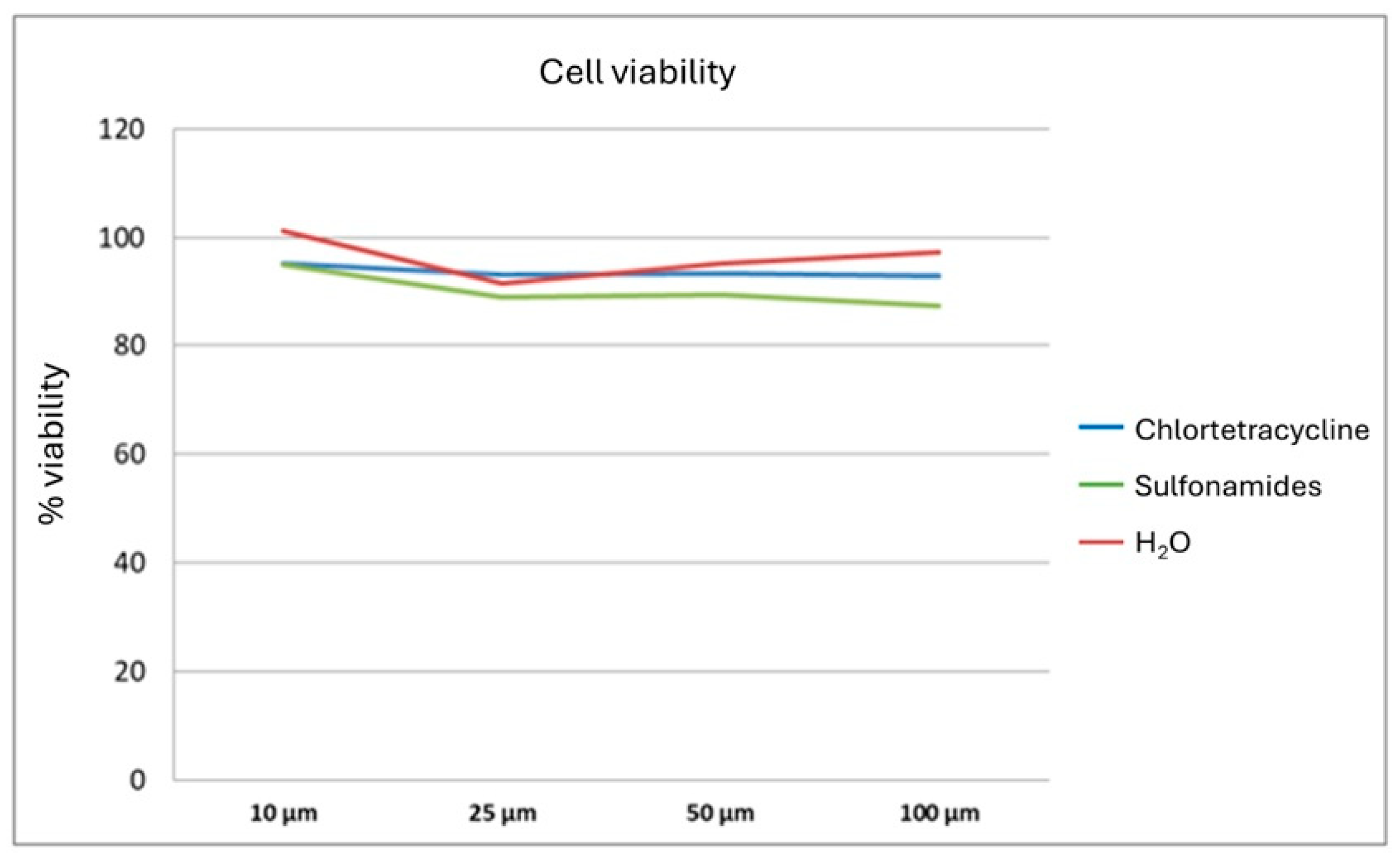
3.2. In Vitro Study
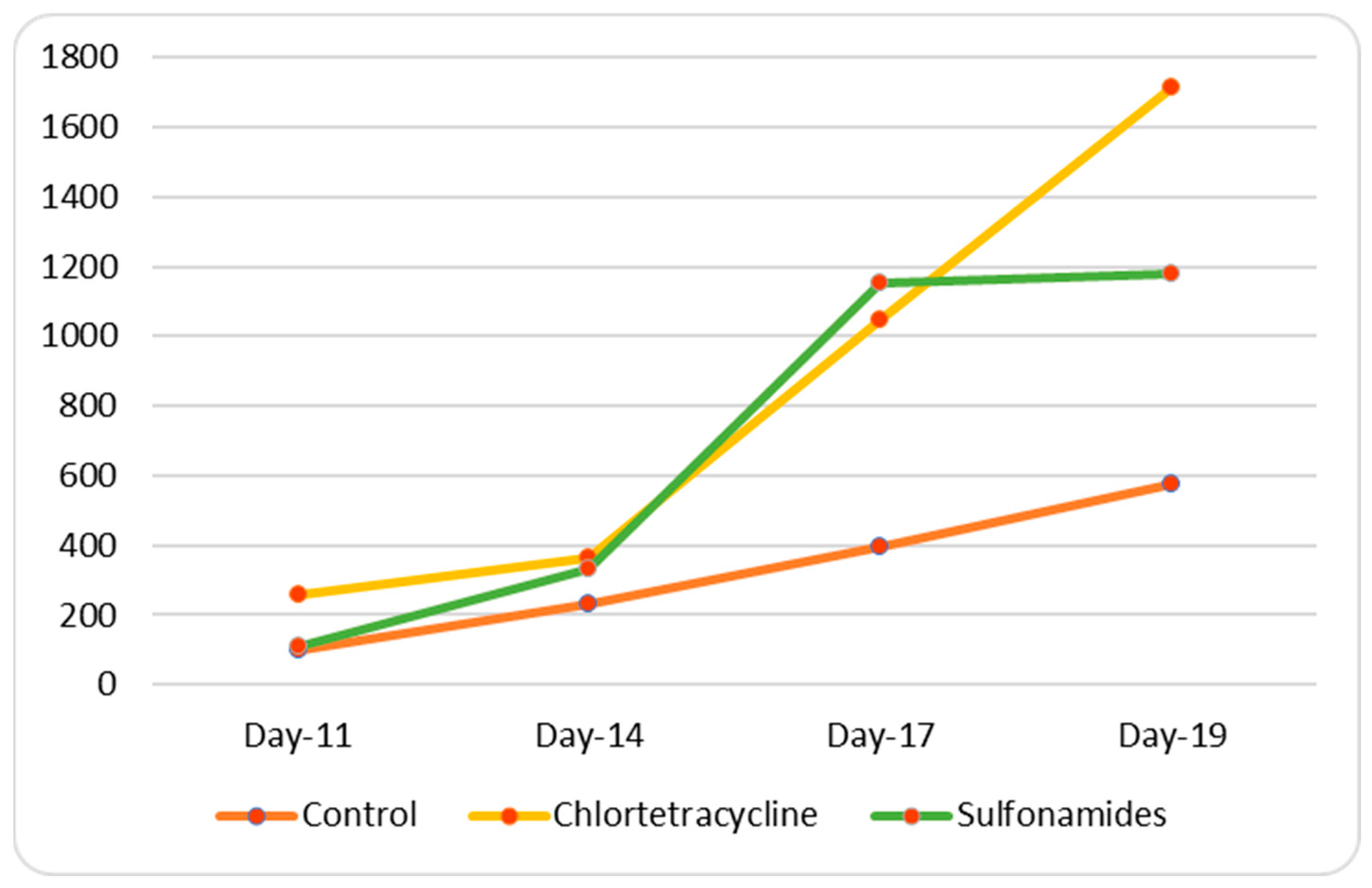
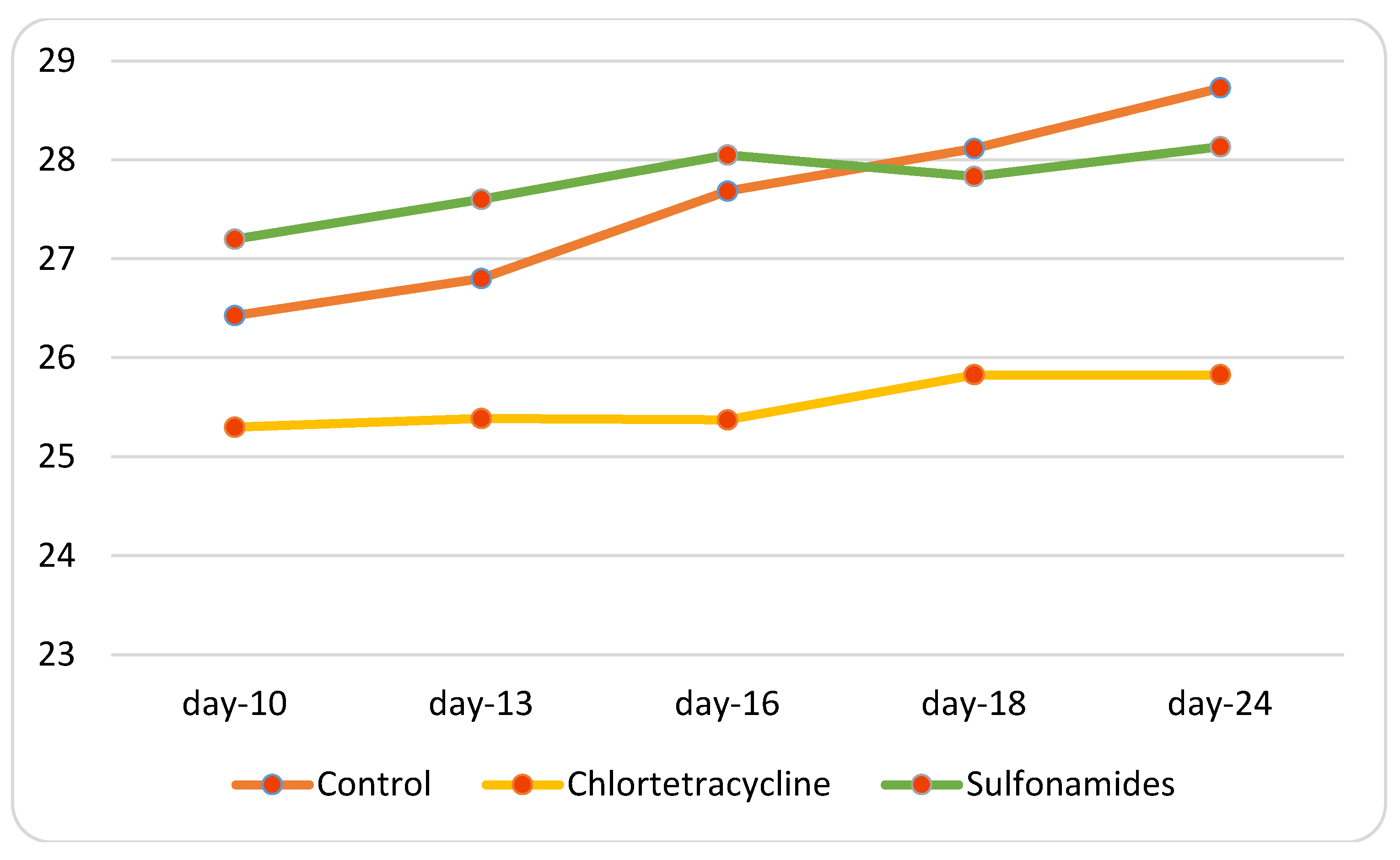
3.3. In Vivo Study
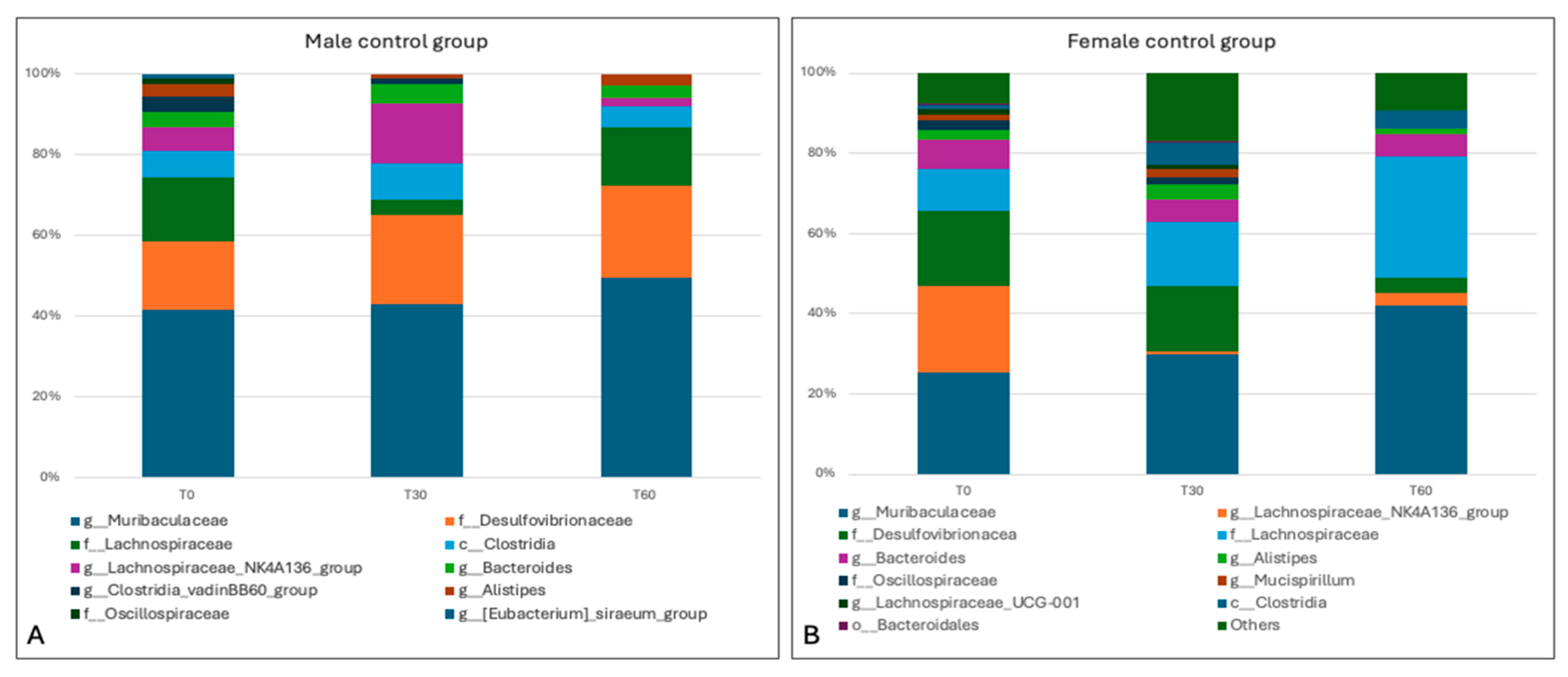
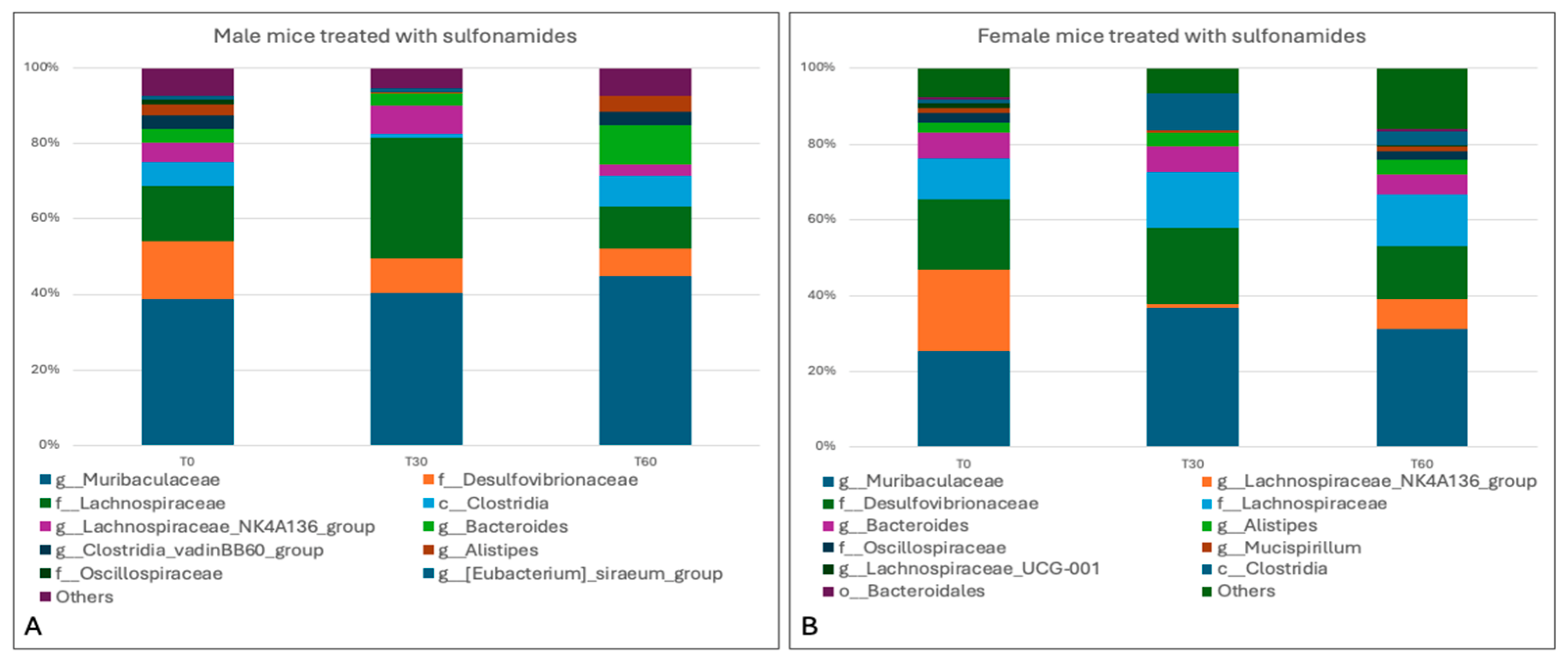
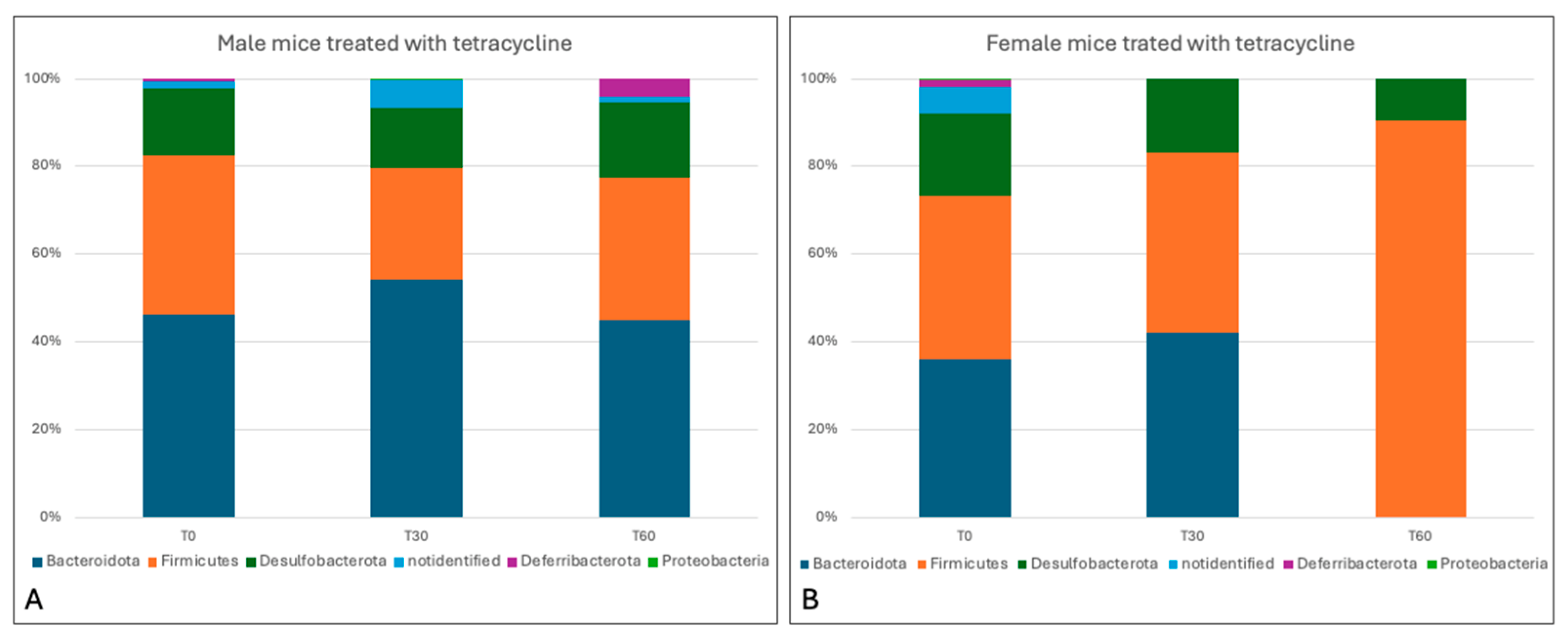
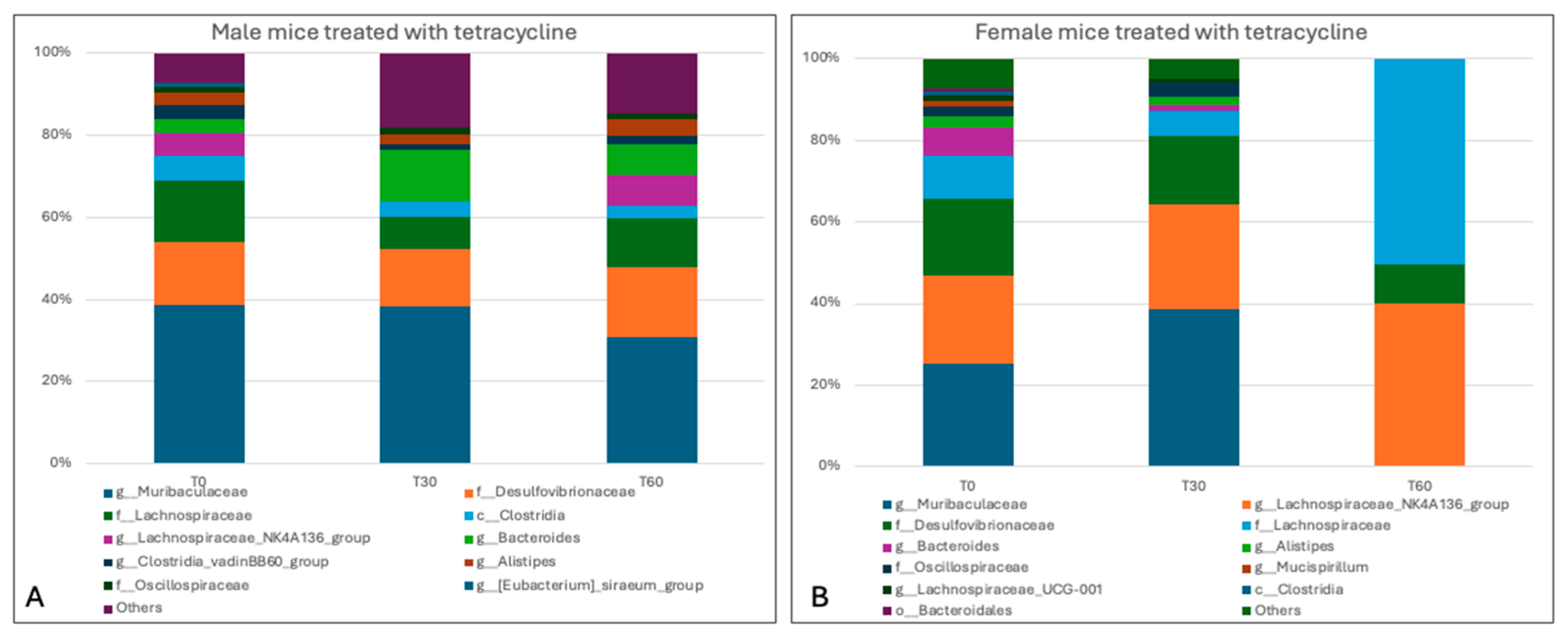
3.4. Sequencing Analysis and Microbiota Characterization
3.5. Characterization of the Effects of Antimicrobials on the Gut Microbiota
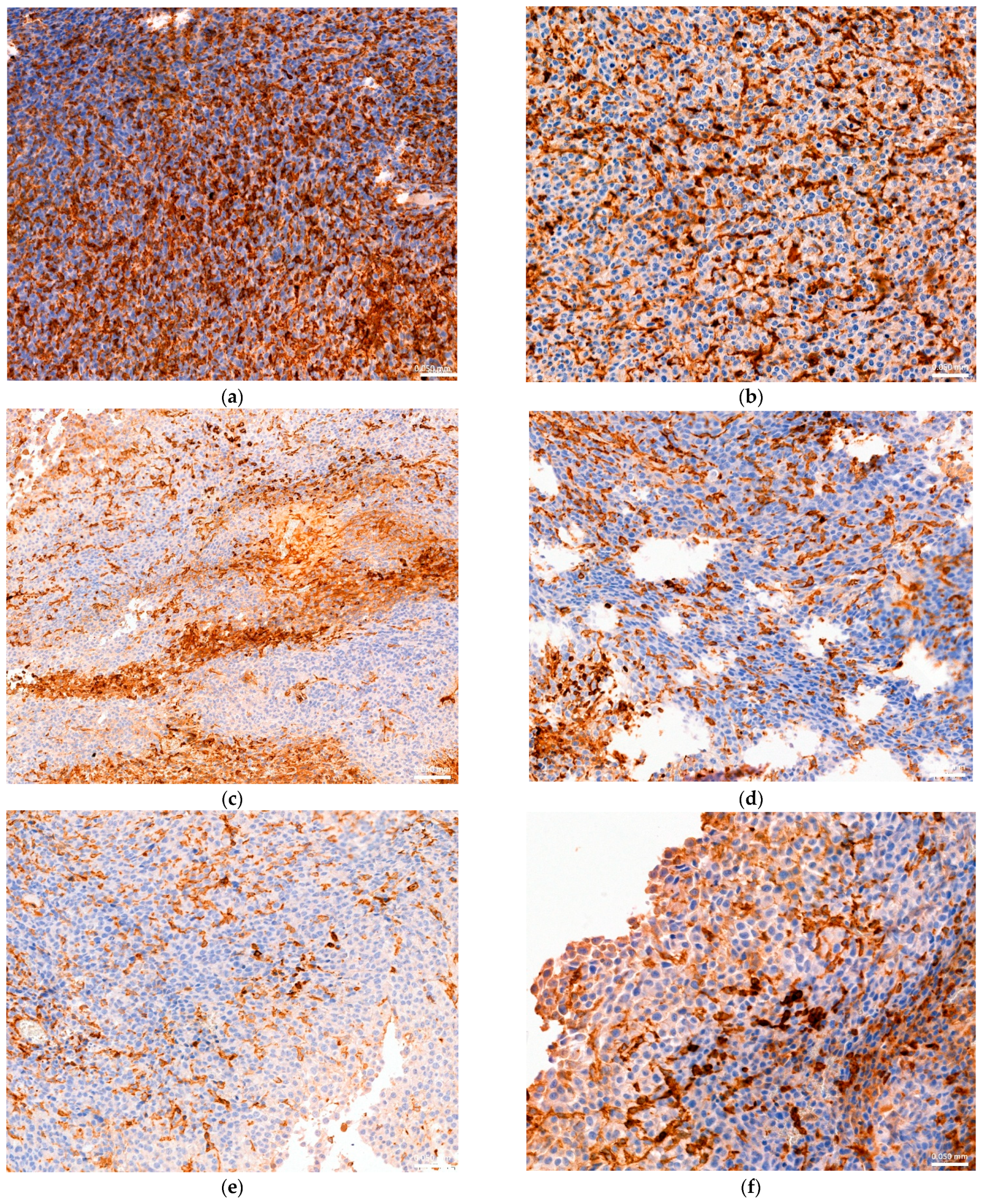
3.6. Histopathological and Immunohistochemical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- One Health. Available online: https://www.who.int/health-topics/one-health (accessed on 10 January 2025).
- Ministero Della Salute. Piano Nazionale Residui. Anno 2023. Available online: https://www.salute.gov.it/imgs/C_17_pubblicazioni_3476_allegato.pdf (accessed on 22 November 2023).
- Commission Regulation (EU) No 37/2010 of 22 December 2009. Available online: https://eur-lex.europa.eu/eli/reg/2010/37(1)/oj/eng (accessed on 10 January 2025).
- Cao, Y.; Wu, K.; Mehta, R.; A Drew, D.; Song, M.; Lochhead, P.; Nguyen, L.H.; Izard, J.; Fuchs, C.S.; Garrett, W.S.; et al. Long-term use of antimicrobials and risk of colorectal adenoma. Gut 2017, 67, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Keum, N.; Giovannucci, E.L. Colorectal Cancer Epidemiology in the Nurses’ Health Study. Am. J. Public. Health 2016, 106, 1599–1607. [Google Scholar] [CrossRef] [PubMed]
- Bud, R. Antimicrobials: The epitome of a wonder drug. BMJ 2007, 334, s6. [Google Scholar] [CrossRef]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antimicrobial consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef] [PubMed]
- Dethlefsen, L.; Relman, D.A. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antimicrobial perturbation. Proc. Natl. Acad. Sci. USA 2011, 108, 4554–4561. [Google Scholar] [CrossRef]
- Lange, K.; Buerger, M.; Stallmach, A.; Bruns, T. Effects of antimicrobials on gutmicrobiota. Dig. Dis. 2016, 34, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Giedraitiene, A.; Vitkauskiene, A.; Naginiene, R.; Pavilonis, A. Antimicrobial resistance mechanisms of clinically important bacteria. Medicina 2011, 47, 137–146. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Singal, A.G.; Chang, L. The gut microbiome and digestive health-a new frontier. Clin. Gastroenterol. Hepatol. 2019, 17, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Guarner, F.; Malagelada, J.-R. Gut flora in health and disease. Lancet 2003, 361, 512–519. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Ravel, J. The vocabulary of microbiomeresearch: A proposal. Microbiome 2015, 3, 31. [Google Scholar] [CrossRef]
- Donaldson, G.P.; Lee, S.M.; Mazmanian, S.K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 2016, 14, 20–32. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current understanding of the human microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Allaband, C.; McDonald, D.; Vázquez-Baeza, Y.; Minich, J.J.; Tripathi, A.; Brenner, D.A.; Loomba, R.; Smarr, L.; Sandborn, W.J.; Schnabl, B.; et al. Microbiome 101: Studying, analyzing, and interpreting gut microbiome data for clinicians. Clin. Gastroenterol. Hepatol. 2019, 17, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Gokulan, K.; Cerniglia, C.E.; Thomas, C.; Pineiro, S.A.; Khare, S. Effects of residual levels of tetracycline on the barrier functions of human intestinal epithelial cells. Food Chem. Toxicol. 2017, 109, 253–263. [Google Scholar] [CrossRef]
- Menkem, Z.E.; Ngangom, B.L.; Tamunjoh, S.S.A.; Boyom, F.F. Antibiotic residues in food animals: Public health concern. Acta Ecol. Sin. 2019, 39, 411–415. [Google Scholar] [CrossRef]
- Serrano-Arias, B.; Araya-Zúñiga, A.; Waterhouse-Garbanzo, J.; Rojas-Barrantes, Z.; Arguedas-Chacón, S.; Zavaleta-Monestel, E. A Comprehensive Review of Sulfonamide Hypersensitivity: Implications for Clinical Practice. Clin. Rev. Allergy Immunol. 2023, 65, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Linee Guida Per Una Sana Alimentazione. Available online: https://www.crea.gov.it/documents/59764/0/ (accessed on 10 January 2025).
- Available online: https://multimedia.efsa.europa.eu/drvs/index.htm (accessed on 18 September 2024).
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; UFAW: Potters Bar, UK, 1992; 238p. [Google Scholar]
- du Sert, N.P.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Almeida, R.R.; Vieira, R.d.S.; Castoldi, A.; Terra, F.F.; Melo, A.C.L.; Canesso, M.C.C.; Lemos, L.; Cipelli, M.; Rana, N.; Hiyane, M.I.; et al. Host dysbiosis negatively impacts IL-9-producing T-cell differentiation and antitumour immunity. Br. J. Cancer 2020, 123, 534–541. [Google Scholar] [CrossRef]
- Benakis, C.; Brea, D.; Caballero, S.; Faraco, G.; Moore, J.; Murphy, M.; Sita, G.; Racchumi, G.; Ling, L.; Pamer, E.G.; et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat. Med. 2016, 22, 516–523. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, M.; Zheng, X.; Fan, J.; Cheng, W.; Yan, T.; Lai, Y.; Zhang, N.; Lu, Y.; Qi, J.; Huo, Z.; et al. Antibiotic-Induced Gut Microbiota Dysbiosis Modulates Host Transcriptome and m6A Epitranscriptome via Bile Acid Metabolism. Adv. Sci. 2024, 11, e2307981. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schlomann, B.H.; Parthasarathy, R. Timescales of gut microbiome dynamics. Curr. Opin. Microbiol. 2019, 50, 56–63. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hong, Y.; Li, H.; Chen, L.; Su, H.; Zhang, B.; Luo, Y.; Li, C.; Zhao, Z.; Shao, Y.; Guo, L. Short-term exposure to antibiotics begets long-term disturbance in gut microbial metabolism and molecular ecological networks. Microbiome 2024, 12, 80. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Han, N.; Zhang, T.; Qiang, Y.; Peng, X.; Li, X.; Zhang, W. Time-scale analysis of the long-term variability of human gut microbiota characteristics in Chinese individuals. Commun. Biol. 2022, 5, 1414. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Doré, J.; Blottière, H. The influence of diet on the gut microbiota and its consequences for health. Curr. Opin. Biotechnol. 2015, 32, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- McDonald, D.; Price, M.N.; Goodrich, J.; Nawrocki, E.P.; DeSantis, T.Z.; Probst, A.; Andersen, G.L.; Knight, R.; Hugenholtz, P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012, 6, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, S.; Lin, H.; Huang, J.; Watkins, P.A.; Moser, A.B.; DeSimone, C.; Song, X.; Diehl, A.M. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology 2003, 37, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Williamson, S.L.; Steward, M.; Milton, I.; Parr, A.; Piggott, N.H.; Krajewski, A.S.; Angus, B.; Horne, C.H. New monoclonal antibodies to the T cell antigens CD4 and CD8. Production and characterization in formalin-fixed paraffin-embedded tissue. Am. J. Pathol. 1998, 152, 1421–1426. [Google Scholar] [PubMed] [PubMed Central]
- CREA Centro di Ricerca Alimenti e Nutrizione. Available online: https://www.alimentinutrizione.it (accessed on 18 September 2024).
- Baraibar, I.; Ros, J.; Saoudi, N.; Salvà, F.; García, A.; Castells, M.R.; Tabernero, J.; Élez, E. Sex and gender perspectives in colorectal cancer. ESMO Open 2023, 8, 101204. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pereira, J.S.; Costa Miguel, M.C.; Queiroz, L.M.G.; Silveira, E.J.D. Analysis of CD8+ and CD4+ cells in oral Squamous Cell Carcinoma and their association with lymph node metastasis and histologic Grade of Malignancy. Appl. Immunoistochem. Mol. Morphol. 2014, 22, 3. [Google Scholar] [CrossRef]
- DiToro, D.; Basu, R. Emerging Complexity in CD4+T Lineage Programming and Its Implications in Colorectal Cancer. Front. Immunol. 2021, 12, 694833. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, R.W.; Harpaz, N.; Itzkowitz, S.H.; Parsons, R.E. Molecular mechanisms in colitis-associated colorectal cancer. Oncogenesis 2023, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Rothenberger, N.J.; Somasundaram, A.; Stabile, L.P. The Role of the Estrogen Pathway in the Tumor Microenvironment. Int. J. Mol. Sci. 2018, 19, 611. [Google Scholar] [CrossRef]






| Pharmacologically Active Substance | Marker Residue | Animals Species | MRL | Target Tissues | Other Provisions | Therapeutic Classification |
|---|---|---|---|---|---|---|
| Chlortetracycline | Sum of parent drug and its 4-epimer | All food-producing species | 100 μg/kg 300 μg/kg 600 μg/kg 100 μg/kg 200 μg/kg | Muscle Liver Kidney Milk Eggs | For fin fish, the muscle MRL relates to ‘muscle and skin in natural proportions’. MRLs for liver and kidney do not apply to fin fish. | Anti-infectious agents/Antimicrobials |
| Sulfonamides (all substances belonging to the sulfonamide group) | Parent drug | All food-producing species | 100 μg/kg 100 μg/kg 100 μg/kg 100 μg/kg | Muscle Fat Liver Kidney | The combined total residues of all substances within the sulfonamide group should not exceed 100 μg/kg. For fin fish, the muscle MRL relates to ‘muscle and skin in natural proportions’. MRLs for fat, liver, and kidney do not apply to fin fish. Not for use in animals from which eggs are produced for human consumption. | Anti-infectious agents/Chemotherapeutics/ |
| Mice | Groups |
|---|---|
| Male | Control Treatment with sulfonamides Treatment with tetracyclines |
| Female | Control Treatment with sulfonamides Treatment with tetracyclines |
| Matrix | Chlortetracycline | Sulfonamides |
|---|---|---|
| Muscle (beef, pork, poultry, fish) | 100 µg/kg | 100 µg/kg |
| Cow’s milk | 100 µg/kg | 100 µg/kg |
| Chicken eggs (for chlortetracycline only) | 200 µg/kg | - |
| Food | Portion | Protein Content | Total (Per Portion) |
|---|---|---|---|
| Milk | 200 g | 3.5 g | 7 g |
| Egg | 1 (53 g CREA data) | 12.4 g | 6.572 g |
| Meat | 100 g | 23.3 g | 23.3 g |
| Fish | 100 g | 17 g | 17 g |
| Total | 453 g of food | 53.872 g of protein |
| Food | Portion | Chlortetracycline (Limit Reg. 37/2010) | Total Chlortetracycline | Sulfonamides (Limit Reg. 37/2010) | Total Sulfonamides | Total Antimicrobial Residues |
|---|---|---|---|---|---|---|
| Milk | 200 g | 100 µg/kg | (100 × 0.2) = 20 µg | 100 µg/kg | (100 × 0.2) = 20 µg | 40 µg |
| Eggs | 1 (53 g, CREA date) | 200 µg/kg | (200 × 0.053) = 10.6 µg | - | - | 10.6 µg |
| Meat | 100 g | 100 µg/kg | (100 × 0.1) = 10 µg | 100 µg/kg | (100 × 0.1) = 10 µg | 20 µg |
| Fish | 100 g | 100 µg/kg | (100 × 0.1) = 10 µg | 100 µg/kg | (100 × 0.1) = 10 µg | 20 µg |
| Total | 453 g of food | - | 50.6 µg | - | 40 µg | 90.6 µg |
| Substance | Total Substance Meal/Day | Weight | Substance/kg Body Weight |
|---|---|---|---|
| Chlortetracycline | 50.6 µg | 60 kg | (50.6/60) = 0.84 µg/kg |
| Sulfonamides | 40 µg | 60 kg | (40/60) = 0.67 µg/kg |
| Days | 11 | 14 | 17 | |
|---|---|---|---|---|
| % rooting females | Controls | 14.2 | 14.2 | 100 |
| Chlortetracycline | 42.8 | 71.4 | 100 | |
| Sulfonamides | 42.8 | 57.1 | 90 |
| Days | 13 | 16 | 18 | |
|---|---|---|---|---|
| % rooting males | Controls | 14.3 | 57 | 57.1 |
| Chlortetracycline | 0 | 42.9 | 42.9 | |
| Sulfonamides | 0 | 42.9 | 57.1 |
| CD4+ | CD8+ | ||||
|---|---|---|---|---|---|
| Groups | N | Minimum | Maximum | Minimum | Maximum |
| Control | 14 | 4.5 | 22.9 | 4.7 | 28.9 |
| Sulfonamides | 12 | 5.3 | 19.6 | 4.9 | 21 |
| Tetracyclines | 14 | 2.3 | 9.8 | 2.7 | 11.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Ambrosio, R.; Cavallo, S.; Brunetti, R.; Pellicanò, R.; Vaccaro, E.; Borriello, G.; Paradiso, R.; Serpe, F.P.; Lambiase, S.; Bruzzese, F.; et al. The Use of Antimicrobials in Animal Husbandry as a Potential Factor for the Increased Incidence of Colorectal Cancer: Food Safety and Kinetics in a Murine Model. Animals 2025, 15, 315. https://doi.org/10.3390/ani15030315
D’Ambrosio R, Cavallo S, Brunetti R, Pellicanò R, Vaccaro E, Borriello G, Paradiso R, Serpe FP, Lambiase S, Bruzzese F, et al. The Use of Antimicrobials in Animal Husbandry as a Potential Factor for the Increased Incidence of Colorectal Cancer: Food Safety and Kinetics in a Murine Model. Animals. 2025; 15(3):315. https://doi.org/10.3390/ani15030315
Chicago/Turabian StyleD’Ambrosio, Rosa, Stefania Cavallo, Roberta Brunetti, Roberta Pellicanò, Emanuela Vaccaro, Giorgia Borriello, Rubina Paradiso, Francesco Paolo Serpe, Sara Lambiase, Francesca Bruzzese, and et al. 2025. "The Use of Antimicrobials in Animal Husbandry as a Potential Factor for the Increased Incidence of Colorectal Cancer: Food Safety and Kinetics in a Murine Model" Animals 15, no. 3: 315. https://doi.org/10.3390/ani15030315
APA StyleD’Ambrosio, R., Cavallo, S., Brunetti, R., Pellicanò, R., Vaccaro, E., Borriello, G., Paradiso, R., Serpe, F. P., Lambiase, S., Bruzzese, F., Palma, G., Rea, D., Barbieri, A., D’Amore, M., Dimatteo, M., degli Uberti, B., Paciello, O., & Baldi, L. (2025). The Use of Antimicrobials in Animal Husbandry as a Potential Factor for the Increased Incidence of Colorectal Cancer: Food Safety and Kinetics in a Murine Model. Animals, 15(3), 315. https://doi.org/10.3390/ani15030315







