Effects of Dietary High-Yield Protease Bacillus subtilis Strain FRE76 on Broiler Growth, Slaughter Performance, Intestinal Morphology, and Gut Microbiota
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics
2.2. Bacillus subtilis FRE76
2.3. Experimental Design, Diets, and Management
2.4. Sample Collection
2.5. Apparent Digestibility Measurements
2.6. Serum Biochemical Parameters Measurements
2.7. Intestinal Morphology Determination
2.8. Protease Activity Measurements
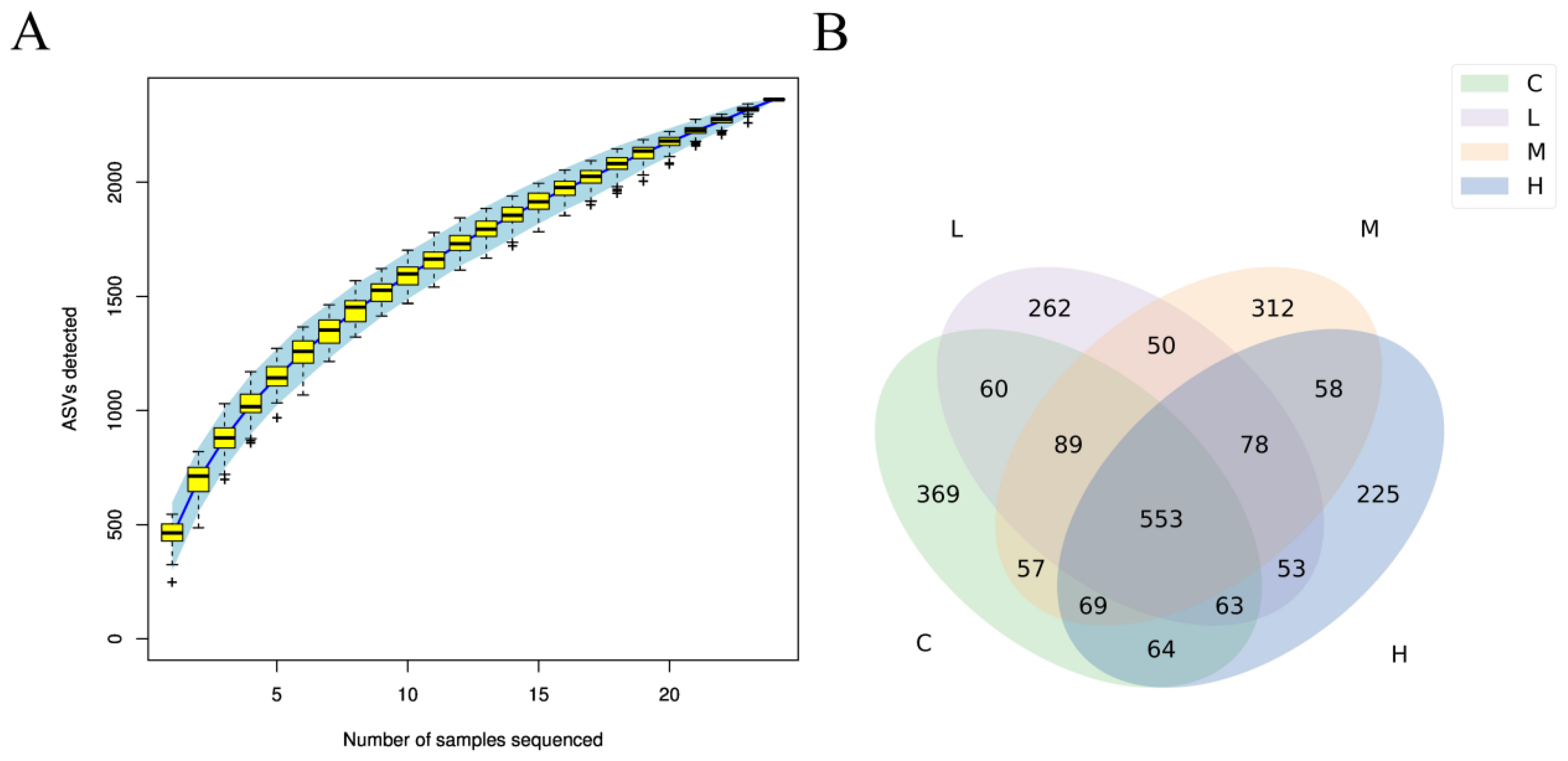
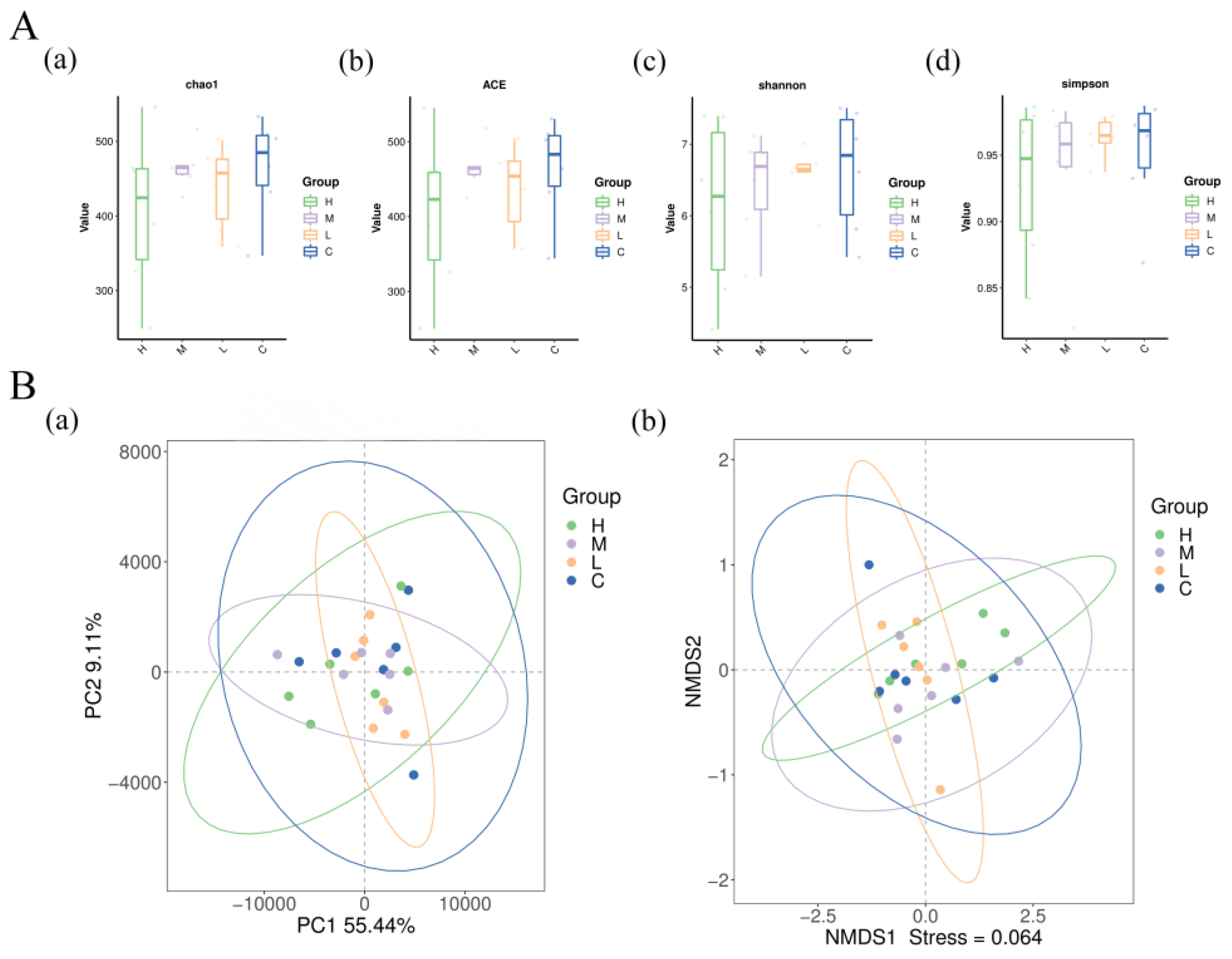
2.9. Cecal Microbiota Analysis
2.10. Statistical Considerations
3. Results
3.1. Growth Parameters
3.2. Slaughter Performance
3.3. Apparent Digestibility
3.4. Serum Biochemical Parameters
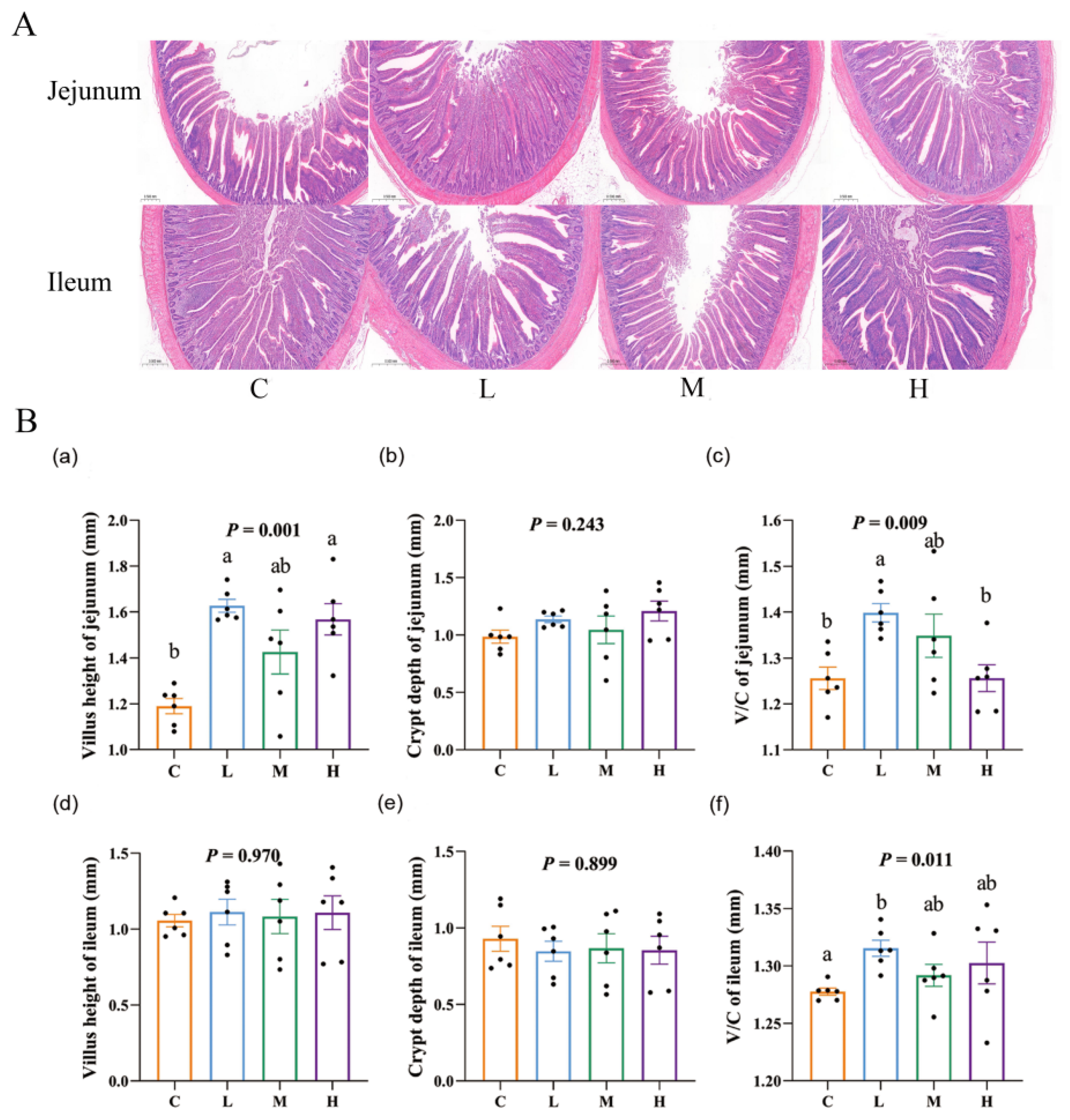
3.5. Intestinal Morphology
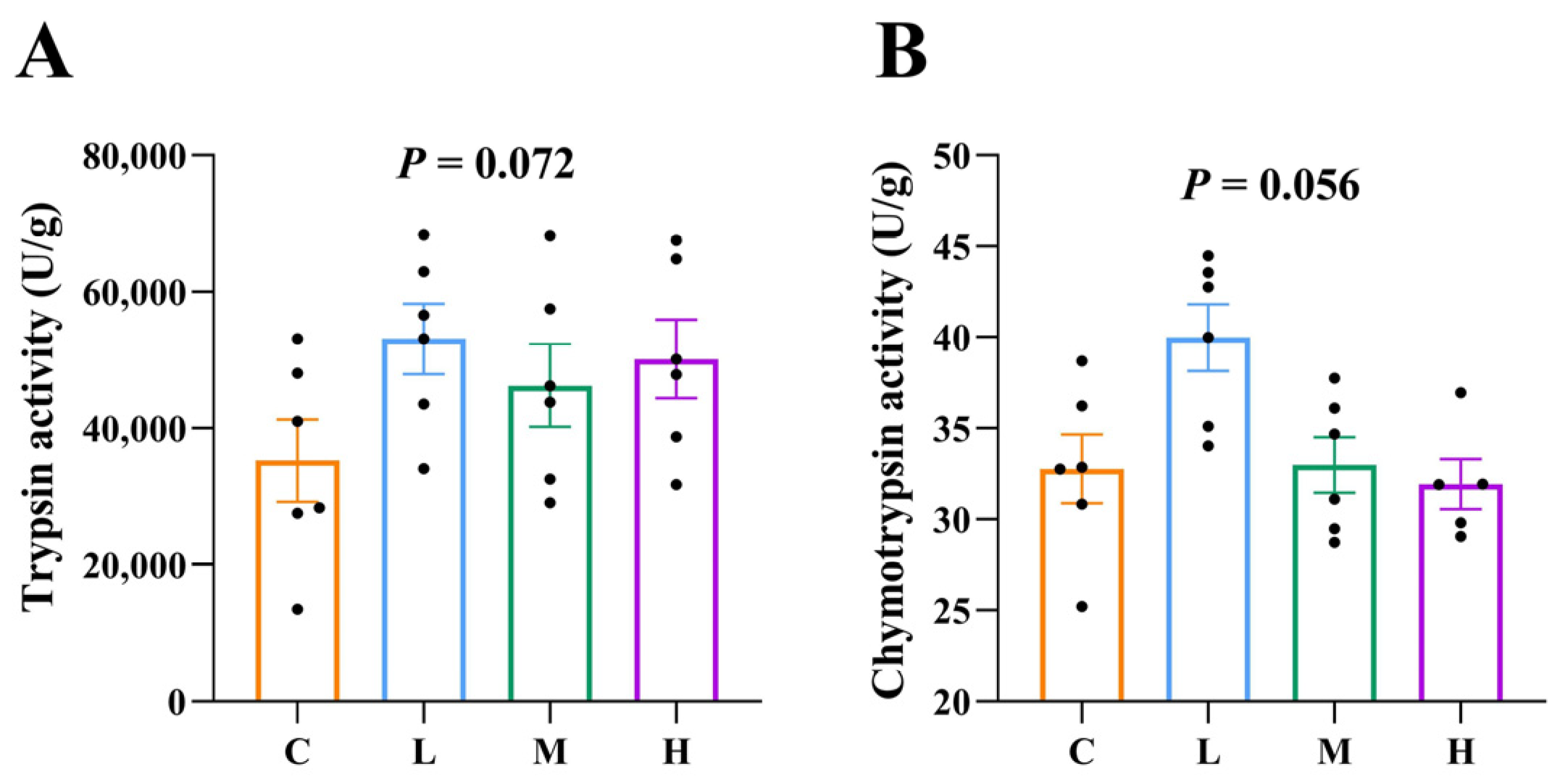
3.6. Protease Activity
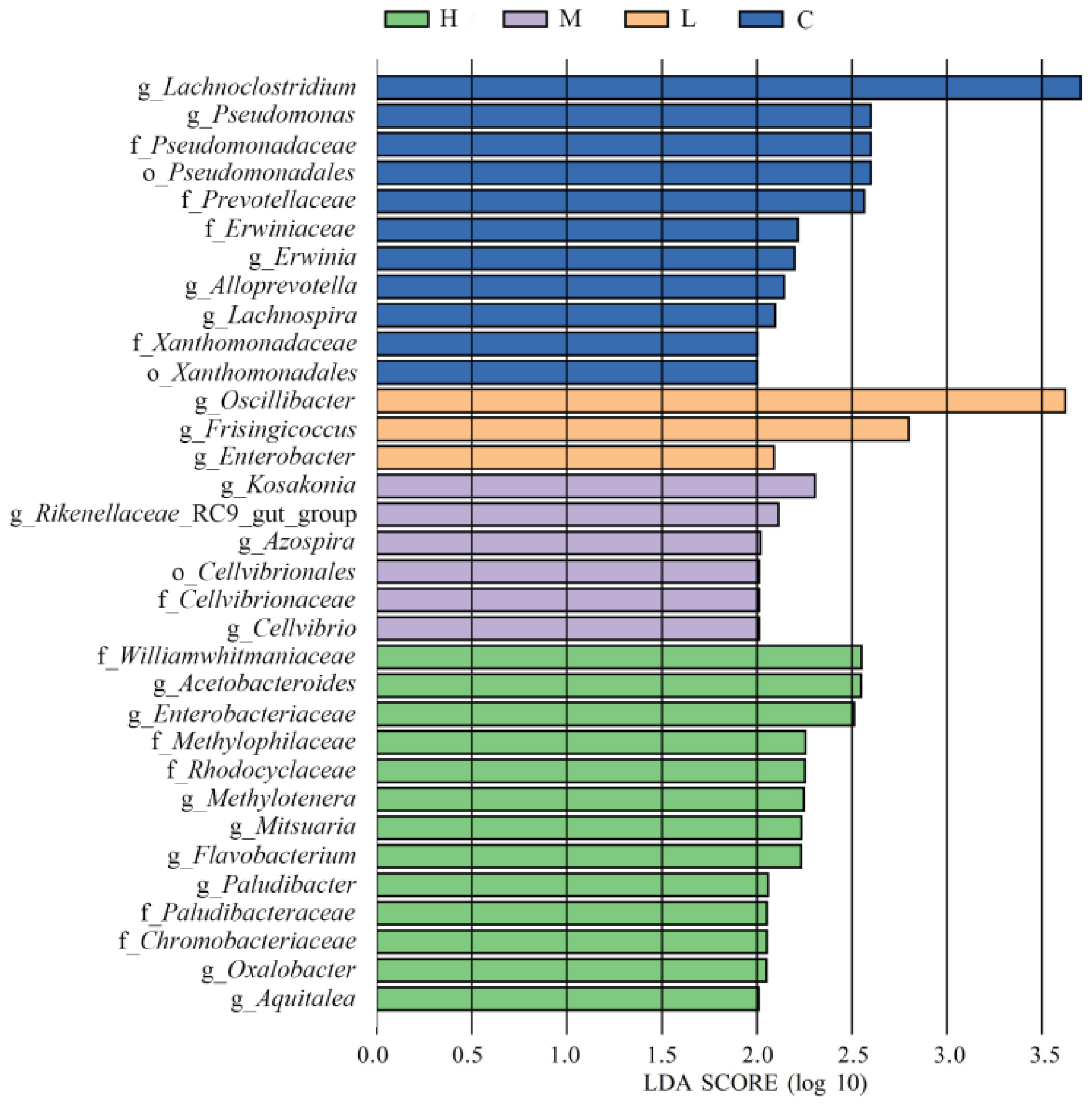
3.7. Cecal Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yi, Z.; Wang, J.; Jiang, T.; Tang, Q.; Cheng, Y. Photocatalytic degradation of sulfamethazine in aqueous solution using ZnO with different morphologies. R. Soc. Open Sci. 2018, 5, 171457. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Xiao, Q.; Zhang, Y.; Wang, X.; Xiao, Y.; Shi, D. Pig liver esterases PLE1 and PLE6: Heterologous expression, hydrolysis of common antibiotics and pharmacological consequences. Sci. Rep. 2019, 9, 15564. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Jha, R. Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J. Anim. Sci. Biotechnol. 2019, 10, 2. [Google Scholar] [CrossRef]
- Jadhav, K.; Sharma, K.S.; Katoch, S.; Sharma, V.K.; Mane, B.G. Probiotics in Broiler Poultry Feeds: A Review. J. Anim. Nutr. Physiol. 2015, 2, 4–16. [Google Scholar]
- Jiang, S.; Yan, F.F.; Hu, J.Y.; Mohammed, A.; Cheng, H.W. Bacillus subtilis-Based Probiotic Improves Skeletal Health and Immunity in Broiler Chickens Exposed to Heat Stress. Animals 2021, 11, 1494. [Google Scholar] [CrossRef]
- Wang, Y.; Heng, C.; Zhou, X.; Cao, G.; Jiang, L.; Wang, J.; Li, K.; Wang, D.; Zhan, X. Supplemental Bacillus subtilis DSM 29784 and enzymes, alone or in combination, as alternatives for antibiotics to improve growth performance, digestive enzyme activity, anti-oxidative status, immune response and the intestinal barrier of broiler chickens. Br. J. Nutr. 2021, 125, 494–507. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Zhang, N.; Han, J.C.; Chang, C.W.; Hsiao, F.S.; Yu, Y.H. Optimization of surfactin production from Bacillus subtilis in fermentation and its effects on Clostridium perfringens-induced necrotic enteritis and growth performance in broilers. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1232–1244. [Google Scholar] [CrossRef]
- Grant, A.; Gay, C.G.; Lillehoj, H.S. Bacillus spp. as direct-fed microbial antibiotic alternatives to enhance growth, immunity, and gut health in poultry. Avian Pathol. 2018, 47, 339–351. [Google Scholar] [CrossRef]
- Dowarah, R.; Verma, A.K.; Agarwal, N.; Singh, P.; Singh, B.R. Selection and characterization of probiotic lactic acid bacteria and its impact on growth, nutrient digestibility, health and antioxidant status in weaned piglets. PLoS ONE 2018, 13, e0192978. [Google Scholar] [CrossRef]
- Gadde, U.; Oh, S.T.; Lee, Y.S.; Davis, E.; Zimmerman, N.; Rehberger, T.; Lillehoj, H.S. The Effects of Direct-fed Microbial Supplementation, as an Alternative to Antibiotics, on Growth Performance, Intestinal Immune Status, and Epithelial Barrier Gene Expression in Broiler Chickens. Probiotics Antimicrob. Proteins 2017, 9, 397–405. [Google Scholar] [CrossRef]
- Rhayat, L.; Jacquier, V.; Brinch, K.S.; Nielsen, P.; Nelson, A.; Geraert, P.A.; Devillard, E. Bacillus subtilis strain specificity affects performance improvement in broilers. Poult. Sci. 2017, 96, 2274–2280. [Google Scholar] [CrossRef] [PubMed]
- Ndazigaruye, G.; Kim, D.H.; Kang, C.W.; Kang, K.R.; Joo, Y.J.; Lee, S.R.; Lee, K.W. Effects of Low-Protein Diets and Exogenous Protease on Growth Performance, Carcass Traits, Intestinal Morphology, Cecal Volatile Fatty Acids and Serum Parameters in Broilers. Animals 2019, 9, 226. [Google Scholar] [CrossRef] [PubMed]
- Jabbar, A.; Tahir, M.; Alhidary, I.A.; Abdelrahman, M.A.; Albadani, H.; Khan, R.U.; Selvaggi, M.; Laudadio, V.; Tufarelli, V. Impact of Microbial Protease Enzyme and Dietary Crude Protein Levels on Growth and Nutrients Digestibility in Broilers over 15-28 Days. Animals 2021, 11, 2499. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Hu, X.; Niu, H.; Wang, Q.; Pei, C.; Li, Y.; Xia, C. Isolation, Identification and Biological Characteristics of Protease-Producing Bacillus subtilis from Bian Chicken. Mod. Anim. Husb. Sci. Technol. 2024, 2, 33–38. (In Chinese) [Google Scholar] [CrossRef]
- Acres, A. Arbor Acres Broiler Nutrition Specifications; Aviagen: Huntsville, AL, USA, 2022. [Google Scholar]
- Hart, G.K.; Dobb, G.J. Effect of a fecal bulking agent on diarrhea during enteral feeding in the critically ill. JPEN J. Parenter. Enter. Nutr. 1988, 12, 465–468. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Qiu, H.; Gao, S.; Hou, L.; Li, A.; Zhu, L.Q.; Dong, J.; Chen, F. Selenium-enriched Bacillus subtilis Improves Growth Performance, Antioxidant Capacity, Immune Status, and Gut Health of Broiler Chickens. Biol. Trace Elem. Res. 2023, 201, 5756–5763. [Google Scholar] [CrossRef]
- Zhang, S.; Zhong, G.; Shao, D.; Wang, Q.; Hu, Y.; Wu, T.; Ji, C.; Shi, S. Dietary supplementation with Bacillus subtilis promotes growth performance of broilers by altering the dominant microbial community. Poult. Sci. 2021, 100, 100935. [Google Scholar] [CrossRef]
- Bai, K.; Huang, Q.; Zhang, J.; He, J.; Zhang, L.; Wang, T. Supplemental effects of probiotic Bacillus subtilis fmbJ on growth performance, antioxidant capacity, and meat quality of broiler chickens. Poult. Sci. 2017, 96, 74–82. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, I.H. Supplemental effect of probiotic Bacillus subtilis B2A on productivity, organ weight, intestinal Salmonella microflora, and breast meat quality of growing broiler chicks. Poult. Sci. 2014, 93, 2054–2059. [Google Scholar] [CrossRef]
- Ciurescu, G.; Dumitru, M.; Gheorghe, A.; Untea, A.E.; Drăghici, R. Effect of Bacillus subtilis on growth performance, bone mineralization, and bacterial population of broilers fed with different protein sources. Poult. Sci. 2020, 99, 5960–5971. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.R.; Briggs, W.; Duff, A.; Chasser, K.; Murugesan, R.; Pender, C.; Ramirez, S.; Valenzuela, L.; Bielke, L. Cecal microbiome composition and metabolic function in probiotic treated broilers. PLoS ONE 2020, 15, e0225921. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Wu, H.; Shi, L.; Zhang, X.; Sheng, R.; Yin, F.; Gooneratne, R. Study of Bacillus subtilis on growth performance, nutrition metabolism and intestinal microflora of 1 to 42 d broiler chickens. Anim. Nutr. 2017, 3, 109–113. [Google Scholar] [CrossRef]
- Wei-Fen, L.I.; Jie, B.; Ya-Li, L.I.; Yan, Q.; Dong-You, Y.U. Effects of Bacillus subtilis on meat quality, nutrient digestibility and serum biochemical parameters of broilers. Chin. J. Vet. Sci. 2014, 34, 1682–1685. [Google Scholar] [CrossRef]
- Chen, W.; Wang, J.P.; Yan, L.; Huang, Y.Q. Evaluation of probiotics in diets with different nutrient densities on growth performance, blood characteristics, relative organ weight and breast meat characteristics in broilers. Br. Poult. 2013, 54, 635–641. [Google Scholar] [CrossRef]
- Wickramasuriya, S.S.; Park, I.; Lee, Y.; Richer, L.M.; Przybyszewski, C.; Gay, C.G.; van Oosterwijk, J.G.; Lillehoj, H.S. Orally delivered Bacillus subtilis expressing chicken NK-2 peptide stabilizes gut microbiota and enhances intestinal health and local immunity in coccidiosis-infected broiler chickens. Poult. Sci. 2023, 102, 102590. [Google Scholar] [CrossRef]
- Gyawali, I.; Zeng, Y.; Zhou, J.; Li, J.; Wu, T.; Shu, G.; Jiang, Q.; Zhu, C. Effect of novel Lactobacillus paracaesi microcapsule on growth performance, gut health and microbiome community of broiler chickens. Poult. Sci. 2022, 101, 101912. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, Y.; Lu, H.; Jiao, N.; Jiang, S.; Li, Y.; Li, J.; Yang, W. Effects of Dietary Bacillus subtilis BC02 Supplementation on Growth Performance, Antioxidant Capacity, and Cecal Microbes in Broilers. Agriculture 2023, 13, 1561. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.E.; Selim, D.A.; Basuony, H.A.; Sabic, E.M.; Saleh, A.A.; Ebeid, T.A. Effect of dietary supplementation of Bacillus subtilis spores on growth performance, oxidative status, and digestive enzyme activities in Japanese quail birds. Trop. Anim. Health Prod. 2020, 52, 671–680. [Google Scholar] [CrossRef]
- Hajjaj, H.; Duboc, P.; Fay, L.B.; Zbinden, I.; Macé, K.; Niederberger, P. Aspergillus oryzae produces compounds inhibiting cholesterol biosynthesis downstream of dihydrolanosterol. FEMS Microbiol. Lett. 2005, 242, 155–159. [Google Scholar] [CrossRef]
- Ooi, L.G.; Liong, M.T. Cholesterol-lowering effects of probiotics and prebiotics: A review of in vivo and in vitro findings. Int. J. Mol. Sci. 2010, 11, 2499–2522. [Google Scholar] [CrossRef] [PubMed]
- Forte, C.; Manuali, E.; Abbate, Y.; Papa, P.; Vieceli, L.; Tentellini, M.; Trabalza-Marinucci, M.; Moscati, L. Dietary Lactobacillus acidophilus positively influences growth performance, gut morphology, and gut microbiology in rurally reared chickens. Poult. Sci. 2018, 97, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Mallo, J.J.; Sol, C.; Puyalto, M.; Bortoluzzi, C.; Applegate, T.J.; Villamide, M.J. Evaluation of sodium butyrate and nutrient concentration for broiler chickens. Poult. Sci. 2021, 100, 101456. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xiao, G.; Wang, Q.; Zhang, Q.; Tian, J.; Li, W.; Gong, L. Effects of Dietary Bacillus subtilis HC6 on Growth Performance, Antioxidant Capacity, Immunity, and Intestinal Health in Broilers. Animals 2023, 13, 2915. [Google Scholar] [CrossRef]
- Leite, B.G.S.; Granghelli, C.A.; Roque, F.A.; Carvalho, R.S.B.; Lopes, M.H.S.; Pelissari, P.H.; Dias, M.T.; Araújo, C.; Araújo, L.F. Evaluation of dietary lignin on broiler performance, nutrient digestibility, cholesterol and triglycerides concentrations, gut morphometry, and lipid oxidation. Poult. Sci. 2024, 103, 103518. [Google Scholar] [CrossRef]
- Gong, L.; Wang, B.; Mei, X.; Xu, H.; Qin, Y.; Li, W.; Zhou, Y. Effects of three probiotic Bacillus on growth performance, digestive enzyme activities, antioxidative capacity, serum immunity, and biochemical parameters in broilers. Anim. Sci. J. 2018, 89, 1561–1571. [Google Scholar] [CrossRef]
- Shang, Y.; Kumar, S.; Oakley, B.; Kim, W.K. Chicken Gut Microbiota: Importance and Detection Technology. Front. Vet. Sci. 2018, 5, 254. [Google Scholar] [CrossRef]
- Rychlik, I. Composition and Function of Chicken Gut Microbiota. Animals 2020, 10, 103. [Google Scholar] [CrossRef]
- Hong, H.A.; Duc, L.H.; Cutting, S.M. The use of bacterial spore formers as probiotics. FEMS Microbiol. Rev. 2005, 29, 813–835. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, Y.; Shen, Y.; Li, Q.; Lan, J.; Wu, Y.; Zhang, R.; Cao, G.; Yang, C. Effects of Bacillus subtilis and Bacillus licheniformis on growth performance, immunity, short chain fatty acid production, antioxidant capacity, and cecal microflora in broilers. Poult. Sci. 2021, 100, 101358. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, W.; Zhang, H.; Wang, J.; Zhang, W.; Gao, J.; Wu, S.; Qi, G. Supplemental Bacillus subtilis DSM 32315 manipulates intestinal structure and microbial composition in broiler chickens. Sci. Rep. 2018, 8, 15358. [Google Scholar] [CrossRef] [PubMed]
- Li, C.L.; Wang, J.; Zhang, H.J.; Wu, S.G.; Hui, Q.R.; Yang, C.B.; Fang, R.J.; Qi, G.H. Intestinal Morphologic and Microbiota Responses to Dietary Bacillus spp. in a Broiler Chicken Model. Front. Physiol. 2018, 9, 1968. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, R.; Kim, H.B. The intestinal microbiome of the pig. Anim. Health Res. Rev. 2012, 13, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Oakley, B.B.; Lillehoj, H.S.; Kogut, M.H.; Kim, W.K.; Maurer, J.J.; Pedroso, A.; Lee, M.D.; Collett, S.R.; Johnson, T.J.; Cox, N.A. The chicken gastrointestinal microbiome. FEMS Microbiol. Lett. 2014, 360, 100–112. [Google Scholar] [CrossRef]
- Singh, K.M.; Shah, T.; Deshpande, S.; Jakhesara, S.J.; Koringa, P.G.; Rank, D.N.; Joshi, C.G. High through put 16S rRNA gene-based pyrosequencing analysis of the fecal microbiota of high FCR and low FCR broiler growers. Mol. Biol. Rep. 2012, 39, 10595–10602. [Google Scholar] [CrossRef]
- Erttmann, S.F.; Gekara, N.O. Hydrogen peroxide release by bacteria suppresses inflammasome-dependent innate immunity. Nat. Commun. 2019, 10, 3493. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, J.; Zhong, H.; Li, N.; Xu, H.; Zhu, Q.; Liu, Y. Effect of probiotics on the meat flavour and gut microbiota of chicken. Sci. Rep. 2017, 7, 6400. [Google Scholar] [CrossRef]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef]
- Macias-Ceja, D.C.; Ortiz-Masiá, D.; Salvador, P.; Gisbert-Ferrándiz, L.; Hernández, C.; Hausmann, M.; Rogler, G.; Esplugues, J.V.; Hinojosa, J.; Alós, R.; et al. Succinate receptor mediates intestinal inflammation and fibrosis. Mucosal Immunol. 2019, 12, 178–187. [Google Scholar] [CrossRef]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The Genus Alistipes: Gut Bacteria With Emerging Implications to Inflammation, Cancer, and Mental Health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, W.; Jiang, Y.; Xie, F.; Mao, S. Response of Milk Performance, Rumen and Hindgut Microbiome to Dietary Supplementation with Aspergillus oryzae Fermentation Extracts in Dairy Cows. Curr. Microbiol. 2022, 79, 113. [Google Scholar] [CrossRef] [PubMed]
- McCormack, U.M.; Curião, T.; Buzoianu, S.G.; Prieto, M.L.; Ryan, T.; Varley, P.; Crispie, F.; Magowan, E.; Metzler-Zebeli, B.U.; Berry, D.; et al. Exploring a Possible Link between the Intestinal Microbiota and Feed Efficiency in Pigs. Appl. Environ. Microbiol. 2017, 83, e00380-17. [Google Scholar] [CrossRef]
- Wang, K.; Liao, M.; Zhou, N.; Bao, L.; Ma, K.; Zheng, Z.; Wang, Y.; Liu, C.; Wang, W.; Wang, J.; et al. Parabacteroides distasonis Alleviates Obesity and Metabolic Dysfunctions via Production of Succinate and Secondary Bile Acids. Cell Rep. 2019, 26, 222–235.e225. [Google Scholar] [CrossRef] [PubMed]
- Zmora, N.; Zilberman-Schapira, G.; Suez, J.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Kotler, E.; Zur, M.; Regev-Lehavi, D.; Brik, R.B.; et al. Personalized Gut Mucosal Colonization Resistance to Empiric Probiotics Is Associated with Unique Host and Microbiome Features. Cell 2018, 174, 1388–1405.e21. [Google Scholar] [CrossRef] [PubMed]

| Element (%) | 1–21 Days | 22–42 Days | Nutrient Levels 2 (%) | 1–21 Days | 22–42 Days |
|---|---|---|---|---|---|
| Corn | 51.85 | 53.57 | ME, (MJ/kg) | 12.27 | 13.91 |
| Soybean meal | 30.00 | 23.89 | Crude protein | 26.26 | 22.95 |
| Flour | 8.00 | 8.00 | Ether extract | 4.20 | 9.27 |
| Powdered soy | 1.50 | 1.50 | Crude fiber | 1.22 | 2.07 |
| Corn gluten meal | 3.05 | 3.42 | Crude ash | 5.71 | 5.03 |
| Duck fat | 1.00 | 6.00 | Calcium | 1.01 | 0.83 |
| Calcium hydrogen phosphate | 1.46 | 0.78 | Phosphorus | 0.61 | 0.57 |
| Fine bluestone powder | 1.21 | 1.11 | Lysine | 1.00 | 0.80 |
| L-Lysine sulphate | 0.65 | 0.65 | Methionine | 0.65 | 0.55 |
| Choline chloride | 0.08 | 0.08 | H2O | 2.17 | 2.17 |
| Premix 1 | 1.20 | 1.00 | |||
| Total | 100.00 | 100.00 |
| Item 1 | Treatment 2 | p-Value | |||
|---|---|---|---|---|---|
| C | L | M | H | ||
| BW, g | |||||
| 1 d | 48.57 ± 0.78 | 47.67 ± 0.67 | 48.15 ± 0.71 | 48.36 ± 0.58 | 0.127 |
| 21 d | 854.80 ± 7.63 b | 881.07 ± 9.14 a | 869.40 ± 16.07 ab | 880.50 ± 6.86 a | 0.005 |
| 42 d | 2321.86 ± 195.79 b | 2613.33 ± 128.47 a | 2522.37 ± 130.18 ab | 2527.99 ± 110.39 ab | 0.038 |
| ADFI, g | |||||
| 1–21 d | 49.01 ± 0.58 | 49.11 ± 0.48 | 49.53 ± 0.89 | 49.30 ± 0.85 | 0.705 |
| 22–42 d | 116.29 ± 11.52 | 129.19 ± 10.72 | 121.17 ± 10.84 | 118.81 ± 8.46 | 0.196 |
| 1–42 d | 82.67 ± 5.98 | 89.25 ± 5.45 | 85.35 ± 5.57 | 84.05 ± 3.94 | 0.195 |
| ADG, g | |||||
| 1–21 d | 38.59 ± 0.34 b | 39.65 ± 0.36 a | 39.10 ± 0.76 ab | 39.64 ± 0.32 a | 0.012 |
| 22–42 d | 74.50 ± 2.76 b | 82.55 ± 6.57 a | 78.49 ± 6.28 ab | 81.15 ± 7.17 ab | 0.040 |
| 1–42 d | 54.23 ± 4.74 b | 61.06 ± 3.08 a | 58.91 ± 3.10 ab | 59.05 ± 2.56 ab | 0.042 |
| FCR | |||||
| 1–21 d | 1.27 ± 0.03 | 1.23 ± 0.01 | 1.26 ± 0.03 | 1.29 ± 0.07 | 0.305 |
| 22–42 d | 1.65 ± 0.12 | 1.64 ± 0.07 | 1.65 ± 0.17 | 1.65 ± 0.12 | 0.997 |
| 1–42 d | 1.52 ± 0.07 | 1.48 ± 0.05 | 1.48 ± 0.06 | 1.50 ± 0.06 | 0.639 |
| Mortality rate, % | |||||
| 1–42 d | 8.33 | 5.00 | 6.67 | 5.00 | 0.710 |
| Item 1 | Treatment 2 | p-Value | |||
|---|---|---|---|---|---|
| C | L | M | H | ||
| CW/kg | 2.25 ± 0.12 | 2.35 ± 0.16 | 2.26 ± 0.16 | 2.22 ± 0.14 | 0.147 |
| DP/% | 89.65 ± 2.24 | 90.20 ± 1.87 | 89.68 ± 4.20 | 89.49 ± 2.36 | 0.983 |
| FBW/kg | 1.79 ± 0.10 | 1.91 ± 0.15 | 1.81 ± 0.12 | 1.81 ± 0.07 | 0.082 |
| HBW/kg | 2.12 ± 0.13 b | 2.29 ± 0.14 a | 2.16 ± 0.13 ab | 2.14 ± 0.09 ab | 0.021 |
| FBP/% | 71.04 ± 1.35 | 72.80 ± 1.63 | 71.24 ± 3.38 | 71.67 ± 1.91 | 0.430 |
| HBP/% | 82.43 ± 2.61 b | 85.06 ± 1.32 a | 84.64 ± 1.67 ab | 84.44 ± 1.95 ab | 0.028 |
| BMP/% | 27.68 ± 0.35 b | 30.33 ± 1.22 a | 28.73 ± 1.22 ab | 28.22 ± 2.17 ab | 0.024 |
| LMP/% | 18.72 ± 0.58 | 18.02 ± 0.55 | 18.49 ± 0.72 | 18.06 ± 0.37 | 0.075 |
| AFP/% | 2.10 ± 0.60 | 2.13 ± 0.29 | 2.19 ± 0.56 | 2.35 ± 0.28 | 0.716 |
| Item 1 | Treatment 2 | p-Value | |||
|---|---|---|---|---|---|
| C | L | M | H | ||
| 21 d | |||||
| DM (%) | 70.45 ± 3.93 | 70.58 ± 2.50 | 70.35 ± 4.61 | 70.56 ± 4.20 | 0.996 |
| OM (%) | 74.29 ± 1.76 | 74.59 ± 2.27 | 74.27 ± 2.30 | 73.40 ± 3.50 | 0.760 |
| CP (%) | 65.47 ± 4.77 | 64.88 ± 2.61 | 66.50 ± 1.55 | 65.29 ± 3.66 | 0.923 |
| EE (%) | 81.34 ± 0.95 b | 83.64 ± 1.90 a | 84.47 ± 1.01 a | 83.73 ± 1.67 a | 0.005 |
| CF (%) | 19.45 ± 6.55 | 20.06 ± 6.49 | 20.66 ± 5.88 | 23.74 ± 5.63 | 0.556 |
| 42 d | |||||
| DM (%) | 79.26 ± 1.02 | 80.34 ± 2.25 | 78.52 ± 2.05 | 79.05 ± 2.49 | 0.481 |
| OM (%) | 81.78 ± 1.89 | 82.35 ± 2.92 | 81.70 ± 1.97 | 82.77 ± 1.33 | 0.893 |
| CP (%) | 69.20 ± 2.00 b | 73.32 ± 2.65 a | 71.08 ± 2.77 ab | 71.71 ± 1.44 ab | 0.036 |
| EE (%) | 87.10 ± 2.67 b | 93.01 ± 2.14 a | 91.31 ± 0.65 a | 91.47 ± 1.42 a | 0.001 |
| CF (%) | 26.72 ± 6.85 | 26.76 ± 8.14 | 25.52 ± 6.44 | 27.99 ± 10.63 | 0.948 |
| Item 1 | Treatment 2 | p-Value | |||
|---|---|---|---|---|---|
| C | L | M | H | ||
| 21 d | |||||
| TP (g/L) | 32.32 ± 3.99 | 32.02 ± 1.57 | 34.73 ± 3.04 | 34.35 ± 3.20 | 0.515 |
| ALB (g/L) | 13.78 ± 0.98 | 12.32 ± 0.76 | 12.35 ± 1.59 | 14.04 ± 1.72 | 0.089 |
| TG (mmol/L) | 0.37 ± 0.04 | 0.35 ± 0.05 | 0.42 ± 0.14 | 0.38 ± 0.16 | 0.838 |
| TC (mmol/L) | 3.31 ± 0.29 | 3.42 ± 0.53 | 3.52 ± 0.24 | 3.08 ± 0.23 | 0.277 |
| LDL-C (mmol/L) | 0.73 ± 0.11 | 0.73 ± 0.13 | 0.67 ± 0.17 | 0.60 ± 0.12 | 0.364 |
| HDL-C (mmol/L) | 2.40 ± 0.25 | 2.50 ± 0.34 | 2.61 ± 0.20 | 2.24 ± 0.12 | 0.088 |
| ALT (U/L) | 8.20 ± 0.72 | 8.50 ± 1.29 | 6.94 ± 0.69 | 7.52 ± 1.10 | 0.102 |
| AST (U/L) | 340.75 ± 20.14 | 351.54 ± 62.68 | 340.45 ± 16.58 | 328.88 ± 20.36 | 0.794 |
| UREA (mmol/L) | 0.61 ± 0.03 a | 0.38 ± 0.13 b | 0.49 ± 0.15 ab | 0.57 ± 0.27 ab | 0.014 |
| UA (μmol/L) | 396.78 ± 66.01 | 413.23 ± 44.15 | 500.76 ± 71.46 | 523.43 ± 190.69 | 0.327 |
| 42 d | |||||
| TP (g/L) | 35.04 ± 2.43 b | 41.84 ± 3.38 a | 38.38 ± 1.57 ab | 39.77 ± 1.68 ab | 0.001 |
| ALB (g/L) | 14.35 ± 1.77 | 14.80 ± 2.01 | 14.71 ± 1.50 | 15.15 ± 1.81 | 0.844 |
| TG (mmol/L) | 0.52 ± 0.04 | 0.61 ± 0.09 | 0.52 ± 0.06 | 0.57 ± 0.10 | 0.162 |
| TC (mmol/L) | 3.42 ± 0.23 c | 4.17 ± 0.33 a | 3.93 ± 0.33 ab | 3.66 ± 0.21 bc | 0.002 |
| LDL-C (mmol/L) | 0.84 ± 0.18 b | 1.15 ± 0.11 a | 1.04 ± 0.15 ab | 1.04 ± 0.16 ab | 0.018 |
| HDL-C (mmol/L) | 2.32 ± 0.16 | 2.51 ± 0.20 | 2.48 ± 0.25 | 2.43 ± 0.24 | 0.423 |
| ALT (U/L) | 5.97 ± 0.60 | 6.47 ± 0.66 | 5.91 ± 1.18 | 5.99 ± 1.10 | 0.703 |
| AST (U/L) | 565.33 ± 85.72 | 497.33 ± 45.99 | 537.53 ± 65.22 | 489.86 ± 73.71 | 0.304 |
| UREA (mmol/L) | 0.43 ± 0.15 | 0.45 ± 0.05 | 0.31 ± 0.03 | 0.44 ± 0.12 | 0.163 |
| UA (μmol/L) | 722.20 ± 98.02 | 627.58 ± 68.85 | 778.42 ± 103.13 | 637.98 ± 115.39 | 0.111 |
| Item 1 | Treatment 2 | p-Value | |||
|---|---|---|---|---|---|
| C | L | M | H | ||
| Phylum (%) | |||||
| Firmicutes | 62.82 ± 11.80 | 52.22 ± 3.31 | 51.50 ± 5.71 | 49.12 ± 15.89 | 0.287 |
| Bacteroidota | 28.45 ± 10.12 b | 45.32 ± 7.76 ab | 46.73 ± 10.02 ab | 50.97 ± 18.88 a | 0.044 |
| Proteobacteria | 0.80 ± 0.43 b | 1.85 ± 0.54 ab | 1.88 ± 0.32 ab | 2.23 ± 1.19 a | 0.039 |
| Desulfobacterota | 1.46 ± 0.16 | 1.57 ± 0.90 | 1.39 ± 0.71 | 1.51 ± 0.53 | 0.975 |
| Genus (%) | |||||
| Barnesiella | 18.81 ± 16.40 | 15.34 ± 7.15 | 20.92 ± 2.87 | 27.62 ± 8.83 | 0.186 |
| Alistipes | 10.53 ± 1.58 b | 18.21 ± 3.43 a | 10.38 ± 3.80 b | 11.21 ± 4.68 ab | 0.024 |
| Clostridia_UCG-014 | 7.29 ± 2.82 | 8.04 ± 4.13 | 7.32 ± 1.56 | 6.72 ± 3.04 | 0.935 |
| Clostridia_vadinBB60_group | 4.44 ± 0.97 b | 6.23 ± 1.12 a | 6.13 ± 0.72 ab | 7.76 ± 0.27 a | 0.001 |
| Bacteroides | 3.22 ± 0.49 | 4.62 ± 2.18 | 3.58 ± 1.01 | 5.36 ± 1.43 | 0.138 |
| [Ruminococcus]_torques_group | 5.30 ± 1.44 | 4.24 ± 0.40 | 4.43 ± 1.24 | 4.73 ± 0.80 | 0.530 |
| Parabacteroides | 2.11 ± 0.92 b | 5.51 ± 1.61 a | 2.17 ± 0.61 b | 4.65 ± 0.50 ab | 0.004 |
| Faecalibacterium | 3.46 ± 1.85 | 3.53 ± 0.57 | 3.05 ± 1.47 | 2.56 ± 1.60 | 0.769 |
| Rikenella | 0.73 ± 0.41 b | 1.36 ± 0.35 ab | 2.75 ± 1.15 a | 2.21 ± 0.73 ab | 0.010 |
| Christensenellaceae_R-7_group | 2.06 ± 1.09 | 1.27 ± 0.83 | 1.01 ± 0.38 | 1.05 ± 0.53 | 0.228 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Bi, H.; Hu, X.; Chen, X.; Li, Y.; Niu, H.; Pei, C.; Zhang, J.; Liu, Q.; Li, J.; et al. Effects of Dietary High-Yield Protease Bacillus subtilis Strain FRE76 on Broiler Growth, Slaughter Performance, Intestinal Morphology, and Gut Microbiota. Animals 2025, 15, 1085. https://doi.org/10.3390/ani15081085
Sun L, Bi H, Hu X, Chen X, Li Y, Niu H, Pei C, Zhang J, Liu Q, Li J, et al. Effects of Dietary High-Yield Protease Bacillus subtilis Strain FRE76 on Broiler Growth, Slaughter Performance, Intestinal Morphology, and Gut Microbiota. Animals. 2025; 15(8):1085. https://doi.org/10.3390/ani15081085
Chicago/Turabian StyleSun, Liping, Haihong Bi, Xinyuan Hu, Xi Chen, Yating Li, Huijing Niu, Caixia Pei, Jing Zhang, Qiang Liu, Jianhui Li, and et al. 2025. "Effects of Dietary High-Yield Protease Bacillus subtilis Strain FRE76 on Broiler Growth, Slaughter Performance, Intestinal Morphology, and Gut Microbiota" Animals 15, no. 8: 1085. https://doi.org/10.3390/ani15081085
APA StyleSun, L., Bi, H., Hu, X., Chen, X., Li, Y., Niu, H., Pei, C., Zhang, J., Liu, Q., Li, J., & Xia, C. (2025). Effects of Dietary High-Yield Protease Bacillus subtilis Strain FRE76 on Broiler Growth, Slaughter Performance, Intestinal Morphology, and Gut Microbiota. Animals, 15(8), 1085. https://doi.org/10.3390/ani15081085





