Calcium Release-Activated Calcium Modulator ORAI1-Sensitive Serine Dehydratase Regulates Fatty Acid-Induced CD4+ Th17/Treg Imbalance in Dairy Cows
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sample Collection
2.2. Isolation and Culture of CD4+ T Cells
2.3. RNA Isolation and qRT-PCR
2.4. Protein Extraction and Western Blotting
2.5. Transcriptomic Testing
2.6. Treatment of siSDS or siORAI1
2.7. ELISA Analysis
2.8. Flow Cytometry
2.9. Statistical Analysis
3. Results
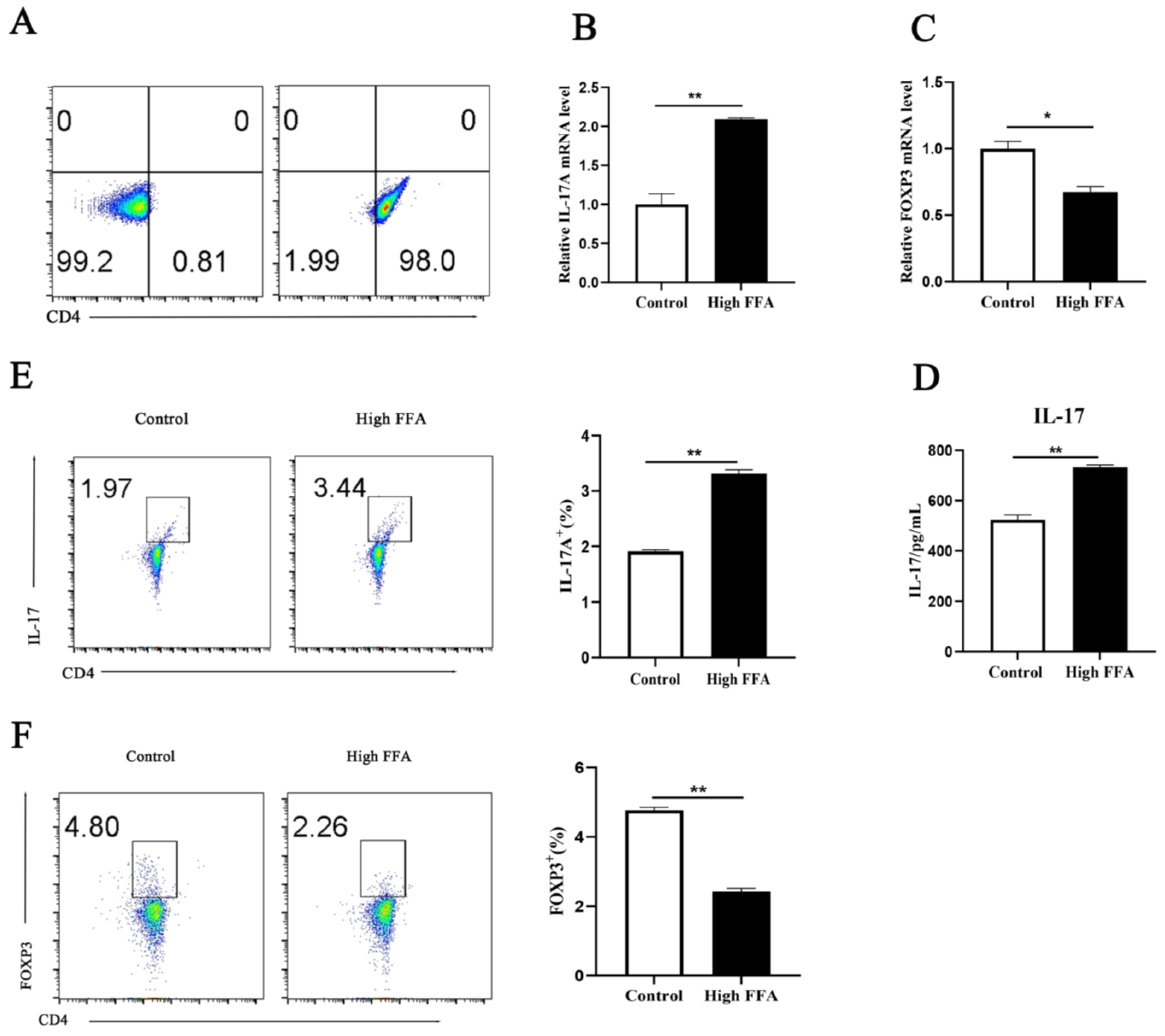
3.1. High FFAs Induced Elevated CD4+ Th17 Cell Production in Dairy Cows
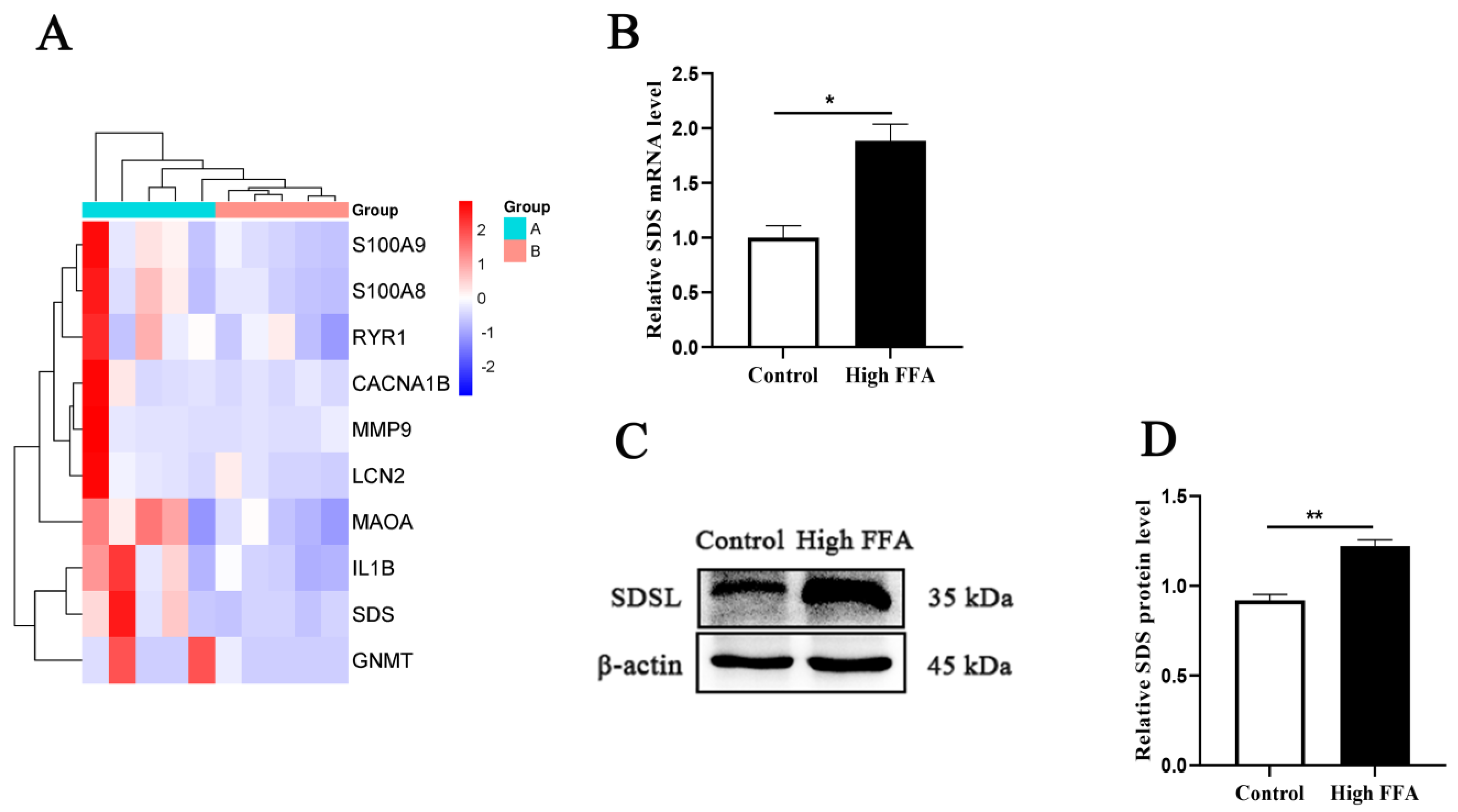
3.2. Elevated SDS Expression in Cows with High FFAs
3.3. Effect of Serine Dehydratase (SDS) on CD4+ Th17/Treg
3.4. Effect of ORAI1 on CD4+ Th17/Treg
3.5. ORAI1 Regulates CD4+ Th17/Treg via Serine Dehydratase (SDS)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FFAs | free fatty acids |
| NEB | negative energy balance |
| ORAI1 | ORAI calcium release-activated calcium modulator 1 |
| SOCE | store-operated calcium entry |
| STIM1 | stromal interaction molecule 1 |
| SDS | serine dehydratase |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
References
- Putman, A.K.; Brown, J.L.; Gandy, J.C.; Wisnieski, L.; Sordillo, L.M. Changes in Biomarkers of Nutrient Metabolism, Inflammation, and Oxidative Stress in Dairy Cows During the Transition into the Early Dry Period. J. Dairy Sci. 2018, 101, 9350–9359. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, M.; Loor, J.J.; Jiang, Q.; Zhu, Y.; Li, W.; Du, X.; Song, Y.; Gao, W.; Lei, L.; et al. Propionate Alleviates Fatty Acid-Induced Mitochondrial Dysfunction, Oxidative Stress, and Apoptosis by Upregulating PPARG Coactivator 1 Alpha in Hepatocytes. J. Dairy Sci. 2022, 105, 4581–4592. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, X.; Li, Y.; Guan, Y.; Song, Y.; Yin, L.; Chen, H.; Lei, L.; Liu, J.; Li, X.; et al. Effects of Nonesterified Fatty Acids on the Synthesis and Assembly of Very Low Density Lipoprotein in Bovine Hepatocytes In Vitro. J. Dairy Sci. 2014, 97, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Guan, Y.; Loor, J.J.; Sha, X.; Coleman, D.N.; Zhang, C.; Du, X.; Shi, Z.; Li, X.; Wang, Z.; et al. Fatty Acid-Induced Endoplasmic Reticulum Stress Promoted Lipid Accumulation in Calf Hepatocytes, and Endoplasmic Reticulum Stress Existed in the Liver of Severe Fatty Liver Cows. J. Dairy Sci. 2019, 102, 7359–7370. [Google Scholar] [CrossRef]
- Cai, F.; Jin, S.; Chen, G. The Effect of Lipid Metabolism on CD4+ T Cells. Mediat. Inflamm. 2021, 2021, 6634532. [Google Scholar] [CrossRef]
- Nakajima, T.; Kanno, T.; Ueda, Y.; Miyako, K.; Endo, T.; Yoshida, S.; Yokoyama, S.; Asou, H.K.; Yamada, K.; Ikeda, K.; et al. Fatty Acid Metabolism Constrains Th9 Cell Differentiation and Antitumor Immunity via the Modulation of Retinoic Acid Receptor Signaling. Cell. Mol. Immunol. 2024, 21, 1266–1281. [Google Scholar] [CrossRef]
- Liang, G.; Huang, J.; Chen, J.; Wen, X.; Li, R.; Xie, H.; Zhang, Z.; Chen, Z.; Chen, Y.; Xian, Z.; et al. Fatty Acid Oxidation Promotes Apoptotic Resistance and Proinflammatory Phenotype of CD4+ Tissue-resident Memory T cells in Crohn’s Disease. Cell. Mol. Gastroenterol. Hepatol. 2024, 17, 939–964. [Google Scholar] [CrossRef]
- Zhang, S.; Gang, X.; Yang, S.; Cui, M.; Sun, L.; Li, Z.; Wang, G. The Alterations in and the Role of the Th17/Treg Balance in Metabolic Diseases. Front. Immunol. 2021, 12, 678355. [Google Scholar] [CrossRef]
- Yang, Y.M.; Kim, S.Y.; Seki, E. Inflammation and Liver Cancer: Molecular Mechanisms and Therapeutic Targets. Semin. Liver Dis. 2019, 39, 26–42. [Google Scholar] [CrossRef]
- Eggenhuizen, P.J.; Ng, B.H.; Ooi, J.D. Treg Enhancing Therapies to Treat Autoimmune Diseases. Int. J. Mol. Sci. 2020, 21, 7015. [Google Scholar] [CrossRef]
- Hochrein, S.M.; Wu, H.; Eckstein, M.; Arrigoni, L.; Herman, J.S.; Schumacher, F.; Gerecke, C.; Rosenfeldt, M.; Grun, D.; Kleuser, B.; et al. The Glucose Transporter Glut3 Controls T Helper 17 Cell Responses Through Glycolytic-Epigenetic Reprogramming. Cell Metab. 2022, 34, 516–532.e11. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.R. The Balance of Th17 versus Treg Cells in Autoimmunity. Int. J. Mol. Sci. 2018, 19, 730. [Google Scholar] [CrossRef] [PubMed]
- Field, C.S.; Baixauli, F.; Kyle, R.L.; Puleston, D.J.; Cameron, A.M.; Sanin, D.E.; Hippen, K.L.; Loschi, M.; Thangavelu, G.; Corrado, M.; et al. Mitochondrial Integrity Regulated by Lipid Metabolism Is a Cell-Intrinsic Checkpoint for Treg Suppressive Function. Cell Metab. 2020, 31, 422–437.e5. [Google Scholar] [CrossRef]
- Gilleron, J.; Bouget, G.; Ivanov, S.; Meziat, C.; Ceppo, F.; Vergoni, B.; Djedaini, M.; Soprani, A.; Dumas, K.; Jacquel, A.; et al. Rab4b Deficiency in T Cells Promotes Adipose Treg/Th17 Imbalance, Adipose Tissue Dysfunction, and Insulin Resistance. Cell Rep. 2018, 25, 3329–3341.e5. [Google Scholar] [CrossRef]
- Li, M.; Zhang, B.; Yin, Y.; Wen, J.; Wang, J.; He, Y.; Jiang, Q.; Loor, J.J.; Wang, S.; Yang, W.; et al. Intracellular Ca2+ Signaling and Calcium Release-Activated Calcium Modulator Orai1 Are Associated with CD4+ T Lymphocytes in Dairy Cows. Front. Immunol. 2022, 13, 835936. [Google Scholar] [CrossRef]
- Ma, E.H.; Bantug, G.; Griss, T.; Condotta, S.; Johnson, R.M.; Samborska, B.; Mainolfi, N.; Suri, V.; Guak, H.; Balmer, M.L.; et al. Serine Is an Essential Metabolite for Effector T Cell Expansion. Cell Metab. 2017, 25, 482. [Google Scholar] [CrossRef]
- Kahlfuss, S.; Kaufmann, U.; Concepcion, A.R.; Noyer, L.; Raphael, D.; Vaeth, M.; Yang, J.; Pancholi, P.; Maus, M.; Muller, J.; et al. Stim1-Mediated Calcium Influx Controls Antifungal Immunity and the Metabolic Function of Non-Pathogenic Th17 Cells. EMBO Mol. Med. 2020, 12, e11592. [Google Scholar] [CrossRef]
- Letizia, M.; Wang, Y.H.; Kaufmann, U.; Gerbeth, L.; Sand, A.; Brunkhorst, M.; Weidner, P.; Ziegler, J.F.; Bottcher, C.; Schlickeiser, S.; et al. Store-Operated Calcium Entry Controls Innate and Adaptive Immune Cell Function in Inflammatory Bowel Disease. EMBO Mol. Med. 2022, 14, e15687. [Google Scholar] [CrossRef]
- Yan, J.B.; Luo, M.M.; Chen, Z.Y.; He, B.H. The Function and Role of the Th17/Treg Cell Balance in Inflammatory Bowel Disease. J. Immunol. Res. 2020, 2020, 8813558. [Google Scholar] [CrossRef]
- Lippolis, J.D.; Putz, E.J.; Reinhardt, T.A.; Casas, E.; Weber, W.J.; Crooker, B.A. Effect of Holstein Genotype on Immune Response to an Intramammary Escherichia coli Challenge. J. Dairy Sci. 2022, 105, 5435–5448. [Google Scholar] [CrossRef]
- Ferguson, J.D.; Galligan, D.T.; Thomsen, N. Principal Descriptors of Body Condition Score in Holstein Cows. J. Dairy Sci. 1994, 77, 2695–2703. [Google Scholar] [CrossRef]
- Manriquez, D.; Thatcher, W.W.; Santos, J.E.P.; Chebel, R.C.; Galvao, K.N.; Schuenemann, G.M.; Bicalho, R.C.; Gilbert, R.O.; Rodriguez-Zas, S.; Seabury, C.M.; et al. Effect of Body Condition Change and Health Status During Early Lactation on Performance and Survival of Holstein Cows. J. Dairy Sci. 2021, 104, 12785–12799. [Google Scholar] [CrossRef] [PubMed]
- Cunha, P.; Vern, Y.L.; Gitton, C.; Germon, P.; Foucras, G.; Rainard, P. Expansion, Isolation and First Characterization of Bovine Th17 Lymphocytes. Sci. Rep. 2019, 9, 16115. [Google Scholar] [CrossRef] [PubMed]
- Ceciliani, F.; Lecchi, C.; Urh, C.; Sauerwein, H. Proteomics and Metabolomics Characterizing the Pathophysiology of Adaptive Reactions to the Metabolic Challenges During the Transition from Late Pregnancy to Early Lactation in Dairy Cows. J. Proteom. 2018, 178, 92–106. [Google Scholar] [CrossRef] [PubMed]
- De Koster, J.; Strieder-Barboza, C.; de Souza, J.; Lock, A.L.; Contreras, G.A. Short Communication: Effects of Body Fat Mobilization on Macrophage Infiltration in Adipose Tissue of Early Lactation Dairy Cows. J. Dairy Sci. 2018, 101, 7608–7613. [Google Scholar] [CrossRef]
- Nguyen, M.; Bourredjem, A.; Piroth, L.; Bouhemad, B.; Jalil, A.; Pallot, G.; Le Guern, N.; Thomas, C.; Pilot, T.; Bergas, V.; et al. High Plasma Concentration of Non-Esterified Polyunsaturated Fatty Acids Is a Specific Feature of Severe COVID-19 Pneumonia. Sci. Rep. 2021, 11, 10824. [Google Scholar] [CrossRef]
- Killeen, N.; Davis, C.B.; Chu, K.; Crooks, M.E.; Sawada, S.; Scarborough, J.D.; Boyd, K.A.; Stuart, S.G.; Xu, H.; Littman, D.R. CD4 Function in Thymocyte Differentiation and T Cell Activation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1993, 342, 25–34. [Google Scholar] [CrossRef]
- Wang, J.; Xia, S.; Ren, H.; Shi, X. The Role and Function of CD4+ T Cells in Hepatic Ischemia-Reperfusion Injury. Expert Rev. Gastroenterol. Hepatol. 2022, 16, 5–11. [Google Scholar] [CrossRef]
- Tortorella, C.; Aiello, A.; Gasperini, C.; Agrati, C.; Castilletti, C.; Ruggieri, S.; Meschi, S.; Matusali, G.; Colavita, F.; Farroni, C.; et al. Humoral- and T-Cell-Specific Immune Responses to SARS-CoV-2 mRNA Vaccination in Patients with MS Using Different Disease-Modifying Therapies. Neurology 2022, 98, e541–e554. [Google Scholar] [CrossRef]
- Kory, N.; Wyant, G.A.; Prakash, G.; Uit de Bos, J.; Bottanelli, F.; Pacold, M.E.; Chan, S.H.; Lewis, C.A.; Wang, T.; Keys, H.R.; et al. Sfxn1 Is a Mitochondrial Serine Transporter Required for One-Carbon Metabolism. Science 2018, 362, eaat9528. [Google Scholar] [CrossRef]
- Nelson, H.A.; Roe, M.W. Molecular Physiology and Pathophysiology of Stromal Interaction Molecules. Exp. Biol. Med. 2018, 243, 451–472. [Google Scholar] [CrossRef]
- Xue, Y.; Zhou, S.; Xie, W.; Meng, M.; Ma, N.; Zhang, H.; Wang, Y.; Chang, G.; Shen, X. STIM1-Orai1 Interaction Exacerbates Lps-Induced Inflammation and Endoplasmic Reticulum Stress in Bovine Hepatocytes through Store-Operated Calcium Entry. Genes 2022, 13, 874. [Google Scholar] [CrossRef]



| Control (n = 6) | High-FFA (n = 6) | |||
|---|---|---|---|---|
| Item | Median | Interquartile Range | Median | Interquartile Range |
| BW (kg) | 645 | 640, 649 | 670 | 663, 693 |
| Milk yield (kg/d) | 37.05 | 34.1, 38.0 | 39.6 | 38.2, 40.4 |
| DMI | 20.85 | 19, 23.4 | 15.35 | 14.7, 15.7 |
| Serum glucose (mM) | 3.51 | 2.93, 3.85 | 2.23 | 1.99, 2.57 |
| Serum NEFA (mM) | 0.38 | 0.26, 0.53 | 1.34 | 1.07, 1.58 |
| Milk fat (%) | 4.03 | 3.14, 4.8 | 4.67 | 4.02, 5.64 |
| Protein (%) | 3.57 | 3.05, 4.06 | 3.21 | 2.86, 3.72 |
| Gene | GenBank Number | Primer (5′ to 3′) | Length (bp) |
|---|---|---|---|
| GAPDH | NM_001034034.2 | Forward: GTCTTCACTACCATGGAGAAGG | 197 |
| Reverse: TCATGGATGACCTTGGCCAG | |||
| ACTB | NM_173979.3 | Forward: GCTAACAGTCCGCCTAGAAGCA | 403 |
| Reverse: GTCATCACCATCGGCAATGAG | |||
| SDS | NM_001075662.1 | Forward: GCCTCTTGTGCGGAGTGGTTC | 109 |
| Reverse: GCCTTGGTGGAAGCGTGGAAG | |||
| IL-17A | NM_001008412.2 | Forward: CACAGCATGTGAGGGTCAAC | 101 |
| Reverse: GTGGAGAGTCCAAGGTGAGG | |||
| FOXP3 | NM_001045933.1 | Forward: TGGTGCAATCTCTGGAGCAA | 116 |
| Reverse: GTCAGATGATGCCGCAGATG | |||
| ORAI1 | NM_001099002.1 | Forward: TTTGCCGTCCACTTCTAC | 285 |
| Reverse: CCTCTTTCCTCCACTTTCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Wang, J.; Li, M.; Wen, J.; Loor, J.J.; Wang, S.; Ji, Z.; Lv, X.; Wang, G.; Xia, C.; et al. Calcium Release-Activated Calcium Modulator ORAI1-Sensitive Serine Dehydratase Regulates Fatty Acid-Induced CD4+ Th17/Treg Imbalance in Dairy Cows. Animals 2025, 15, 388. https://doi.org/10.3390/ani15030388
Zhang B, Wang J, Li M, Wen J, Loor JJ, Wang S, Ji Z, Lv X, Wang G, Xia C, et al. Calcium Release-Activated Calcium Modulator ORAI1-Sensitive Serine Dehydratase Regulates Fatty Acid-Induced CD4+ Th17/Treg Imbalance in Dairy Cows. Animals. 2025; 15(3):388. https://doi.org/10.3390/ani15030388
Chicago/Turabian StyleZhang, Bingbing, Jingjing Wang, Ming Li, Jianan Wen, Juan J. Loor, Shuang Wang, Ziwei Ji, Xinquan Lv, Guihua Wang, Cheng Xia, and et al. 2025. "Calcium Release-Activated Calcium Modulator ORAI1-Sensitive Serine Dehydratase Regulates Fatty Acid-Induced CD4+ Th17/Treg Imbalance in Dairy Cows" Animals 15, no. 3: 388. https://doi.org/10.3390/ani15030388
APA StyleZhang, B., Wang, J., Li, M., Wen, J., Loor, J. J., Wang, S., Ji, Z., Lv, X., Wang, G., Xia, C., Yang, W., & Xu, C. (2025). Calcium Release-Activated Calcium Modulator ORAI1-Sensitive Serine Dehydratase Regulates Fatty Acid-Induced CD4+ Th17/Treg Imbalance in Dairy Cows. Animals, 15(3), 388. https://doi.org/10.3390/ani15030388








