In Vitro Investigation of the Effects of Bacillus subtilis-810B and Bacillus licheniformis-809A on the Rumen Fermentation and Microbiota
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Origin and Sampling of Rumen and Digestive Content
2.2. Strains, Media and Culture Conditions
2.3. Compatibility with Monensin
2.4. Enzymatic Activity Assay
2.5. Pathogen Inhibition Assay
2.6. Batch Fermentation
2.7. Gas Composition, Biochemical, Ammonia-N and SCFA Analyses
2.8. DNA Extraction from Batch Fermentation Samples
2.9. MiSeq 16S rDNA Sequencing and Bioinformatic Analysis
3. Results
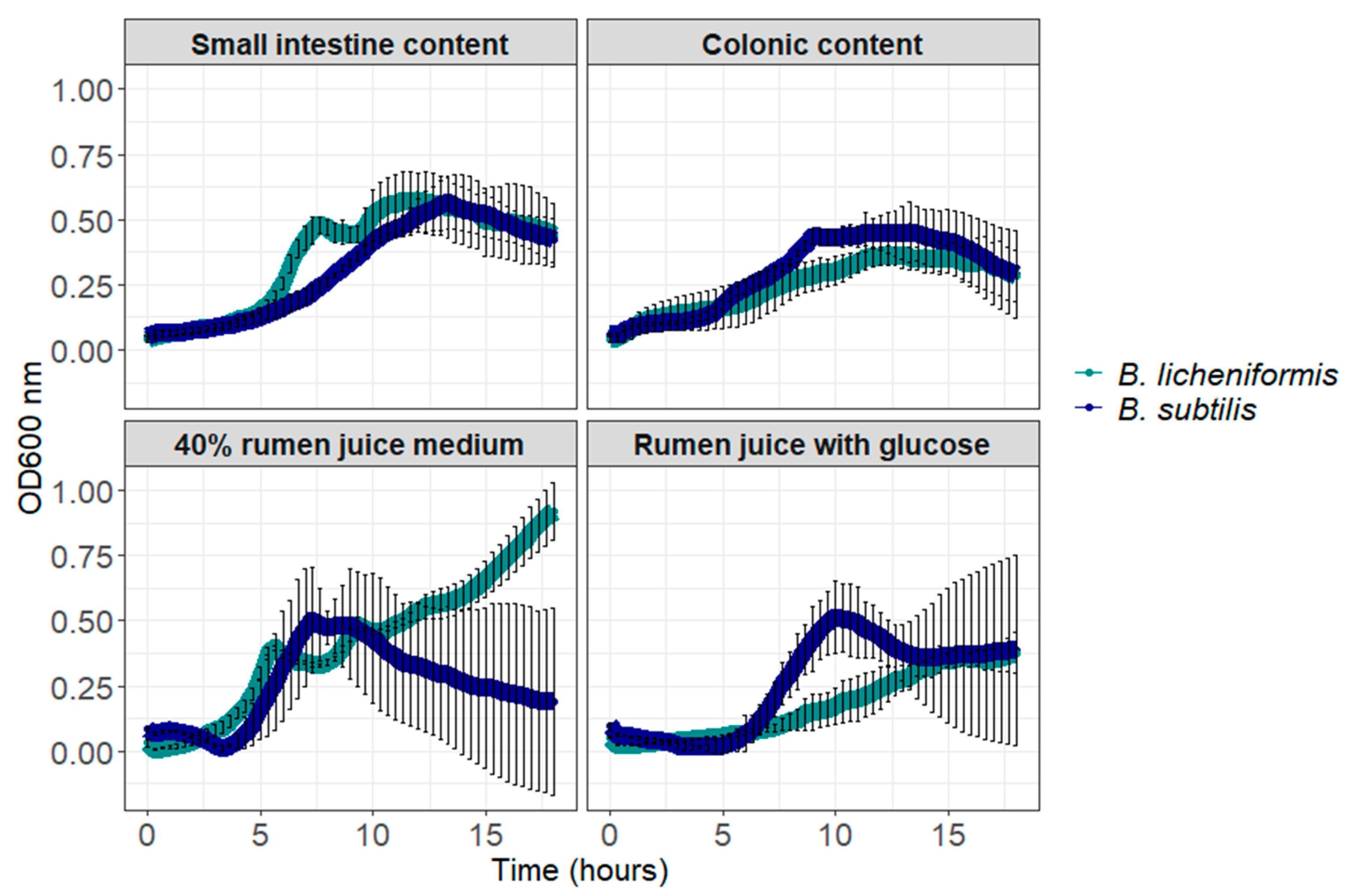
3.1. B. licheniformis and B. subtilis Can Germinate and Grow in Digestive Contents
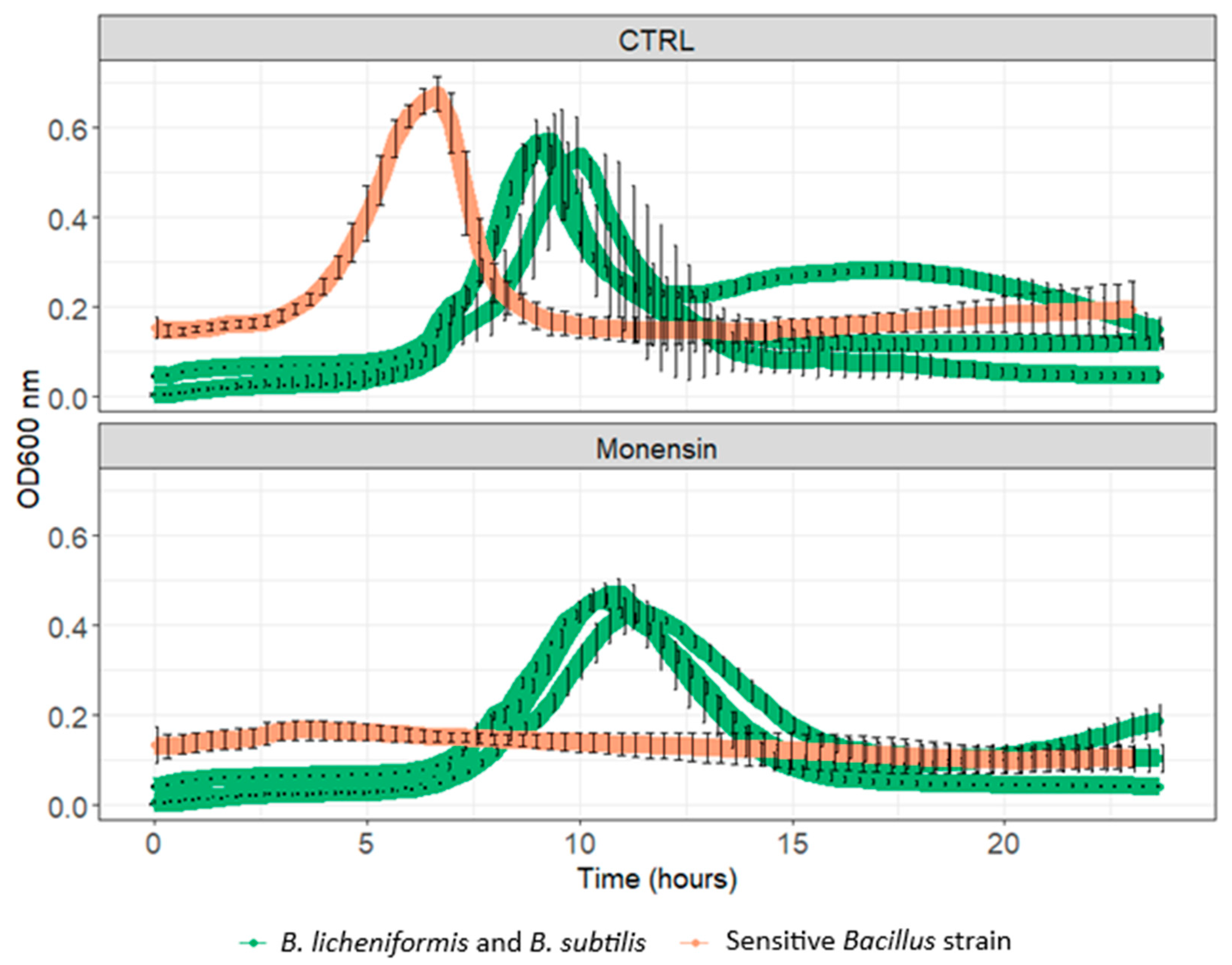
3.2. Resistance to In-Feed Antibiotics
3.3. Growth and Germination of B. licheniformis and B. subtilis Favors Anaerobic Environment
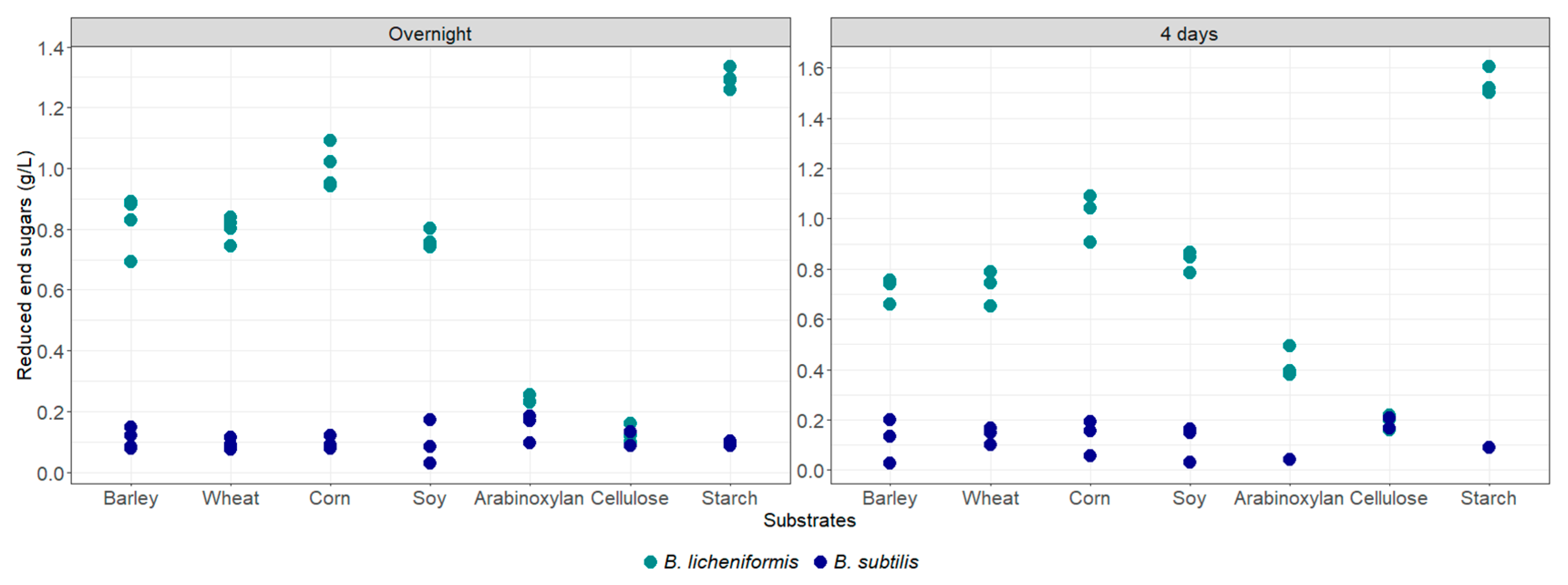
3.4. Enzymatic Capacity of B. licheniformis and B. subtilis in Rumen Content
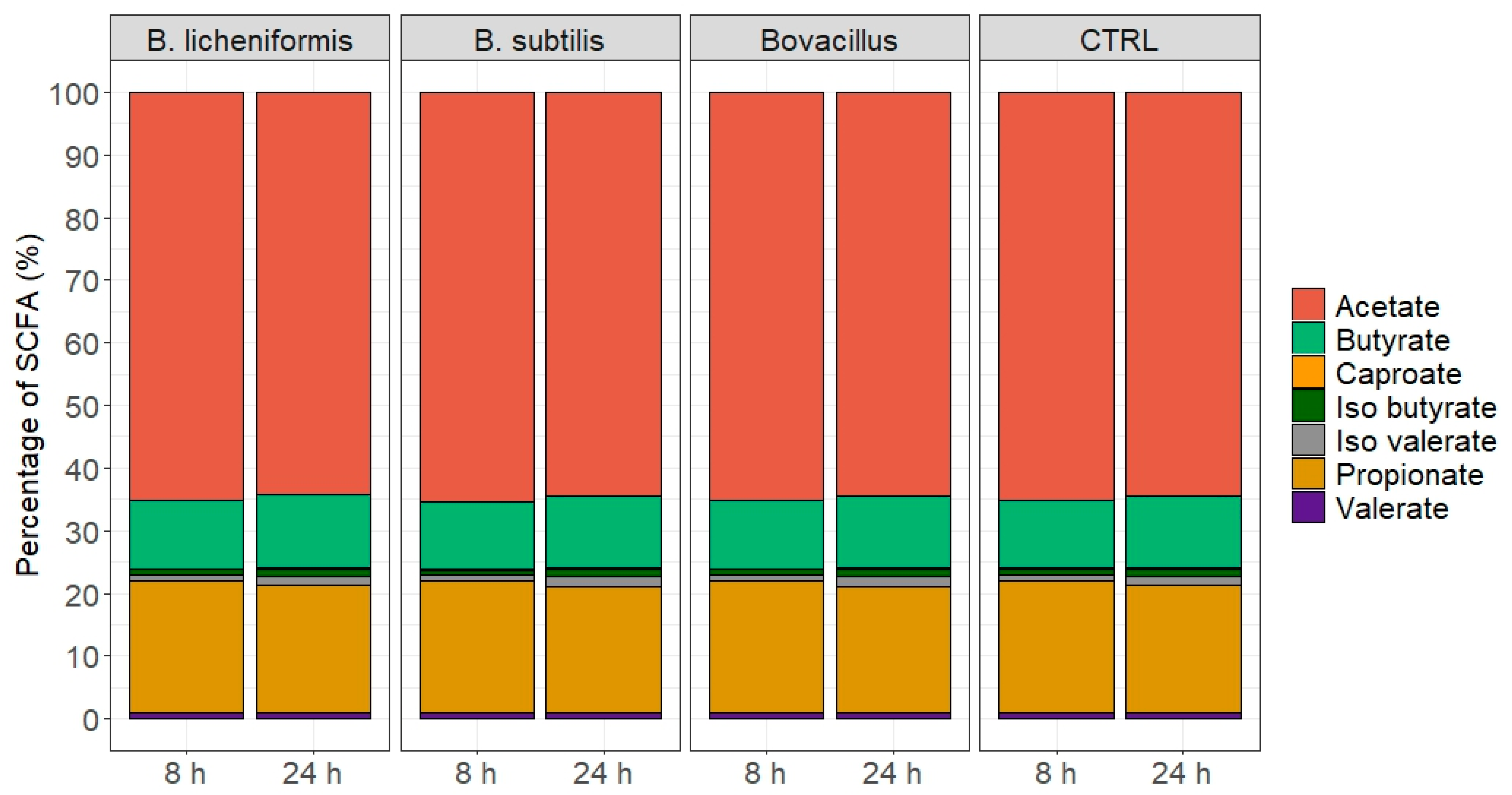
3.5. Effects of Single Strains and Cocktails of B. licheniformis and B. subtilis on Rumen Batch Fermentation
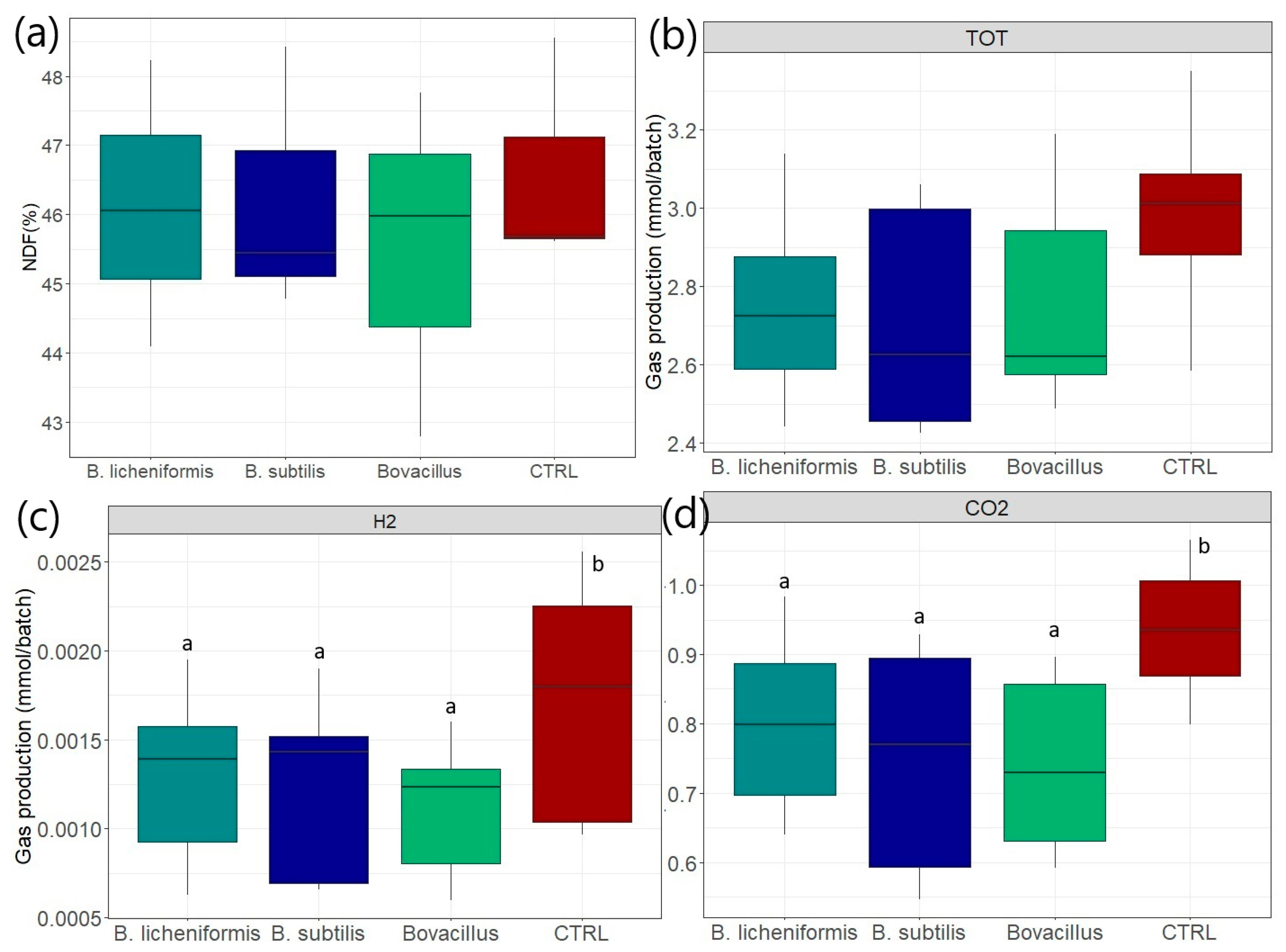
3.6. Effects on Fiber Degradation
3.7. Effects on Gas Production After 8 h
3.8. In Vitro Effects of B. licheniformis and B. subtilis on the Rumen Microbiota Composition
3.9. Inhibition Capacity of B. licheniformis and B. subtilis on S. Typhimurium
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ban, Y.; Guan, L.L. Implication and challenges of direct-fed microbial supplementation to improve ruminant production and health. J. Anim. Sci. Biotechnol. 2021, 12, 109. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bernardeau, M.; Lehtinen, M.J.; Forssten, S.D.; Nurminen, P. Importance of the gastrointestinal life cycle of Bacillus for probiotic functionality. J. Food Sci. Technol. 2017, 54, 2570–2584. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Luise, D.; Bosi, P.; Raff, L.; Amatucci, L.; Virdis, S.; Trevisi, P. Bacillus spp. Probiotic Strains as a Potential Tool for Limiting the Use of Antibiotics, and Improving the Growth and Health of Pigs and Chickens. Front. Microbiol. 2022, 13, 801827. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Elshaghabee, F.M.F.; Rokana, N.; Gulhane, R.D.; Sharma, C.; Panwar, H. Bacillus As Potential Probiotics: Status, Concerns, and Future Perspectives. Front. Microbiol. 2017, 8, 1490. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Su, Y.; Liu, C.; Fang, H.; Zhang, D. Bacillus subtilis: A universal cell factory for industry, agriculture, biomaterials and medicine. Microb. Cell Fact. 2020, 19, 173. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ruiz Sella, S.R.B.; Bueno, T.; de Oliveira, A.A.B.; Grace Karp, S.; Ricardo Soccol, C. Bacillus subtilis natto as a potential probiotic in animal nutrition. Crit. Rev. Biotechnol. 2021, 41, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, J.; Cappellozza, B.I.; Dos Santos, T.C.; Inoe, F.; Pessoa Araújo Júnior, J.; Kurissio, J.K.; Queiroz, O.; Joergensen, J.N.; Cooke, R.F.; Vasconcelos, C.G.C.; et al. Effects of supplementing direct-fed microbials on health and growth of preweaning Gyr × Holstein dairy calves. J. Dairy Sci. 2024, 107, 6117–6130. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Wang, J.Q.; Deng, L.F. Effects of Bacillus subtilis natto on milk production, rumen fermentation and ruminal microbiome of dairy cows. Animal. 2013, 7, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Smock, T.M.; Samuelson, K.L.; Hergenreder, J.E.; Rounds, P.W.; Richeson, J.T. Effects of Bacillus subtilis PB6 and/or chromium propionate supplementation on clinical health, growth performance, and carcass traits of high-risk cattle during the feedlot receiving and finishing periods. Transl. Anim. Sci. 2020, 4, 163. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, P.; Wang, J.Q.; Zhang, H.T. Effects of supplementation of Bacillus subtilis natto Na and N1 strains on rumen development in dairy calves. Anim. Feed Sci. Technol. 2011, 164, 154–160. [Google Scholar] [CrossRef]
- Izquierdo, V.S.; Cappellozza, B.I.; Silva, J.V.L.; Santos, G.C.M.; Miranda, A.; Bittar, J.H.J.; Pickett, A.; Mackey, S.; Cooke, R.F.; Vendramini, J.M.B.; et al. Maternal pre- and postpartum supplementation of a Bacillus-based DFM enhanced cow and calf performance. J. Anim. Sci. 2024, 102, 110. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fazelnia, K.; Fakhraei, J.; Yarahmadi, H.M. Dietary Supplementation of Potential Probiotics Bacillus subtilis, Bacillus licheniformis, and Saccharomyces cerevisiae and Synbiotic Improves Growth Performance and Immune Responses by Modulation in Intestinal System in Broiler Chicks Challenged with Salmonella Typhimurium. Probiotics Antimicrob. Proteins 2021, 13, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- Ostovan, R.; Pourmontaseri, M.; Hosseinzadeh, S.; Shekarforoush, S.S. Interaction between the probiotic Bacillus subtilis and Salmonella Typhimurium in Caco-2 cell culture. Iran. J. Microbiol. 2021, 13, 91–97. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sandvang, D.; Skjoet-Rasmussen, L.; Cantor, M.D.; Mathis, G.F.; Lumpkins, B.S.; Blanch, A. Effects of feed supplementation with 3 different probiotic Bacillus strains and their combination on the performance of broiler chickens challenged with Clostridium perfringens. Poult. Sci. 2021, 100, 100982. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Salehi, T.Z.; Tadjbakhsh, H.; Atashparvar, N.; Nadalian, M.G.; Mahzounieh, M.R. Detection and identification of Salmonella Typhimurium in bovine diarrhoeic fecal samples by immunomagnetic separation and multiplex PCR assay. Zoonoses Public Health 2007, 54, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Tamamura, Y.; Uchida, I.; Tanaka, K.; Okazaki, H.; Tezuka, S.; Hanyu, H.; Kataoka, N.; Makino, S.; Kishima, M.; Kubota, T.; et al. Molecular epidemiology of Salmonella enterica serovar typhimurium isolates from cattle in hokkaido, Japan: Evidence of clonal replacement and characterization of the disseminated clone. Appl. Environ. Microbiol. 2011, 77, 1739–1750. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nyberg, K.A.; Andersson, G.M.; Elving, J. Long-term survival of Escherichia coli O157:H7 and Salmonella Typhimurium in cowpats on pasture. J. Appl. Microbiol. 2019, 126, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Segura, A.; Bertoni, M.; Auffret, P.; Klopp, C.; Bouchez, O.; Genthon, C. Transcriptomic analysis reveals specific metabolic pathways of enterohemorrhagic Escherichia coli O157:H7 in bovine digestive contents. BMC Genom. 2018, 19, 766. [Google Scholar] [CrossRef] [PubMed]
- Leedle, J.A.; Hespell, R.B. Differential carbohydrate media and anaerobic replica plating techniques in delineating carbohydrate-utilizing subgroups in rumen bacterial populations. Appl. Environ. Microbiol. 1980, 39, 709–719. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bertin, Y.; Deval, C.; de la Foye, A.; Masson, L.; Gannon, V.; Harel, J. The gluconeogenesis pathway is involved in maintenance of enterohaemorrhagic Escherichia coli O157:H7 in bovine intestinal content. PLoS ONE. 2014, 9, 98367. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Macheboeuf, D.; Morgavi, D.P.; Papon, Y.; Mousset, J.L.; Arturo-Schaan, M. Dose-response effects of essential oils on in vitro fermentation activity of the rumen microbial population. Anim. Feed Sci. Technol. 2008, 145, 335–350. [Google Scholar] [CrossRef]
- Theodorou, M.K.; Williams, B.A.; Dhanoa, M.S.; McAllan, A.B.; France, J. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim. Feed Sci. Technol. 1994, 48, 185–197. [Google Scholar] [CrossRef]
- Weatherburn, M.W. Phenol Hipochlorite Reaction for Determination of Ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods of Dietary Fiber, Neutral Detergent Fiber and Non-Starch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Lee, C.; Kim, J.; Hwang, S. Group-specific primer and probe sets to detect methanogenic communities using quantitative real-time polymerase chain reaction. Biotechnol. Bioeng. 2005, 89, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.J.; Pace, B.; Olsen, G.J.; Stahl, D.A.; Sogin, M.L.; Pace, N.R. Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proc. Natl. Acad. Sci. USA 1985, 82, 6955–6959. [Google Scholar] [CrossRef]
- Ohene-Adjei, S.; Chaves, A.V.; McAllister, T.A.; Benchaar, C.; Teather, R.M.; Forster, R.J. Evidence of increased diversity of methanogenic archaea with plant extract supplementation. Microb. Ecol. 2008, 56, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Theil, S.; Rifa, E. rANOMALY: AmplicoN wOrkflow for Microbial community AnaLYsis. F1000Research 2021, 10, 7. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cooley, N.P.; Wright, E.S. Accurate annotation of protein coding sequences with IDTAXA. NAR Genom. Bioinform. 2021, 16, 3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, 590–596. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Foster, Z.S.; Sharpton, T.J.; Grünwald, N.J. Metacoder: An R package for visualization and manipulation of community taxonomic diversity data. PLoS Comput. Biol. 2017, 13, 1005404. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Berni Canani, R.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Cutting, S.M. Bacillus probiotics. Food Microbiol. 2011, 28, 214–220. [Google Scholar] [CrossRef]
- Cappellozza, B.I.; Segura, A.; Milora, N.; Galschioet, C.; Schjelde, M.; Copani, G. Stability of Bacillus and Enterococcus faecium 669 Probiotic Strains When Added to Different Feed Matrices Used in Dairy Production. Animals 2023, 13, 2350. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carresi, C.; Marabelli, R.; Roncada, P.; Britti, D. Is the Use of Monensin Another Trojan Horse for the Spread of Antimicrobial Resistance? Antibiotics 2024, 13, 129. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thomas, M.; Webb, M.; Ghimire, S.; Blair, A.; Olson, K.; Fenske, G.; Scaria, J. Metagenomic characterization of the effect of feed additives on the gut microbiome and antibiotic resistome of feedlot cattle. Sci. Rep. 2017, 7, 1. [Google Scholar] [CrossRef]
- Rezaei Ahvanooei, M.R.; Norouzian, M.A.; Piray, A.H.; Vahmani, P.; Ghaffari, M.H. Effects of monensin supplementation on rumen fermentation, methane emissions, nitrogen balance, and metabolic responses of dairy cows: A systematic review and dose-response meta-analysis. J. Dairy Sci. 2024, 107, 607–624. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.; Chen, C.; Knowlton, K.; Pruden, A.; Xia, K. Fate and effect of antibiotics in beef and dairy manure during static and turned composting. J. Environ. Qual. 2017, 46, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Schären, M.; Drong, C.; Kiri, K.; Riede, S.; Gardener, M.; Meyer, U.; Hummel, J.; Urich, T.; Breves, G.; Dänicke, S. Differential effects of monensin and a blend of essential oils on rumen microbiota composition of transition dairy cows. J. Dairy Sci. 2017, 100, 2765–2783. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.C.; Devine, A.A.; Ellis, J.C.; Grunden, A.M.; Fellner, V. Effects of antibiotics and oil on microbial profiles and fermentation in mixed cultures of ruminal microorganisms. J. Dairy Sci. 2009, 92, 4467–4480. [Google Scholar] [CrossRef] [PubMed]
- Simjee, S.; Heffron, A.L.; Pridmore, A.; Shryock, T.R. Reversible monensin adaptation in Enterococcus faecium, Enterococcus faecalis and Clostridium perfringens of cattle origin: Potential impact on human food safety. J. Antimicrob. Chemother. 2012, 67, 2388–2395. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, H.; Zheng, H.; Dewhurst, R.; Roehe, R. A knowledge-driven network-based analytical framework for the identification of rumen metabolites. IEEE Trans. Nanobiosci. 2020, 19, 518–526. [Google Scholar] [CrossRef]
- Zhang, H. Microbial composition play the leading role in volatile fatty acid production in the fermentation of different scale of corn stover with rumen fluid. Front. Bioeng. Biotechnol. 2024, 11, 1275454. [Google Scholar] [CrossRef]
- Otani, M.; Ihara, N.; Umezawa, C.; Sano, K. Predominance of gluconate formation from glucose during germination of Bacillus megaterium qm b1551 spores. J. Bacteriol. 1986, 167, 148–152. [Google Scholar] [CrossRef]
- Focardi, S. Development and maturation of microbiota in cow rumen, plant-fibers degradation and influences on the immune system and cow health. Corpus J. Vet. Dairy Sci. 2022, 3, 1–2. [Google Scholar] [CrossRef]
- Latorre, J.; Hernández-Velasco, X.; Wolfenden, R.; Vicente, J.; Wolfenden, A.; Menconi, A.; Téllez, G. Evaluation and selection of bacillus species based on enzyme production, antimicrobial activity, and biofilm synthesis as direct-fed microbial candidates for poultry. Front. Vet. Sci. 2016, 3, 95. [Google Scholar] [CrossRef]
- Acharya, A.; Khanal, A.; Bajracharya, M.; Timalsina, A.; Bishwokarma, A.; Basnet, A. Production, purification and optimisation of amylase by submerged fermentation using Bacillus subtilis. Int. J. Sci. Res. Sci. Eng. Technol. 2019, 6, 265–275. [Google Scholar] [CrossRef]
- Adelina, A.; Feliatra, F.; Siregar, Y.; Putra, I.; Suharman, I. Use of chicken feather meal fermented with Bacillus subtilis in diets to increase the digestive enzymes activity and nutrient digestibility of silver pompano trachinotus blochii (lacepede, 1801). F1000Research 2021, 10, 25. [Google Scholar] [CrossRef]
- Mohammed, K.A.; Goji, A.D.T.; Tanko, Y.; Muhammed, A.; Salisu, I.A. Protective Effects of Magnesium Chloride on Liver Enzymes and Biomarkers of Oxidative Stress in high fat diet fed Rats. Niger. J. Physiol. Sci. 2019, 34, 149–157. [Google Scholar] [PubMed]
- Fuerniss, L.K.; Kreikemeier, K.K.; Reed, L.D.; Cravey, M.D.; Johnson, B.J. Cecal microbiota of feedlot cattle fed a four-species Bacillus supplement. J. Anim. Sci. 2022, 100, 10. [Google Scholar] [CrossRef] [PubMed]
- Lamontagne, J.; Rico, D.E.; Perdomo, C.M.; Ronholm, J.; Gervais, R.; Chouinard, P.Y. Effects of direct-fed Bacillus subtilis and Bacillus licheniformis on production performance and milk fatty acid profile in dairy cows. J. Dairy Sci. 2023, 106, 1815–1825. [Google Scholar] [CrossRef] [PubMed]
- Silva, K.G.S.; Sarturi, J.O.; Johnson, B.J.; Woerner, D.R.; Lopez, A.M.; Rodrigues, B.M.; Nardi, K.T.; Rush, C.J. Effects of bacterial direct-fed microbial mixtures offered to beef cattle consuming finishing diets on intake, nutrient digestibility, feeding behavior, and ruminal kinetics/fermentation profile. J. Anim. Sci. 2024, 3, 102. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hristov, A.N.; Lee, C.; Hristova, R.; Huhtanen, P.; Firkins, J.L. A meta-analysis of variability in continuous-culture ruminal fermentation and digestibility data. J. Dairy Sci. 2012, 95, 5299–5307. [Google Scholar] [CrossRef] [PubMed]
- Menke, K.H.; Steingass, H. Estimation of the energetic feed value obtained from chemical analysis and gas production using rumen fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]
- Getachew, G.; DePeters, E.; Robinson, P.; Fadel, J. Use of an in vitro rumen gas production technique to evaluate microbial fermentation of ruminant feeds and its impact on fermentation products. Anim. Feed Sci. Technol. 2005, 123, 547–559. [Google Scholar] [CrossRef]
- Pan, L.; Harper, K.; Queiroz, O.; Copani, G.; Cappellozza, B.I. Effects of a Bacillus-based direct-fed microbial on in vitro nutrient digestibility of forage and high-starch concentrate substrates. Transl. Anim. Sci. 2022, 6, 67. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cappellozza, B.I.; Joergensen, J.N.; Copani, G.; Bryan, K.A.; Fantinati, P.; Bodin, J.C.; Khahi, M.M.; NinoDeGuzman, C.; Arriola, K.G.; Lima, L.O.; et al. Evaluation of a Bacillus-based direct-fed microbial probiotic on in vitro rumen gas production and nutrient digestibility of different feedstuffs and total mixed rations. Transl. Anim. Sci. 2023, 7, 44. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bowen, J.M.; McCabe, M.S.; Lister, S.J.; Cormican, P.; Dewhurst, R.J. Evaluation of microbial communities associated with the liquid and solid phases of the rumen of cattle offered a diet of perennial ryegrass or white clover. Front. Microbiol. 2018, 9, 2389. [Google Scholar] [CrossRef]
- Zhao, W.; Abdelsattar, M.M.; Wang, X.; Zhang, N.; Chai, J. In Vitro Modulation of Rumen Fermentation by Microbiota from the Recombination of Rumen Fluid and Solid Phases. Microbiol. Spectr. 2023, 11, e03387-22. [Google Scholar] [CrossRef]
- Miller, A.C.; Mezzomo, R.; Gomes, D.I.; Loh, H.Y.; Levenson, J.R.; Guimaraes, O.; Tangredi, B.V.; Zuchegno, S.M.; Chek, E.; Cappellozza, B.I.; et al. Influence of BOVAMINE DEFEND Plus on growth performance, carcass characteristics, estimated dry matter digestibility, rumen fermentation characteristics, and immune function in finishing beef steers. Transl. Anim. Sci. 2024, 8, txae045. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pan, L.; Eyre, K.; Harper, K.; Prada e Silva, L. Using specific Bacillus strains to improve digestibility and performance of growing steers. In Proceedings of the ASAS Annual 2023 Meeting, Athens, Greece, 13–14 January 2023; Oxford University Press: Cary, NC, USA, 2023. [Google Scholar] [CrossRef]
- Qiao, G.H.; Shan, A.S.; Ma, N.; Ma, Q.Q.; Sun, Z.W. Effect of supplemental Bacillus cultures on rumen fermentation and milk yield in Chinese Holstein cows. J. Anim. Physiol. Nutr. 2010, 1, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Cummings, K.J.; Warnick, L.D.; Elton, M.; Gröhn, Y.T.; McDonough, P.L.; Siler, J.D. The effect of clinical outbreaks of salmonellosis on the prevalence of fecal Salmonella shedding among dairy cattle in New York. Foodborne Pathog. Dis. 2010, 7, 815–823. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Majowicz, S.E.; Musto, J.; Scallan, E.; Angulo, F.J.; Kirk, M.; O’Brien, S.J. The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis. 2010, 50, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, L.R.; Baggesen, D.L.; Aabo, S.; Moos, M.K.; Rattenborg, E. Prevalence and risk factors for Salmonella in veal calves at Danish cattle abattoirs. Epidemiol. Infect. 2011, 139, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Abriouel, H.; Franz, C.M.; Ben Omar, N.; Gálvez, A. Diversity and applications of Bacillus bacteriocins. FEMS Microbiol. Rev. 2011, 35, 201–232. [Google Scholar] [CrossRef] [PubMed]
- Basi-Chipalu, S.; Sthapit, P.; Dhital, S. A review on characterization, applications and structure-activity relationships of Bacillus species-produced bacteriocins. Drug Discov. Ther. 2022, 16, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Schoster, A.; Kokotovic, B.; Permin, A.; Pedersen, P.D.; Dal Bello, F.; Guardabassi, L. In vitro inhibition of Clostridium difficile and Clostridium perfringens by commercial probiotic strains. Anaerobe 2013, 20, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Cull, C.; Singu, V.K.; Cull, B.J.; Lechtenberg, K.F.; Amachawadi, R.G.; Schutz, J.S.; Bryan, K.A. Efficacy of two probiotic products fed daily to reduce Clostridium perfringens-based adverse health and performance effects in dairy calves. Antibiotics 2022, 11, 1513. [Google Scholar] [CrossRef]



| Ingredients | % of DM |
|---|---|
| Beet pulp | 30.15 |
| Wheat | 20 |
| Barley | 20 |
| Rapeseed | 15 |
| Soy | 7.5 |
| Corn | 3 |
| Molasses | 1.5 |
| Calcium phosphate | 1 |
| Vitamins and minerals | 0.75 |
| Salt | 0.6 |
| Magnesia | 0.5 |
| pH at 8-h Fermentation | pH at 24-h Fermentation | |
|---|---|---|
| Bovacillus | 6.51 ± 0.12 | 6.16 ± 0.12 |
| B. Licheniformis | 6.49 ± 0.11 | 6.16 ± 0.13 |
| B. Subtilis | 6.51 ± 0.09 | 6.17 ± 0.13 |
| Control | 6.50 ± 0.11 | 6.17 ± 0.12 |
| NH3 (mmol/L) at 8-h Fermentation | NH3 (mmol/L) at 24-h Fermentation | |
|---|---|---|
| Bovacillus | 1.51 ± 0.87 | 13.35 ± 1.49 |
| B. Licheniformis | 1.69 ± 0.95 | 12.70 ± 1.10 |
| B. Subtilis | 1.65 ± 1.10 | 12.83 ± 1.53 |
| Control | 1.77 ± 0.81 | 13.16 ± 1.15 |
| Principal phyla at 8 h of Fermentation | Bovacillus | B. licheniformis | B. subtilis | Control |
|---|---|---|---|---|
| p__Firmicutes | 44.77 | 44.78 | 45.63 | 45.43 |
| p__Bacteroidota | 35.50 | 36.72 | 36.54 | 36.34 |
| p__Proteobacteria | 3.89 | 2.70 | 2.13 | 3.20 |
| p__Verrucomicrobiota | 3.74 | 3.82 | 3.73 | 3.61 |
| p__Actinobacteriota | 2.65 | 2.68 | 2.91 | 2.93 |
| p__Methanobacteriota | 1.48 | 1.90 | 1.70 | 1.40 |
| p__Fibrobacterota | 1.36 | 0.90 | 0.89 | 1.06 |
| Principal phyla at 24 h of Fermentation | Bovacillus | B. licheniformis | B. subtilis | Control |
| p__Firmicutes | 44.70 | 43.65 | 44.11 | 44.32 |
| p__Bacteroidota | 30.55 | 31.40 | 31.28 | 31.42 |
| p__Verrucomicrobiota | 8.39 | 8.67 | 8.44 | 8.60 |
| p__Actinobacteriota | 3.54 | 3.18 | 3.09 | 3.44 |
| p__Methanobacteriota | 2.22 | 2.39 | 2.06 | 1.97 |
| p__Proteobacteria | 2.22 | 2.35 | 2.46 | 2.05 |
| p__Fibrobacterota | 1.44 | 1.54 | 2.12 | 1.54 |
| Principal genera at 8 h of Fermentation | Bovacillus | B. licheniformis | B. subtilis | Control |
| g__Prevotella | 14.78 | 15.47 | 15.55 | 15.83 |
| g__Rikenellaceae_RC9_gut_group | 10.13 | 10.58 | 9.99 | 9.98 |
| g__Christensenellaceae_R-7_group | 8.18 | 8.54 | 8.85 | 8.71 |
| g__NK4A214_group | 4.14 | 4.60 | 4.57 | 4.41 |
| g__Clostridia_genus | 3.87 | 4.42 | 4.48 | 4.60 |
| g__F082_genus | 2.94 | 2.96 | 3.06 | 2.94 |
| g__WCHB1-41_genus | 2.86 | 2.94 | 2.81 | 2.81 |
| g__Ruminococcus | 3.00 | 2.74 | 2.87 | 2.78 |
| g__Olsenella | 2.23 | 2.23 | 2.40 | 2.47 |
| Principal genera at 24 h of Fermentation | Bovacillus | B. licheniformis | B. subtilis | Control |
| g__Prevotella | 9.78 | 10.10 | 10.02 | 10.59 |
| g__Rikenellaceae_RC9_gut_group | 9.65 | 9.76 | 9.57 | 9.38 |
| g__Christensenellaceae_R-7_group | 9.49 | 8.88 | 9.16 | 9.15 |
| g__WCHB1-41_genus | 6.55 | 6.85 | 6.83 | 6.64 |
| g__NK4A214_group | 5.20 | 5.12 | 5.11 | 5.05 |
| g__Clostridia_genus | 3.76 | 4.20 | 4.13 | 4.31 |
| g__F082_genus | 3.19 | 3.36 | 3.34 | 3.30 |
| g__Olsenella | 2.94 | 2.67 | 2.54 | 2.90 |
| g__Lachnospiraceae_NK3A20_group | 2.02 | 1.86 | 1.92 | 1.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gresse, R.; Cappellozza, B.I.; Macheboeuf, D.; Torrent, A.; Danon, J.; Capern, L.; Sandvang, D.; Niderkorn, V.; Copani, G.; Forano, E. In Vitro Investigation of the Effects of Bacillus subtilis-810B and Bacillus licheniformis-809A on the Rumen Fermentation and Microbiota. Animals 2025, 15, 476. https://doi.org/10.3390/ani15040476
Gresse R, Cappellozza BI, Macheboeuf D, Torrent A, Danon J, Capern L, Sandvang D, Niderkorn V, Copani G, Forano E. In Vitro Investigation of the Effects of Bacillus subtilis-810B and Bacillus licheniformis-809A on the Rumen Fermentation and Microbiota. Animals. 2025; 15(4):476. https://doi.org/10.3390/ani15040476
Chicago/Turabian StyleGresse, Raphaële, Bruno Ieda Cappellozza, Didier Macheboeuf, Angélique Torrent, Jeanne Danon, Lena Capern, Dorthe Sandvang, Vincent Niderkorn, Giuseppe Copani, and Evelyne Forano. 2025. "In Vitro Investigation of the Effects of Bacillus subtilis-810B and Bacillus licheniformis-809A on the Rumen Fermentation and Microbiota" Animals 15, no. 4: 476. https://doi.org/10.3390/ani15040476
APA StyleGresse, R., Cappellozza, B. I., Macheboeuf, D., Torrent, A., Danon, J., Capern, L., Sandvang, D., Niderkorn, V., Copani, G., & Forano, E. (2025). In Vitro Investigation of the Effects of Bacillus subtilis-810B and Bacillus licheniformis-809A on the Rumen Fermentation and Microbiota. Animals, 15(4), 476. https://doi.org/10.3390/ani15040476





