Risk Assessment of Global Animal Melioidosis Under Current and Future Climate Scenarios
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
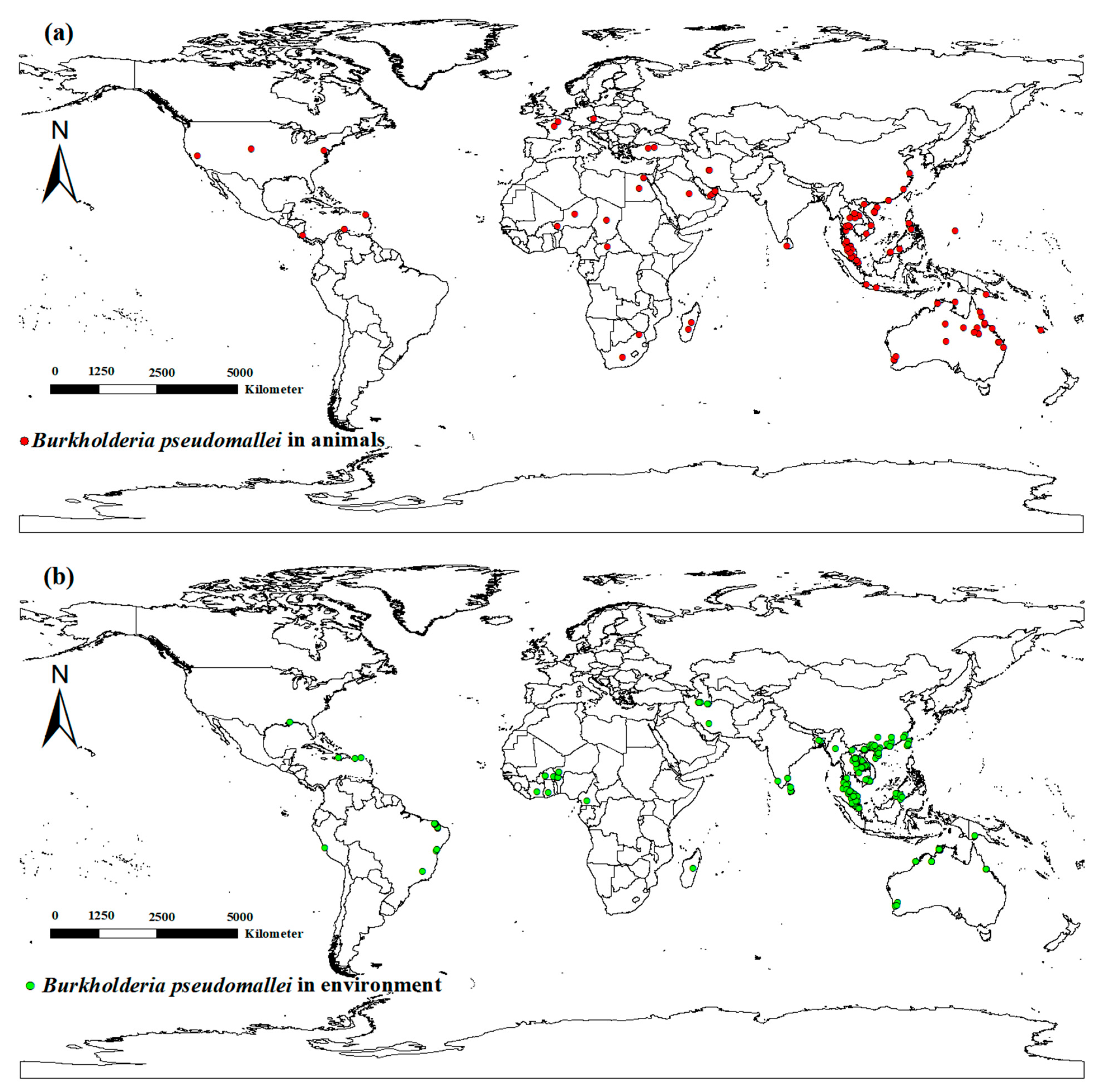
2.1. Occurrence Sites of Animal Melioidosis
2.2. Collection and Processing of Environmental Variables
2.3. Eliminating Spatial Autocorrelation of Environmental Variables
2.4. Establishment of the MaxEnt Model of Animal Melioidosis
2.5. Model Evaluation and Interpretation
3. Results
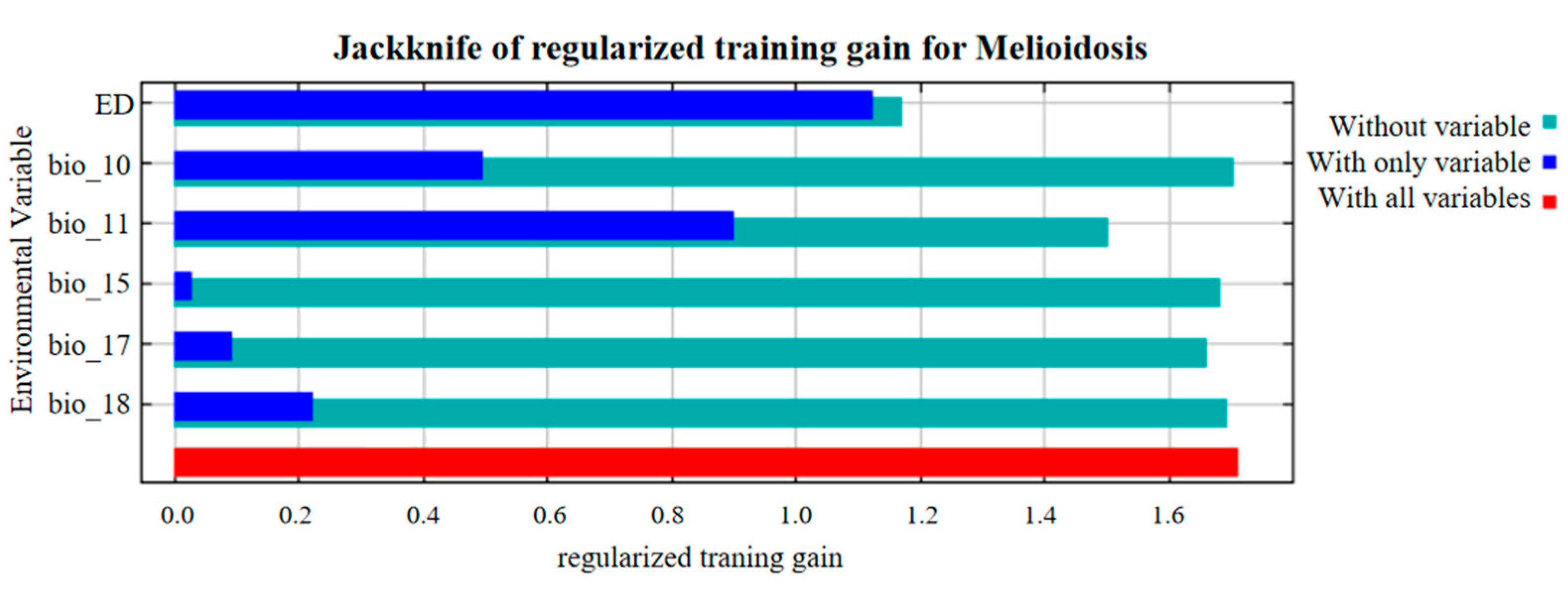
3.1. Environmental Variables Used in the Animal Melioidosis Model
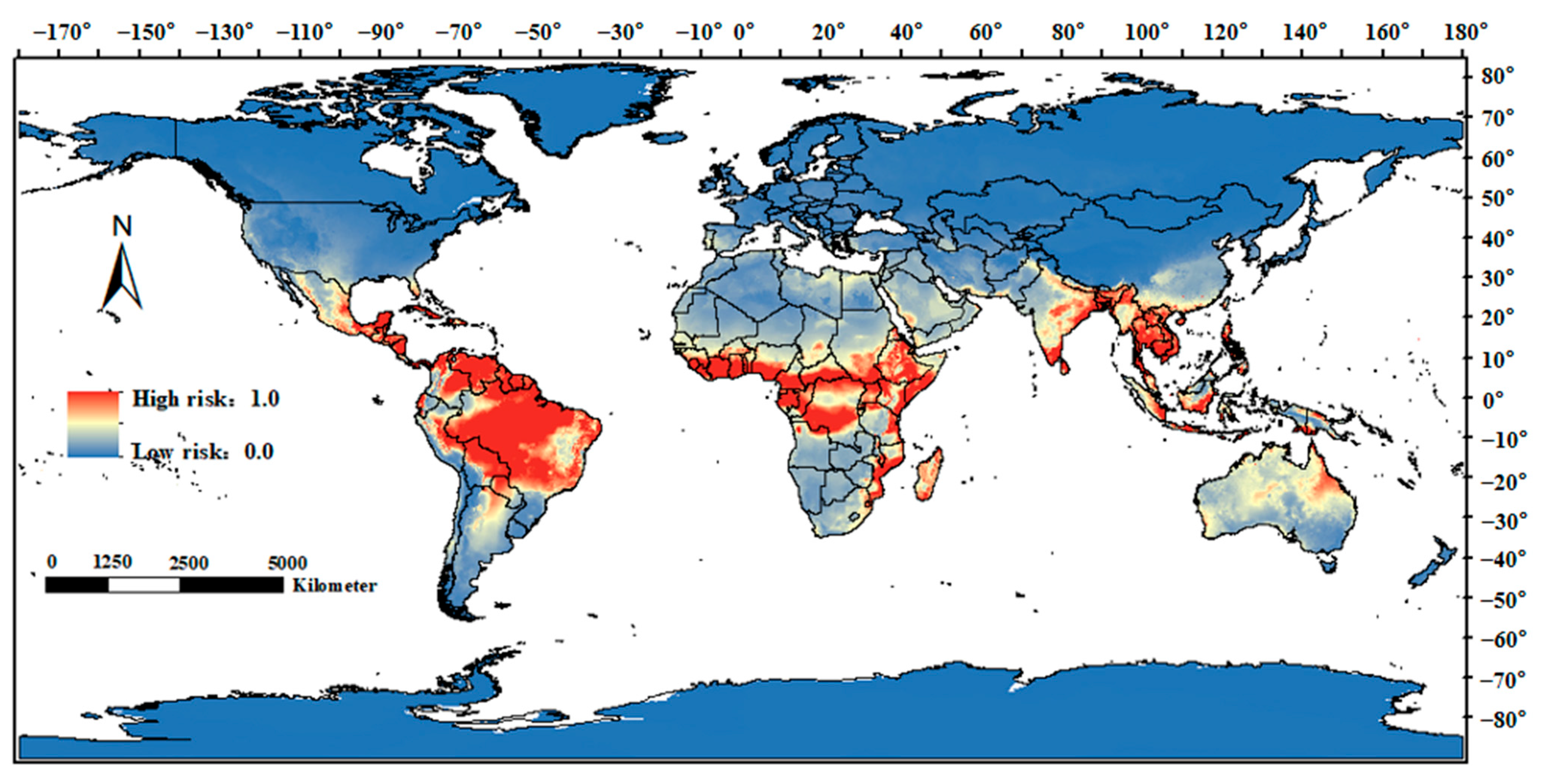
3.2. Global Risk Regions for Animal Melioidosis
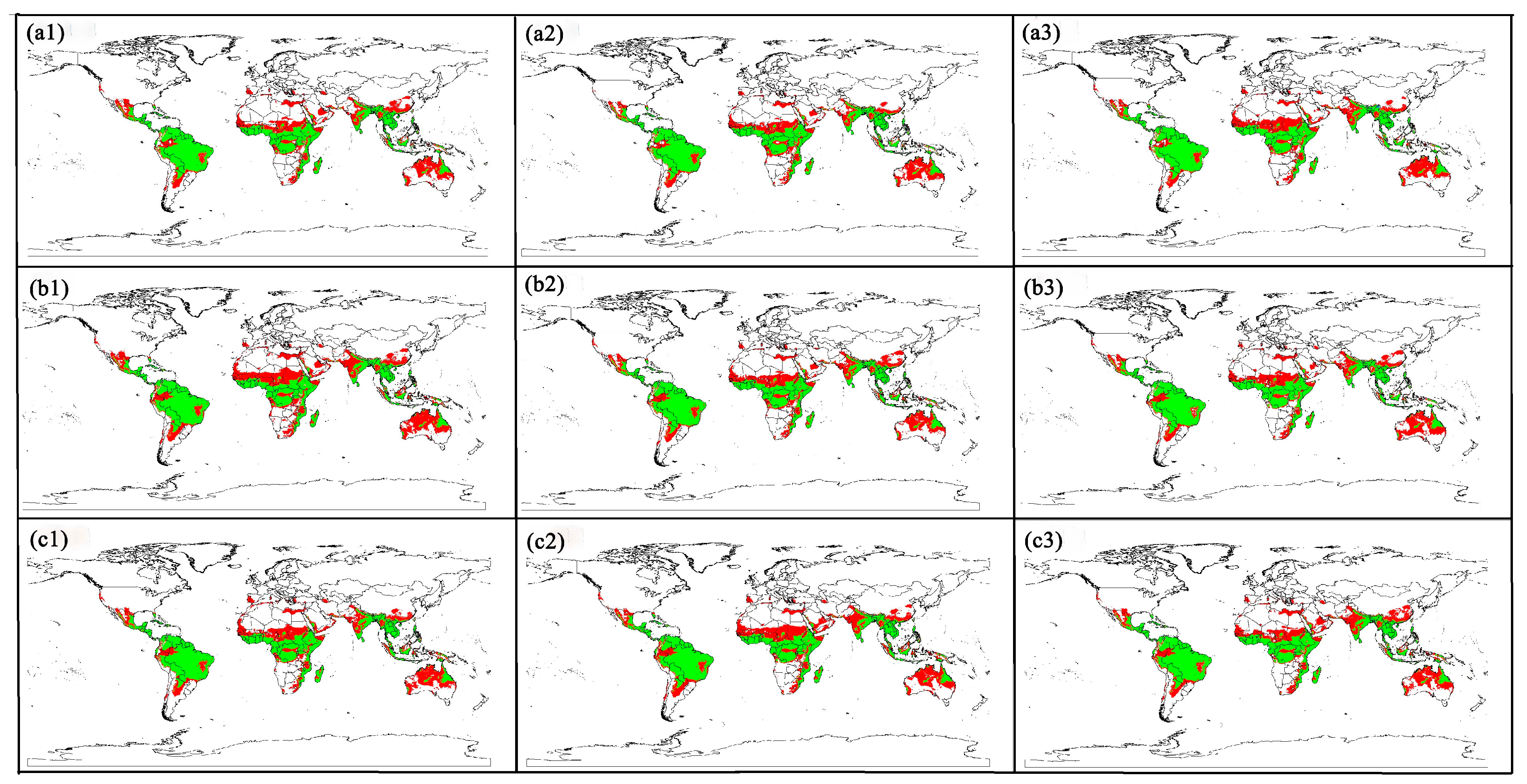
3.3. Risk Distribution Changes for Animal Melioidosis Under Future Climate Scenarios
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chewapreecha, C.; Holden, M.T.G.; Vehkala, M.; Välimäki, N.; Yang, Z.; Harris, S.R.; Mather, A.E.; Tuanyok, A.; De Smet, B.; Le Hello, S.; et al. Global and regional dissemination and evolution of Burkholderia pseudomallei. Nat. Microbiol. 2017, 2, 16263. [Google Scholar] [CrossRef] [PubMed]
- Cottew, G.S. Melioidosis in sheep in Queens land; a description of the causal organism. Aust. J. Exp. Biol. Med. Sci. 1950, 28, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Limmathurotsakul, D.; Golding, N.; Dance, D.A.B.; Messina, J.P.; Pigott, D.M.; Moyes, C.L.; Rolim, D.B.; Bertherat, E.; Day, N.P.J.; Peacock, S.J.; et al. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat. Microbiol. 2016, 1, 15008. [Google Scholar] [CrossRef]
- Currie, B.J. Melioidosis and Burkholderia pseudomallei: Progress in epidemiology, diagnosis, treatment and vaccination. Curr. Opin. Infect. Dis. 2022, 35, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Meumann, E.M.; Limmathurotsakul, D.; Dunachie, S.J.; Wiersinga, W.J.; Currie, B.J. Burkholderia pseudomallei and melioidosis. Nat. Rev. Microbiol. 2024, 22, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Limmathurotsakul, D.; Thammasart, S.; Warrasuth, N.; Thapanagulsak, P.; Jatapai, A.; Pengreungrojanachai, V.; Anun, S.; Joraka, W.; Thongkamkoon, P.; Saiyen, P.; et al. Melioidosis in animals, Thailand, 2006–2010. Emerg. Infect. Dis. 2012, 18, 325–327. [Google Scholar] [CrossRef]
- Van der Lugt, J.J.; Henton, M.M. Melioidosis in a goat. J. S. Afr. Vet. Assoc. 1995, 66, 71–73. [Google Scholar]
- Limmathurotsakul, D.; Kanoksil, M.; Wuthiekanun, V.; Kitphati, R.; Destavola, B.; Day, N.P.J.; Peacock, S.J. Activities of daily living associated with acquisition of melioidosis in northeast Thailand: A matched case-control study. PLoS Negl. Trop. Dis. 2013, 7, e2072. [Google Scholar] [CrossRef]
- Chen, P.S.; Chen, Y.-S.; Lin, H.-H.; Liu, P.-J.; Ni, W.-F.; Hsueh, P.-T.; Liang, S.-H.; Chen, C. Airborne Transmission of Melioidosis to Humans from Environmental Aerosols Contaminated with B. pseudomallei. PLoS Negl. Trop. Dis. 2015, 9, e0003834. [Google Scholar] [CrossRef]
- Currie, B.J.; Ward, L.; Cheng, A.C. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin prospective study. PLoS Negl. Trop. Dis. 2010, 4, e900. [Google Scholar] [CrossRef]
- Gassiep, I.; Armstrong, M.; Norton, R. Human Melioidosis. Clin. Microbiol. Rev. 2020, 33, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; He, L.; Kuang, H.; Li, H.; Li, S.; Chen, H.; Zheng, X.; Wang, L.; Chen, R. Correlation analysis between meteorological factors and the incidence of melioidosis in Sanya, Hainan Province from 2013 to 2017. Dis. Monit. 2020, 35, 156–161. [Google Scholar]
- Gassiep, I.; Grey, V.; Thean, L.J.; Farquhar, D.; Clark, J.E.; Ariotti, L.; Graham, R.; Jennison, A.V.; Bergh, H.; Anuradha, S.; et al. Expanding the Geographic Boundaries of Melioidosis in Queensland, Australia. Am. J. Trop. Med. Hyg. 2023, 108, 1215–1219. [Google Scholar] [CrossRef] [PubMed]
- Ganeshalingam, V.; Kaestli, M.; E Norton, R.; Gassiep, I. The effect of climate on melioidosis incidence in Townsville, Australia: A dry tropical region. Environ. Health Prev. Med. 2023, 28, 33. [Google Scholar] [CrossRef]
- Kaestli, M.; Grist, E.P.; Ward, L.; Hill, A.; Mayo, M.; Currie, B.J. The association of melioidosis with climatic factors in Darwin, Australia: A 23-year time-series analysis. J. Infect. 2016, 72, 687–697. [Google Scholar] [CrossRef]
- Shaw, T.; Assig, K.; Tellapragada, C.; Wagner, G.E.; Choudhary, M.; Göhler, A.; Eshwara, V.K.; Steinmetz, I.; Mukhopadhyay, C. Environmental Factors Associated with Soil Prevalence of the Melioidosis Pathogen Burkholderia pseudomallei: A Longitudinal Seasonal Study From South West India. Front. Microbiol. 2022, 13, 902996. [Google Scholar] [CrossRef]
- Naidoo, S. Commentary on the contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. S. Afr. J. Sci. 2022, 118, 1–4. [Google Scholar] [CrossRef]
- Dyez, K.A.; Cole, J.E.; Lough, J.M. Rainfall variability increased with warming in northern Queensland, Australia, over the past 280 years. Commun. Earth Environ. 2024, 5, 117. [Google Scholar] [CrossRef]
- Mallesh, R.; Srinivasan, J. How is the relationship between rainfall and water vapor in the Indian monsoon influenced by changes in lapse rate during global warming? Environ. Res. Commun. 2024, 6, 031001. [Google Scholar] [CrossRef]
- Ciarlo, J.; Giorgi, F. An increase in global daily precipitation records in response to global warming based on reanalysis and observations. Open Res. Eur. 2024, 4, 114. [Google Scholar] [CrossRef]
- Currie, B.J.; Jacups, S.P. Intensity of rainfall and severity of melioidosis, Australia. Emerg. Infect. Dis. 2003, 9, 1538–1542. [Google Scholar] [CrossRef] [PubMed]
- Sillero, N. What does ecological modelling model? A proposed classification of ecological niche models based on their underlying methods. Ecol. Model. 2011, 222, 1343–1346. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Li, H.; Pan, H.; Xu, L.; Li, S.; Li, S.; Chen, S.; Man, C.; Du, L.; Chen, Q.; Xiao, J.; et al. Predicting Risk Areas of Classical Scrapie in China Based on Environmental Suitability. Transbound. Emerg. Dis. 2023, 2023, 2826256. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Sun, Z.; Li, Y.-P.; An, Q.; Gao, X.; Wang, H.-B. Risk Factors Contributing to Highly Pathogenic Avian Influenza H5N6 in China, 2014–2021: Based on a MaxEnt Model. Transbound. Emerg. Dis. 2023, 2023, 6449392. [Google Scholar] [CrossRef]
- Elith, J.; HGraham, C.P.; Anderson, R.; Dudík, M.; Ferrier, S.; Guisan, A.; JHijmans, R.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel Methods Improve Prediction of Species’ Distributions from Occurrence Data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Morales-Castilla, I.; Pappalardo, P.; Farrell, M.J.; Aguirre, A.A.; Huang, S.; Gehman, A.-L.M.; Dallas, T.; Gravel, D.; Davies, T.J. Forecasting parasite sharing under climate change. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2021, 376, 20200360. [Google Scholar] [CrossRef]
- Estes, L.D.; Bradley, B.A.; Beukes, H.; Hole, D.G.; Lau, M.; Oppenheimer, M.G.; Schulze, R.; Tadross, M.A.; Turner, W.R. Comparing mechanistic and empirical model projections of crop suitability and productivity: Implications for ecological forecasting. Glob. Ecol. Biogeogr. 2013, 22, 1007–1018. [Google Scholar] [CrossRef]
- Fortini, L.B.; Kaiser, L.R.; Xue, L.; Wang, Y. Bioclimatic variables dataset for baseline and future climate scenarios for climate change studies in Hawai’i. Data Brief 2022, 45, 108572. [Google Scholar] [CrossRef]
- Ma, J.; Gao, X.; Liu, B.; Chen, H.; Xiao, J.; Wang, H. Peste des petits ruminants in China: Spatial risk analysis. Transbound. Emerg. Dis. 2019, 66, 1784–1788. [Google Scholar] [CrossRef] [PubMed]
- Mollalo, A.; Sadeghian, A.; Israel, G.D.; Rashidi, P.; Sofizadeh, A.; Glass, G.E. Machine learning approaches in GIS-based ecological modeling of the sand fly Phlebotomus papatasi, a vector of zoonotic cutaneous leishmaniasis in Golestan province, Iran. Acta Trop. 2018, 188, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Fekede, R.J.; Gils, H.; Huang, L.; Wang, X. High probability areas for ASF infection in China along the Russian and Korean borders. Transbound. Emerg. Dis. 2018, 66, 852–864. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, L.; Zhou, X.; Chen, R.; Zhao, G.; Zhang, F. Prediction of the potentially suitable areas of Leonurus japonicus in China based on future climate change using the optimized MaxEnt model. Ecol. Evol. 2023, 13, e10597. [Google Scholar] [CrossRef]
- Moss, R.H.; Edmonds, J.A.; Hibbard, K.A.; Manning, M.R.; Rose, S.K.; Van Vuuren, D.P.; Carter, T.R.; Emori, S.; Kainuma, M.; Kram, T. The next generation of scenarios for climate change research and assessment. Nature 2010, 463, 747–756. [Google Scholar] [CrossRef]
- Xu, W.; Xiao, Y.; Zhang, J.; Yang, W.; Zhang, L.; Hull, V.; Wang, Z.; Zheng, H.; Liu, J.; Polasky, S.; et al. Strengthening protected areas for biodiversity and ecosystem services in China. Proc. Natl. Acad. Sci. USA 2017, 114, 1601–1606. [Google Scholar] [CrossRef]
- Lozier, J.D.; Mills, N.J. Ecological niche models and coalescent analysis of gene flow support recent allopatric isolation of parasitoid wasp populations in the Mediterranean. PLoS ONE 2009, 4, e5901. [Google Scholar] [CrossRef]
- Lippi, C.A.; Mundis, S.J.; Sippy, R.; Flenniken, J.M.; Chaudhary, A.; Hecht, G.; Carlson, C.J.; Ryan, S.J. Trends in mosquito species distribution modeling: Insights for vector surveillance and disease control. Parasites Vectors 2023, 16, 302. [Google Scholar] [CrossRef]
- Wen, F.; Lu, L.; Nie, C.; Sun, Z.; Liu, R.; Huang, W.; Ye, H. Analysis of Spatiotemporal Variation in Habitat Suitability for Oedaleus decorus asiaticus Bei-Bienko on the Mongolian Plateau Using Maxent and Multi-Source Remote Sensing Data. Insects 2023, 14, 492. [Google Scholar] [CrossRef]
- Balceniuk, M.D.; Motyl, C.M.; Ayers, B.C.; Geiger, J.T.; Sebastian, A.; Doyle, A.J.; Glocker, R.J.; Stoner, M.C. Association Between the Atherosclerotic Disease Risk Score and Carotid Artery Stenosis. J. Surg. Res. 2021, 257, 189–194. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Venzal, J.M. Climate niches of tick species in the Mediterranean region: Modeling of occurrence data, distributional constraints, and impact of climate change. J. Med. Entomol. 2007, 44, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.P.; van Klinken, R.D.; Metternicht, G. Comparison of alternative strategies for invasive species distribution modeling. Ecol. Model. 2010, 221, 2261–2269. [Google Scholar] [CrossRef]
- Çorbacıoğlu, Ş.K.; Aksel, G. Receiver operating characteristic curve analysis in diagnostic accuracy studies: A guide to interpreting the area under the curve value. Turk. J. Emerg. Med. 2023, 23, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Dodin, A.; Galimand, M. Origin, course and recession of an infectious disease, melioidosis, in temperate countries. Arch. Inst. Pasteur. Tunis 1986, 63, 69–73. [Google Scholar] [PubMed]
- Dance, D.A.; King, C.; Aucken, H.; Knott, C.D.; West, P.G.; Pitt, T.L. An outbreak of melioidosis in imported primates in Britain. Vet. Rec. 1992, 130, 525–529. [Google Scholar] [CrossRef]
- Taetzsch, S.J.; Swaney, E.M.; Gee, J.E.; Hidalgo, P.M.; Broussard, K.R.; Martines, R.B.; Blaney, D.D.; Galland, G.G.; Gulvik, C.A.; Marston, C.K.; et al. Melioidosis in Cynomolgus Macaques (Macaca Fascicularis) Imported to the United States from Cambodia. Comp. Med. 2022, 72, 394–402. [Google Scholar] [CrossRef]
- Zehnder, A.M.; Hawkins, M.G.; Koski, M.A.; Lifland, B.; Byrne, B.A.; Swanson, A.A.; Rood, M.P.; Gee, J.E.; Elrod, M.G.; Beesley, C.A.; et al. Burkholderia pseudomallei isolates in 2 pet iguanas, California, USA. J. Emerg. Infect. Dis. 2014, 20, 304–306. [Google Scholar] [CrossRef]
- Savelkoel, J.; Dance, D.A.B.; Currie, B.J.; Limmathurotsakul, D.; Wiersinga, W.J. A call to action: Time to recognise melioidosis as a neglected tropical disease. Lancet Infect. Dis. 2022, 22, e176–e182. [Google Scholar] [CrossRef]
- Cheng, A.C.; Currie, B.J. Melioidosis: Epidemiology, pathophysiology, and management. Clin. Microbiol. Rev. 2005, 18, 383–416. [Google Scholar] [CrossRef]
- Manivanh, L.; Pierret, A.; Rattanavong, S.; Kounnavongsa, O.; Buisson, Y.; Elliott, I.; Maeght, J.L.; Xayyathip, K.; Silisouk, J.; Vongsouvath, M.; et al. Burkholderia pseudomallei in a lowland rice paddy: Seasonal changes and influence of soil depth and physico-chemical properties. Sci. Rep. 2017, 7, 3031. [Google Scholar] [CrossRef]
- Hinwan, Y.; Chareonsudjai, P.; Reungsang, P.; Kraiklang, R.; Chetchotisakd, P.; Chareonsudjai, S.; Sirichoat, A.; Nithichanon, A.; Wonglakorn, L.; Sermswan, R.W.; et al. Analysis of fine-scale phylogeny of Burkholderia pseudomallei in relation to regional geography and drug susceptibility in Thailand. Sci. Rep. 2024, 14, 19961. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Pang, L.; Sim, S.H.; Goh, K.T.; Ravikumar, S.; Win, M.S.; Tan, G.; Cook, A.R.; Fisher, D.; Chai, L.Y.A. Association of melioidosis incidence with rainfall and humidity, Singapore, 2003–2012. Emerg. Infect. Dis. 2015, 21, 159–162. [Google Scholar] [CrossRef]
- Chen, Y.L.; Yen, Y.-C.; Yang, C.-Y.; Lee, M.S.; Ho, C.-K.; Mena, K.D.; Wang, P.-Y.; Chen, P.-S. The concentrations of ambient Burkholderia pseudomallei during typhoon season in endemic area of melioidosis in Taiwan. PLoS Negl. Trop. Dis. 2014, 8, e2877. [Google Scholar] [CrossRef]
- Currie, B.J.; Mayo, M.; Ward, L.M.; Kaestli, M.; Meumann, E.M.; Webb, J.R.; Woerle, C.; Baird, R.W.; Price, R.N.; Marshall, C.S.; et al. The Darwin Prospective Melioidosis Study: A 30-year prospective, observational investigation. Lancet Infect. Dis. 2021, 21, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Bulterys, P.L.; A Bulterys, M.; Phommasone, K.; Luangraj, M.; Mayxay, M.; Kloprogge, S.; Miliya, T.; Vongsouvath, M.; Newton, P.N.; Phetsouvanh, R.; et al. Climatic drivers of melioidosis in Laos and Cambodia: A 16-year case series analysis. Lancet Planet Health 2018, 2, e334–e343. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.D.; Forbes-Faulkner, J.; Parker, M. Isolation of Pseudomonas pseudomallei from clay layers at defined depths. Am. J. Epidemiol. 1979, 110, 515–521. [Google Scholar] [CrossRef]
- Tong, S.; Yang, S.; Lu, Z.; He, W. Laboratory investigation of ecological factors influencing the environmental presence of Burkholderia pseudomallei. Microbiol. Immunol. 1996, 40, 451–453. [Google Scholar] [CrossRef]
- Chen, I.C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- He, X.; Burgess, K.S.; Yang, X.; Ahrends, A.; Gao, L.; Li, D. Upward elevation and northwest range shifts for alpine Meconopsis species in the Himalaya-Hengduan Mountains region. Ecol. Evol. 2019, 9, 4055–4064. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef]


| Variable | Description | Data Processing | Included |
|---|---|---|---|
| bio 1 | Annual mean temperature | N | |
| bio 2 | Mean diurnal range | N | |
| bio 3 | Isothermality (bio2/bio7) | N | |
| bio 4 | Temperature seasonality | N | |
| bio 5 | Maximum temperature of the warmest month | N | |
| bio 6 | Minimum temperature of the coldest month | N | |
| bio 7 | Temperature annual range (bio 5–bio 6) | N | |
| bio 8 | Mean temperature of the wettest quarter | N | |
| bio 9 | Mean temperature of the driest quarter | N | |
| bio 10 | Mean temperature of the warmest quarter | Y | |
| bio 11 | Mean temperature of the coldest quarter | Y | |
| bio 12 | Annual precipitation | N | |
| bio 13 | Precipitation of the wettest month | N | |
| bio 14 | Precipitation of the driest month | N | |
| bio 15 | Precipitation seasonality | Y | |
| bio 16 | Precipitation of the wettest quarter | N | |
| bio 17 | Precipitation of the driest quarter | Y | |
| bio 18 | Precipitation of the warmest quarter | Y | |
| bio 19 | Precipitation of the coldest quarter | N | |
| ED | Density of Burkholderia pseudomallei in the environment (soil, water, and air) | Kernel density analysis | Y |
| 2050s | 2070s | 2090s | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SSPs | Expansion | Stable | Contraction | Expansion | Stable | Contraction | Expansion | Stable | Contraction |
| SSP 126 | 18,316.1 | 28,105.3 | 5.7 | 17,901.0 | 28,099.0 | 12.0 | 18,886.7 | 28,033.3 | 77.7 |
| SSP 245 | 23,237.7 | 28,107.4 | 3.6 | 19,265.0 | 28,105.3 | 5.6 | 18,715.6 | 28,077.0 | 34.0 |
| SSP 585 | 19,607.1 | 28,106.2 | 4.8 | 21,805.9 | 21,808.3 | 2.7 | 19,855.5 | 28,105.3 | 5.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Xu, L.; Jiao, Y.; Li, S.; Yang, Y.; Lan, F.; Chen, S.; Man, C.; Du, L.; Chen, Q.; et al. Risk Assessment of Global Animal Melioidosis Under Current and Future Climate Scenarios. Animals 2025, 15, 455. https://doi.org/10.3390/ani15030455
Li S, Xu L, Jiao Y, Li S, Yang Y, Lan F, Chen S, Man C, Du L, Chen Q, et al. Risk Assessment of Global Animal Melioidosis Under Current and Future Climate Scenarios. Animals. 2025; 15(3):455. https://doi.org/10.3390/ani15030455
Chicago/Turabian StyleLi, Suya, Le Xu, Yuqing Jiao, Shiyuan Li, Yingxue Yang, Feng Lan, Si Chen, Churiga Man, Li Du, Qiaoling Chen, and et al. 2025. "Risk Assessment of Global Animal Melioidosis Under Current and Future Climate Scenarios" Animals 15, no. 3: 455. https://doi.org/10.3390/ani15030455
APA StyleLi, S., Xu, L., Jiao, Y., Li, S., Yang, Y., Lan, F., Chen, S., Man, C., Du, L., Chen, Q., Wang, F., & Gao, H. (2025). Risk Assessment of Global Animal Melioidosis Under Current and Future Climate Scenarios. Animals, 15(3), 455. https://doi.org/10.3390/ani15030455







