Body-Size Change in a Rodent Is Affected by Environmental Warming and Population-Specific Thermoneutral Zone
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Rodent Data
2.2. Measuring Resting Metabolic Rate (RMR) and Estimating Thermoneutral Zone (TNZ)
2.3. Weather Data
2.4. Data Analysis
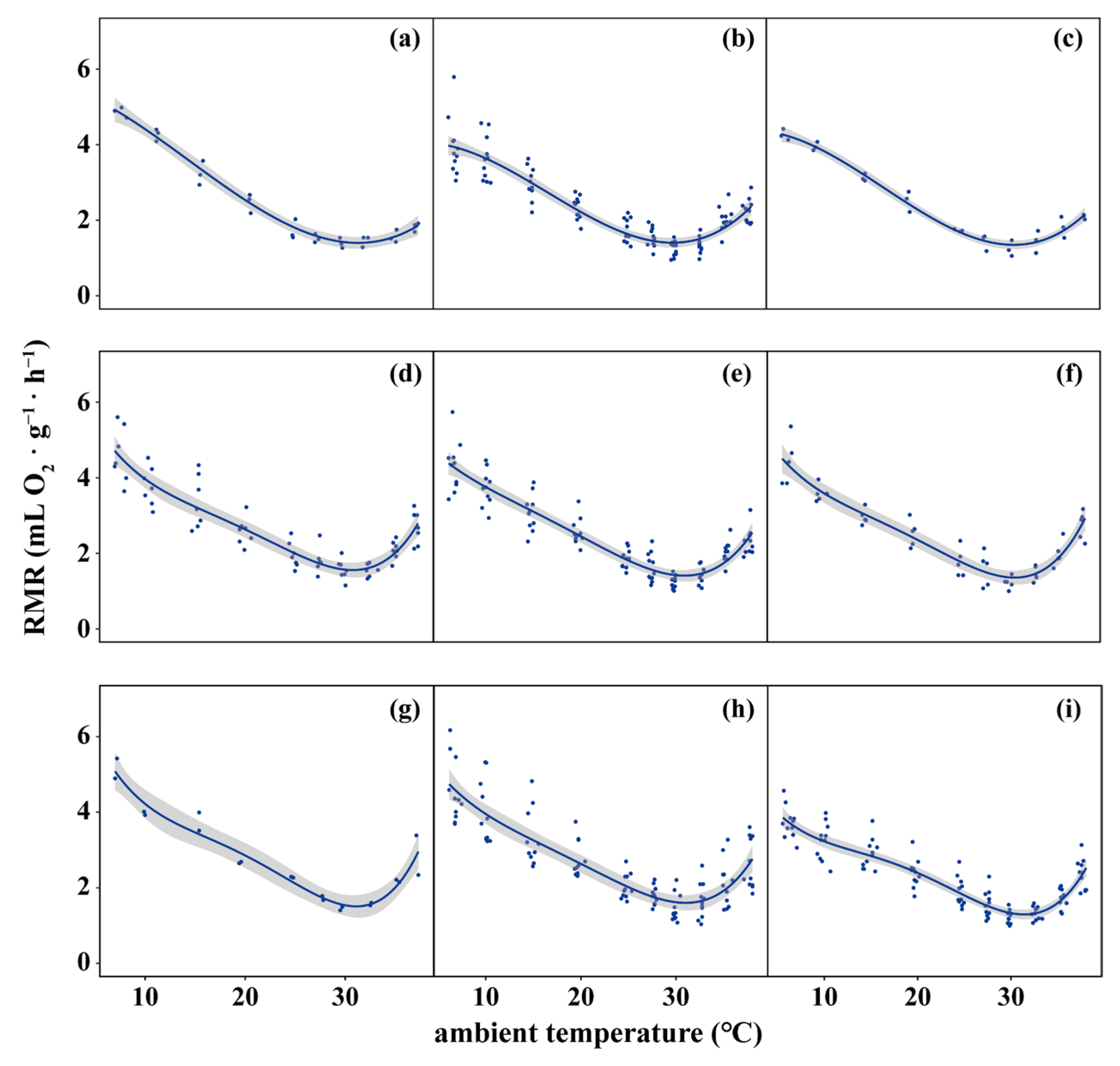
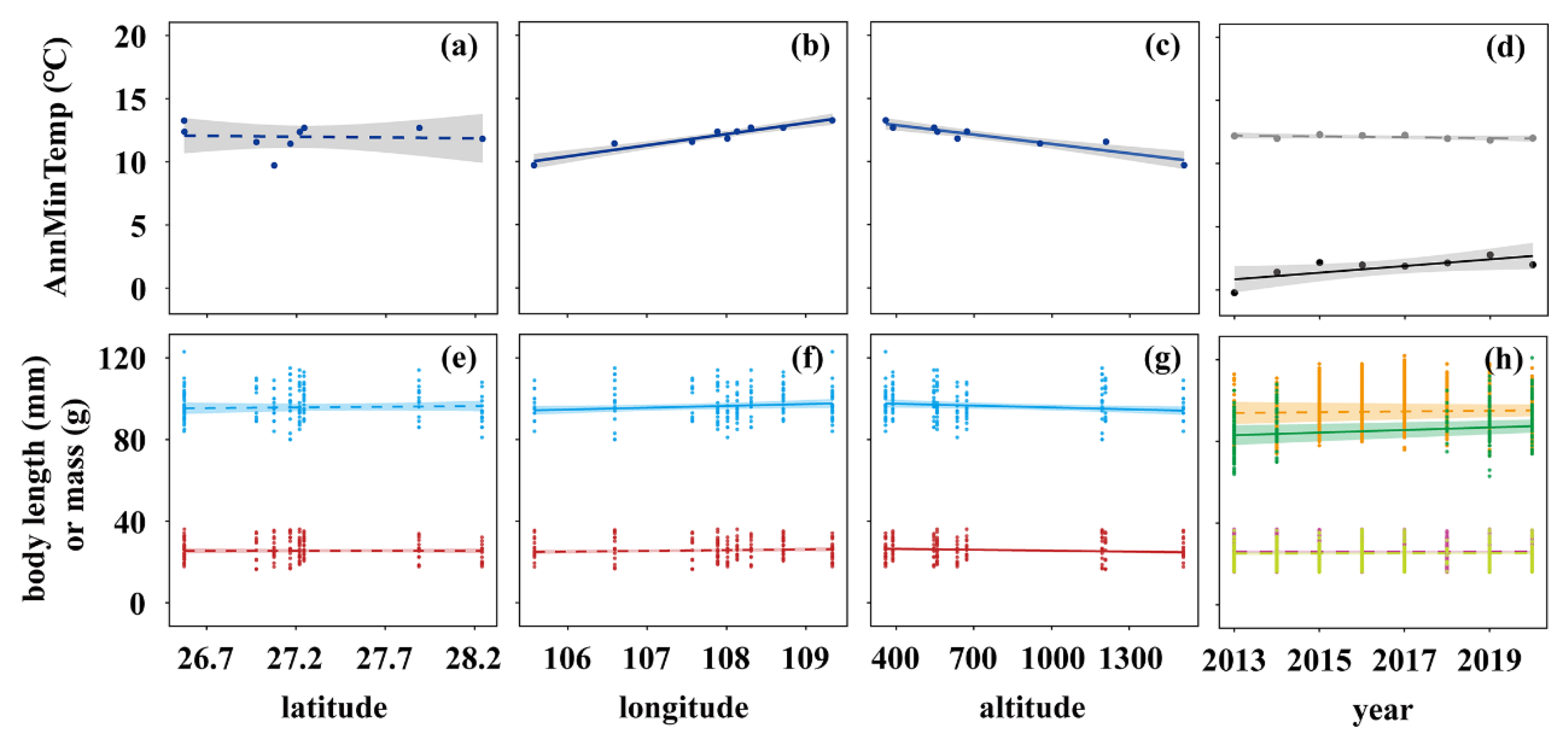
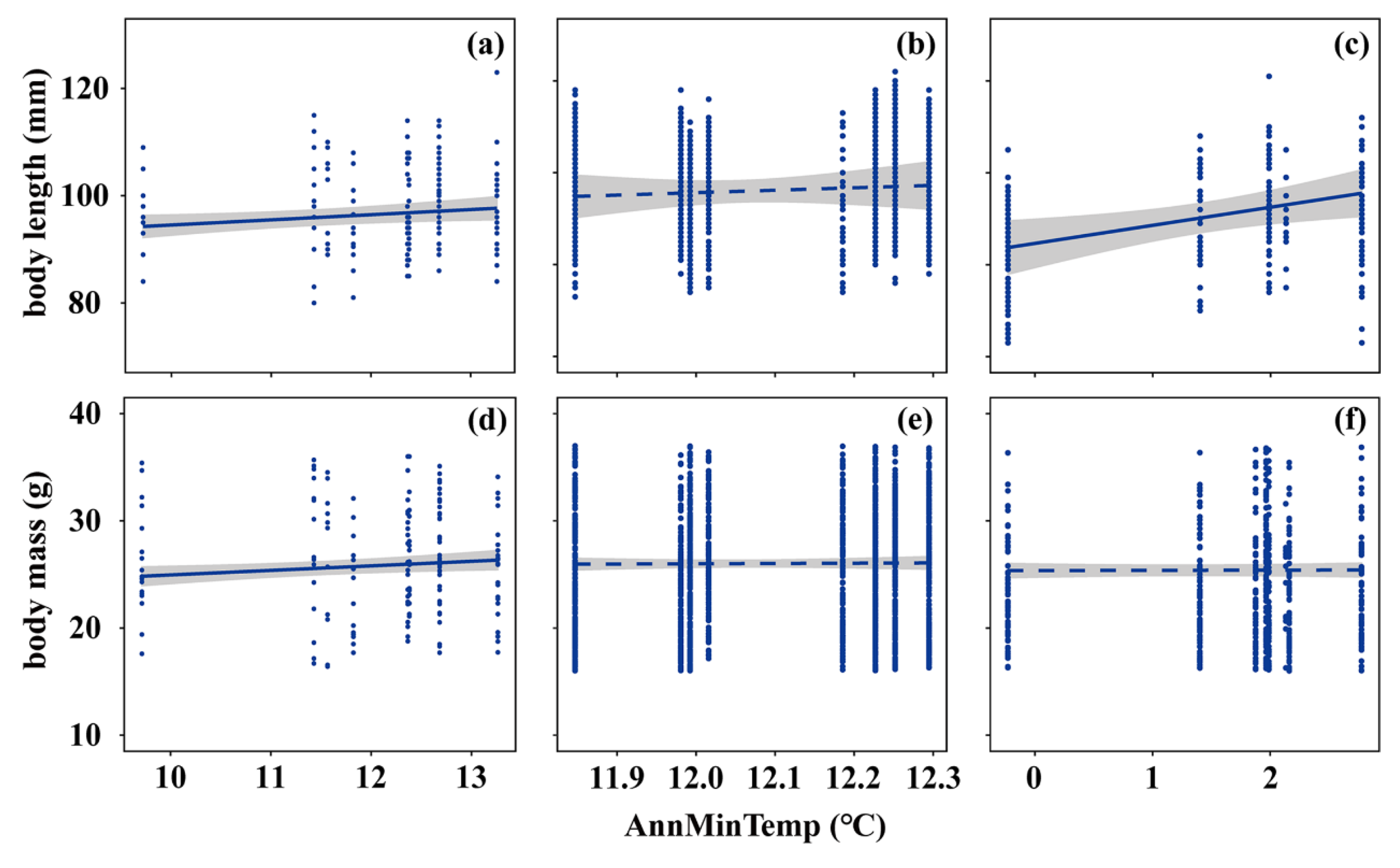
3. Results
3.1. Spatial and Temporal Trends in Temperature and Body Size
3.2. Spatial and Temporal Trends of Body Size Along Temperature Gradients
3.3. Interrelations Among Ambient Temperature, Thermoneutral Zone, and Body Size
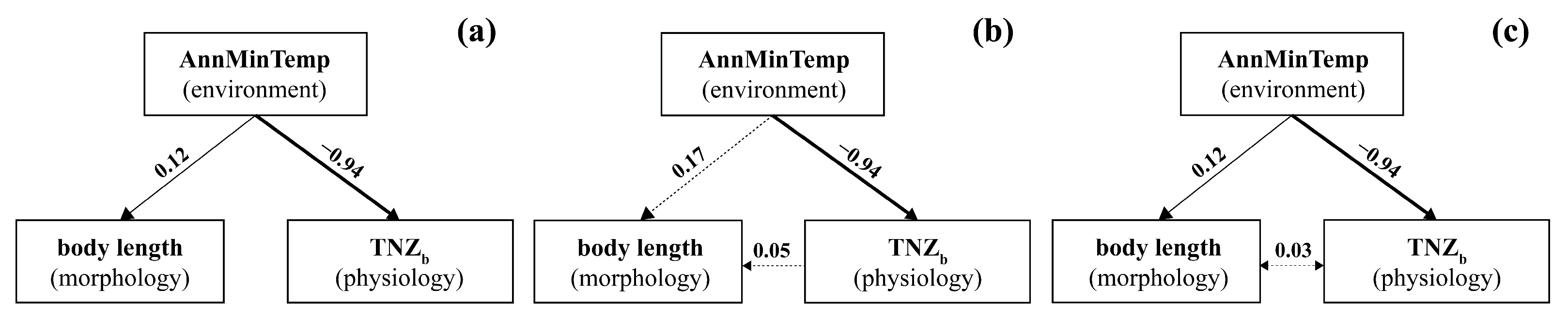
3.4. Structural Equation Modelling
4. Discussion
4.1. Effects of Ambient Temperature on Body Size
4.2. Relationship Between the Thermoneutral Zone and Body Size
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TNZ | Thermoneutral zone |
| RMR | Resting metabolic rate |
| LLT | Lower-limit temperature |
| ULT | Upper-limit temperature |
| TNZb | The breadth of the TNZ |
| AnnMinTemp | Annual mean minimum temperature |
| ECMWF | European Centre for Medium-Range Weather Forecasts |
| GLMM | Generalised linear mixed-effects model |
| LM | Linear model |
| SEM | Structural equation model |
| AIC | Akaike information criterion |
Appendix A
Appendix A.1. Methods
Appendix A.1.1. Measuring Resting Metabolic Rate (RMR)
Appendix A.1.2. Calculating the Thermoneutral Zone (TNZ)
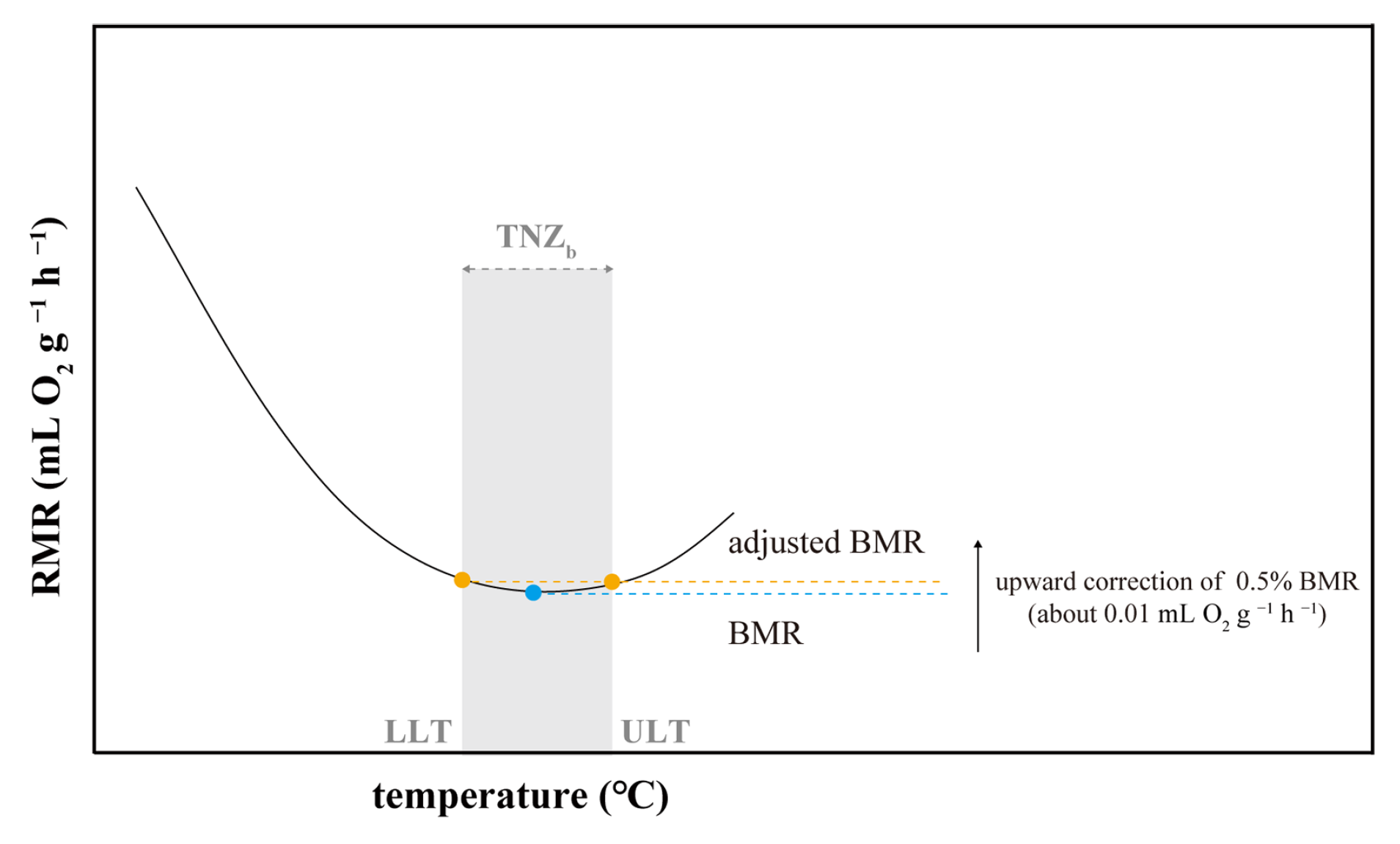
Appendix A.2. Results
| Site | Model | AICc | wi | logLik |
|---|---|---|---|---|
| Dafang | = 4.91 − 1.06 × 10−1t | 52.31 | 0.00 | −22.69 |
| = 6.92 − 3.41 × 10−1t + 5.38 × 10−3t2 | 3.57 | 0.02 | 3.01 | |
| = 5.86 − 1.37 × 10−1t − 5.13 × 10−3t2 + 1.58 × 10−4t3 | −3.61 | 0.77 | 8.05 | |
| = 5.97 − 1.68 × 10−1t − 2.51 × 10−3t2 + 7.02 × 10−5t3 + 1.00 × 10−6t4 | −0.49 | 0.16 | 8.07 | |
| = 7.31 − 6.21 × 10−1t + 5.14 × 10−2t2 − 2.80 × 10−3t3 + 7.13 × 10−5t4 − 6.43 × 10−7t5 | 2.14 | 0.04 | 8.48 | |
| Xifeng | = 4.01 − 7.25 × 10−2t | 214.02 | 0.00 | −103.90 |
| = 5.91 − 2.94 × 10−1t + 5.04 × 10−3t2 | 142.71 | 0.00 | −67.17 | |
| = 3.95 + 7.60 × 10−2t − 1.38 × 10−2t2 + 2.80 × 10−4t3 | 119.20 | 0.55 | −54.31 | |
| = 4.85 − 1.60 × 10−1t + 5.86 × 10−3t2 − 3.65 × 10−4t3 + 7.27 × 10−6t4 | 120.24 | 0.33 | −53.71 | |
| = 5.99 − 5.32 × 10−1t + 4.89 × 10−2t2 − 2.61 × 10−3t3 + 6.11 × 10−5t4 − 4.85 × 10−7t5 | 122.15 | 0.13 | −53.53 | |
| Weng’an | = 4.48 − 9.02 × 10−2t | 51.24 | 0.00 | −22.16 |
| = 6.41 − 3.14 × 10−1t + 5.08 × 10−3t2 | 15.29 | 0.00 | −2.84 | |
| = 4.12 + 1.17 × 10−1t − 1.69 × 10−2t2 + 3.28 × 10−4t3 | −22.19 | 0.70 | 17.34 | |
| = 4.73 − 4.17 × 10−2t − 3.72 × 10−3t 2 − 1.03 × 10−4t3 + 4.86 × 10−6t4 | −20.13 | 0.25 | 17.89 | |
| = 5.11 − 1.65 × 10−1t + 1.04 × 10−2t2 − 8.40 × 10−3t3 + 2.26 × 10−5t4 − 1.61 × 10−7t5 | −16.78 | 0.05 | 17.93 | |
| Dejiang | = 4.47 − 7.97 × 10−2t | 148.48 | 0.00 | −71.06 |
| = 6.60 − 3.28 × 10−1t + 5.63 × 10−3t2 | 98.06 | 0.00 | −44.72 | |
| = 4.73 + 2.51 × 10−2t − 1.23 × 10−2t2 + 2.67 × 10−4t3 | 87.19 | 0.17 | −38.13 | |
| RMR = 7.13 − 5.97 × 10−1t + 3.92 × 10−2t2 − 1.42 × 10−3t3 + 1.90 × 10−5t4 | 85.03 | 0.51 | −35.85 | |
| + 1.52 × 10−1t2 − 7.29 × 10−3t3 + 1.60 × 10−4t4 − 1.28 × 10−6t5 | 85.95 | 0.32 | −35.07 | |
| Yuqing | = 4.32 − 7.97 × 10−2t | 203.01 | 0.00 | −98.38 |
| = 6.41 − 3.19 × 10−1t + 5.40 × 10−3t2 | 133.99 | 0.00 | −62.78 | |
| = 4.19 + 9.05 × 10−2t − 1.52 × 10−2t2 + 3.05 × 10−4t3 | 110.59 | 0.20 | −49.98 | |
| RMR = 6.07 − 3.91 × 10−1t + 2.45 × 10−2t2 − 9.93 × 10−4t3 + 1.46 × 10−5t4 | 108.75 | 0.49 | −47.92 | |
| + 1.16 × 10−1t2 − 5.73 × 10−3t3 + 1.28 × 10−4t4 −1.02 × 10−6t5 | 109.65 | 0.31 | −47.22 | |
| Kaili | = 4.17 − 7.31 × 10−2t | 113.91 | 0.00 | −53.69 |
| = 6.68 − 3.63 × 10−1t + 6.54 × 10−3t2 | 68.83 | 0.00 | −29.97 | |
| = 4.18 + 1.02 × 10−1t− 1.69 × 10−2t2 + 3.48 × 10−4t3 | 52.94 | 0.07 | −20.79 | |
| = 7.34 − 7.05 × 10−1t + 4.96 × 10−2t2 − 1.82 × 10−3t3 + 2.44 × 10−5t4 | 48.31 | 0.73 | −17.18 | |
| = 8.05 − 9.34 × 10−1t + 7.56 × 10−2t2 − 3.17 × 10−3t3 + 5.66 × 10−5t4 − 2.89 × 10−7t5 | 50.95 | 0.20 | −17.14 | |
| Sinan | = 4.98 − 9.29 × 10−2t | 49.64 | 0.00 | −21.07 |
| = 7.63 − 3.95 × 10−1t + 6.82 × 10−3t2 | 32.31 | 0.01 | −10.82 | |
| = 4.82 + 1.19 × 10−1t − 1.90 × 10−2t2 + 3.84 × 10−4t3 | 27.20 | 0.19 | −6.46 | |
| = 10.20 − 1.22 + 8.87 × 10−2t2 − 3.09 × 10−3t3 + 3.88 × 10−5t4 | 24.63 | 0.68 | −3.08 | |
| = + 2.58 × 10−1t2 − 1.18 × 10−2t3 + 2.45 × 10−4t4 −1.86 × 10−6t5 | 28.14 | 0.12 | −2.40 | |
| Jinping | 167.41 | 0.00 | −80.58 | |
| + 4.41 × 10−32 | 100.3 | 0.00 | −45.94 | |
| 23 | 55.69 | 0.03 | −22.53 | |
| = 4.89 − 3.02 × 10−1 + 2.20 × 10−22 − 9.73 × 10−43 + 1.47 × 10−54 | 50.18 | 0.53 | −18.64 | |
| 2345 | 50.59 | 0.43 | −17.69 | |
| Cengon | = 4.47 − 7.94 × 10−2t | 192.29 | 0.00 | −93.03 |
| = 6.70 − 3.38 × 10−1t + 5.87 × 10−3t2 | 141.47 | 0.00 | −66.54 | |
| = 4.73 + 3.03 × 10−2t−1 − 1.28 × 10−2t2 + 2.78 × 10−4t3 | 108.35 | 0.00 | −48.89 | |
| = 7.23 − 6.14 × 10−1t + 4.05 × 10−2t2 − 1.46 × 10−3t3 + 1.96 × 10−5t4 | 95.14 | 0.63 | −41.16 | |
| + 1.75 × 10−1t1 − 8.46 × 10−3t3 + 1.87 × 10−4t4 − 1.51 × 10−6t5 | 96.18 | 0.37 | −40.54 |
| Site | Sample Size | BMR (mL O2 g −1 h −1) | ULT (°C) | LLT (°C) | TNZb (°C) |
|---|---|---|---|---|---|
| Dafang | 3 | 1.39 | 32.21 | 29.72 | 2.49 |
| Xifeng | 9 | 1.37 | 30.97 | 28.64 | 2.33 |
| Wengan | 3 | 1.27 | 31.37 | 29.50 | 1.87 |
| Dejiang | 7 | 1.54 | 31.88 | 29.83 | 2.05 |
| Yuqing | 8 | 1.39 | 31.65 | 30.01 | 1.64 |
| Kaili | 5 | 1.45 | 31.00 | 29.40 | 1.60 |
| Sinan | 2 | 1.40 | 31.88 | 30.30 | 1.58 |
| Jinping | 10 | 1.25 | 31.39 | 30.05 | 1.34 |
| Cengon | 10 | 1.71 | 31.09 | 29.47 | 1.62 |
| Total/Average | 57 | 1.42 | 31.49 | 29.66 | 1.84 |
References
- Bergmann, C. Ueber die Verhältnisse der Wärmeökonomie der Thiere zu ihrer Grösse, Göttinger Studien. Göttingen 1847, 3, 595–708. [Google Scholar]
- Teplitsky, C.; Millien, V. Climate warming and Bergmann’s rule through time: Is there any evidence? Evol. Appl. 2014, 7, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Villar, C.H.; Naya, D.E. Climate change and temporal trends in body size: The case of rodents. Oikos 2018, 127, 1186–1194. [Google Scholar] [CrossRef]
- Li, K.; Sommer, S.; Yang, Z.; Guo, Y.; Yue, Y.; Ozgul, A.; Wang, D. Distinct body-size responses to warming climate in three rodent species. Proc. R. Soc. B 2022, 289, 20220015. [Google Scholar] [CrossRef]
- Mayr, E. Geographical character gradients and climatic adaptation. Evolution 1956, 10, 105–108. [Google Scholar] [CrossRef]
- James, F.C. Geographic size variation in birds and its relationship to climate. Ecology 1970, 51, 365–390. [Google Scholar] [CrossRef]
- Thornton, M.M.; Thornton, P.E.; Wei, Y.; Mayer, B.W.; Cook, R.B.; Vose, R.S. Daymet: Monthly Climate Summaries for a 1-km Grid for North America; ORNL DAAC: Oak Ridge, TN, USA, 2020. [Google Scholar]
- Schiaffini, M.I. A test of the Resource’s and Bergmann’s rules in a widely distributed small carnivore from southern South America, Conepatus chinga (Molina, 1782) (Carnivora: Mephitidae). Mamm. Biol. 2016, 81, 73–81. [Google Scholar] [CrossRef]
- Blackburn, T.M.; Gaston, K.J.; Loder, N.; Jul, N. Geographic gradients in body size: A clarification of Bergmann’s rule. Divers. Distrib. 1999, 5, 165–174. [Google Scholar] [CrossRef]
- Henry, E.; Santini, L.; Huijbregts, M.A.J.; Benítez-López, A. Unveiling the environmental drivers of intraspecific body size variation in terrestrial vertebrates. Glob. Ecol. Biogeogr. 2023, 32, 267–280. [Google Scholar] [CrossRef]
- Millien, V.; Lyons, S.K.; Olson, L.; Smith, F.A.; Wilson, A.B.; Yom-Tov, Y. Ecotypic variation in the context of global climate change: Revisiting the rules. Ecol. Lett. 2006, 9, 853–869. [Google Scholar] [CrossRef]
- van Jaarsveld, B.; Bennett, N.C.; Kemp, R.; Czenze, Z.J.; McKechnie, A.E. Heat tolerance in desert rodents is correlated with microclimate at inter- and intraspecific levels. J. Comp. Physiol. B 2021, 191, 575–588. [Google Scholar] [CrossRef] [PubMed]
- IUPS Thermal Commission. Glossary of terms for thermal physiology, Third ed. Revised by The Commission for Thermal Physiology of the International Union of Physiological Sciences. Jpn. J. Physiol. 2001, 51, 245–280. [Google Scholar]
- Spencer, H. Principles of Biology; Williams and Norgate: London, UK, 1864; Volume 1, p. 444. [Google Scholar]
- Tran, L.T.; Park, S.; Kim, S.K.; Lee, J.S.; Kim, K.W.; Kwon, O. Hypothalamic control of energy expenditure and thermogenesis. Exp. Mol. Med. 2022, 54, 358–369. [Google Scholar] [CrossRef] [PubMed]
- McNab, B.K. Extreme Measures: The Ecological Energetics of Birds and Mammals; University of Chicago Press: Chicago, IL, USA, 2012; p. 53. [Google Scholar]
- Buckley, L.B.; Khaliq, I.; Swanson, D.L.; Hof, C. Does metabolism constrain bird and mammal ranges and predict shifts in response to climate change? Ecol. Evol. 2018, 8, 12375–12385. [Google Scholar] [CrossRef]
- Carneiro, L.D.O.; Mellado, B.; Nogueira, M.R.; Cruz-Neto, A.P.D.; Monteiro, L.R. Flight performance and wing morphology in the bat Carollia perspicillata: Biophysical models and energetics. Integr. Zool. 2023, 18, 876–890. [Google Scholar] [CrossRef]
- Schaeffer, P.J.; O’Mara, M.T.; Breiholz, J.; Keicher, L.; Lázaro, J.; Muturi, M.; Dechmann, D.K. Metabolic rate in common shrews is unaffected by temperature, leading to lower energetic costs through seasonal size reduction. Proc. R. Soc. B 2020, 7, 191989. [Google Scholar] [CrossRef]
- Names, G.R.; Grindstaff, J.L.; Westneat, D.F.; Heidinger, B.J. Climate change and its effects on body size and shape: The role of endocrine mechanisms. Phil. Trans. R. Soc. B 2024, 379, 20220509. [Google Scholar] [CrossRef]
- Lovegrove, B.G. The influence of climate on the basal metabolic rate of small mammals: A slow-fast metabolic continuum. J. Comp. Physiol. B 2003, 173, 87–112. [Google Scholar] [CrossRef]
- Naya, D.E.; Naya, H.; White, C.R. On the interplay among ambient temperature, basal metabolic rate, and body mass. Am. Nat. 2018, 192, 518–524. [Google Scholar] [CrossRef]
- Menéndez, J.; Ruperto, E.F.; Taraborelli, P.A.; Sassi, P.L. Phenotypic plasticity acclimation and developmental conditions. J. Exp. Zool. A. Ecol. Integr. Physiol. 2022, 337, 303–315. [Google Scholar] [CrossRef]
- Gardner, J.L.; Amano, T.; Mackey, B.G.; Sutherland, W.J.; Clayton, M.; Peters, A. Dynamic size responses to climate change: Prevailing effects of rising temperature drive long-term body size increases in a semi-arid passerine. Glob. Change Biol. 2014, 20, 2062–2075. [Google Scholar] [CrossRef] [PubMed]
- Trillmich, F.; Guenther, A. Shifts in energy allocation and reproduction in response to temperature in a small precocial mammal. BMC Zool. 2023, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Komar, E.; Fasel, N.J.; Szafrańska, P.A.; Dechmann, D.K.N.; Zegarek, M.; Ruczyński, I. Energy allocation shifts from sperm production to self-maintenance at low temperatures in male bats. Sci. Rep. 2022, 12, 2138. [Google Scholar] [CrossRef] [PubMed]
- Khaliq, I.; Hof, C. Testing the heat dissipation limitation hypothesis: Basal metabolic rates of endotherms decrease with increasing upper and lower critical temperatures. PeerJ 2018, 6, e5725. [Google Scholar] [CrossRef]
- Bennett, J.M.; Sunday, J.; Calosi, P.; Villalobos, F.; Martínez, B.; Molina-Venegas, R.; Araújo, M.B.; Algar, A.C.; Clusella-Trullas, S.; Hawkins, B.A.; et al. The evolution of critical thermal limits of life on Earth. Nat. Commun. 2021, 12, 1198. [Google Scholar] [CrossRef]
- Castellanos-Frías, E.; García-Perea, R.; Gisbert, J.; Bozinovic, F.; Virgós, E. Intraspecific variation in the energetics of the Cabrera vole. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 190, 32–38. [Google Scholar] [CrossRef]
- Zhao, Z.J.; Chi, Q.S.; Liu, Q.S.; Zheng, W.H.; Liu, J.S.; Wang, D.H. The shift of thermoneutral zone in striped hamster acclimated to different temperatures. PLoS ONE 2014, 9, e84396. [Google Scholar] [CrossRef][Green Version]
- Liao, S.; Tan, S.; Jiang, M.; Wen, J.; Liu, J.; Cao, J.; Li, M.; Zhao, Z. Temperature determines the shift of thermal neutral zone and influences thermogenic capacity in striped hamsters. Integr. Zool. 2023, 18, 353–371. [Google Scholar] [CrossRef]
- Wang, D.; Anderson, D.P.; Li, K.; Guo, Y.; Yang, Z.; Pech, R.P. Predicted population dynamics of an indigenous rodent, Apodemus agrarius, in an agricultural system. Crop Prot. 2021, 147, 105683. [Google Scholar] [CrossRef]
- Liu, C.S.; Wu, W.N.; Guo, S.K.; Meng, J.H. A study of the subspecies classification of Apodemus agrarius in eastern continental China. Acta Theriol. Sin. 1991, 11, 294–299. (In Chinese) [Google Scholar]
- Yang, Z.X. Methods of Determining Age in Small Mammals of China; China Agriculture Press: Beijing, China, 2016; p. 59. (In Chinese) [Google Scholar]
- Withers, P.C. Measurement of VO2, VCO2, and evaporative water loss with a flow-through mask. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1977, 42, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yang, R.; Li, M.; Bao, M.; Huo, D.; Cao, J.; Speakman, J.R. Effects of ambient temperatures between 5 and 35 °C on energy balance, body mass and body composition in mice. Mol. Metab. 2022, 64, 101551. [Google Scholar] [CrossRef] [PubMed]
- Mandic, D.P. A generalized normalized gradient descent algorithm. IEEE Signal Process. Lett. 2004, 11, 115–118. [Google Scholar] [CrossRef]
- Zuur, A.; Ieno, E.N.; Smith, G.M. Analysing Ecological Data; Springer: New York, NY, USA, 2007; pp. 79–88. [Google Scholar]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Automat. Contr. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Shipley, B. Cause and Correlation in Biology; Cambridge University Press: Cambridge, UK, 2000; pp. 153–187. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 1 March 2024).
- Grothendieck, G.; Grothendieck, M.G. Package ‘nls2’. Non-Linear Regression with Brute Force. 2013. Available online: https://cran.r-project.org/web/packages/nls2/index.html (accessed on 1 March 2024).
- Hasselman, B. nleqslv: Solve Systems of Nonlinear Equations. 2017. Available online: https://cran.r-project.org/web/packages/nleqslv/index.html (accessed on 1 March 2024).
- Gilbert, P.; Varadhan, R.; Gilbert, M.P. Package ‘numDeriv’. 2009. Available online: https://cran.r-project.org/web/packages/numDeriv/index.html (accessed on 15 March 2024).
- Bates, D.; Maechler, M.; Bolker, B. Lme4: Linear Mixed-Effects Models Using S4 Classes R Package. 2012. Available online: http://cran.r-project.org/web/packages/lme4/index.html (accessed on 15 March 2024).
- Venables, W.N.; Ripley, B.D. 2002 Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Mazerolle, M.J. AICcmodavg: Model Selection and Multimodel Inference Based on (Q)AIC(c). 2013. Available online: https://cran.r-project.org/package=AICcmodavg (accessed on 15 March 2024).
- Lefcheck, J.S. piecewiseSEM: Piecewise structural equation modelling in r for ecology, evolution, and systematics. Methods Ecol. Evol. 2016, 7, 573–579. [Google Scholar] [CrossRef]
- Rind, D. Latitudinal temperature gradients and climate change. J. Geophys. Res. 1998, 103, 5943–5971. [Google Scholar] [CrossRef]
- Gu, S.; Shi, H. Temporal and Spatial Variation of Summer Extreme High Temperature in Guizhou Province from 1970 to 2020. J. Geosci. Environ. Prot. 2023, 11, 62–72. [Google Scholar] [CrossRef]
- Gardner, J.L.; Peters, A.; Kearney, M.R.; Joseph, L.; Heinsohn, R. Declining body size: A third universal response to warming? Trends. Ecol. Evol. 2011, 26, 285–291. [Google Scholar] [CrossRef]
- Hantak, M.M.; McLean, B.S.; Li, D.; Guralnick, R.P. Mammalian body size is determined by interactions between climate, urbanization, and ecological traits. Commun. Biol. 2021, 4, 972. [Google Scholar] [CrossRef]
- Speakman, J.R. The cost of living: Field metabolic rates of small mammals. Adv. Ecol. Res. 1999, 30, 177–297. [Google Scholar] [CrossRef]
- Speakman, J.R.; Ergon, T.; Cavanagh, R.; Reid, K.A.; Scantlebury, D.M.; Lambin, X. Resting and daily energy expenditures of free-living field voles are positively correlated but reflect extrinsic rather than intrinsic effects. Proc. Natl. Acad. Sci. USA 2003, 100, 14057–14062. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a metabolic theory of ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- White, C.R.; Blackburn, T.M.; Martin, G.R.; Butler, P.J. Basal metabolic rate of birds is associated with habitat temperature and precipitation, not primary productivity. Proc. R. Soc. B 2007, 274, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Hudson, L.N.; Isaac, N.J.B.; Reuman, D.C. The relationship between body mass and field metabolic rate among individual birds and mammals. J. Anim. Ecol. 2013, 82, 1009–1020. [Google Scholar] [CrossRef]
- Naya, D.E.; Spangenberg, L.; Naya, H.; Bozinovic, F. Thermal conductance and basal metabolic rate are part of a coordinated system for heat transfer regulation. Proc. R. Soc. B 2013, 280, 20131629. [Google Scholar] [CrossRef]
- Sakka, H.; Quéré, J.P.; Kartavtseva, I.; Pavlenko, M.; Chelomina, G.; Atopkin, D.; Bogdanov, A.; Michaux, J. Comparative phylogeography of four Apodemus species (Mammalia: Rodentia) in the Asian Far East: Evidence of Quaternary climatic changes in their genetic structure. Biol. J. Linn. Soc. 2010, 100, 797–821. [Google Scholar] [CrossRef]
- McNab, B.K. The Physiological Ecology of Vertebrates: A View from Energetics; Cornell University Press: Ithaca, NY, USA, 2002; pp. 85–87. [Google Scholar]
- Porter, W.P.; Kearney, M. Size, shape, and the thermal niche of endotherms. Proc. Natl. Acad. Sci. USA 2009, 106, 19666–19672. [Google Scholar] [CrossRef]
- Kobbe, S.; Nowack, J.; Dausmann, K.H. Torpor is not the only option: Seasonal variations of the thermoneutral zone in a small primate. J. Comp. Physiol. B 2014, 184, 789–797. [Google Scholar] [CrossRef]
- Diao, Y.; Yang, M.; Chi, Q.S. Winter energy metabolism and thermoregulation of Apodemus agrarius from northeast China. In Proceedings of the 3rd International Conference on Rodent Biology and Management, Hanoi, Vietnam, 28 August–1 September 2006; p. 13. [Google Scholar]
- Bennett, N.C.; Cotterill, F.P.D.; Spinks, A.C. Thermoregulation in two population of the Matabeleland mole-rat (Cryptomys hottentotus nimrodi) and remarks on the general thermoregulatory trends within the genus Cryptomys (Rodentia: Bathyergidae). J. Zool. 1996, 239, 17–27. [Google Scholar] [CrossRef]
- Medina-Bello, K.I.; Orozco-Lugo, C.L.; Ayala-Berdon, J. Differences in thermal energetics of the cave myotis (Myotis velifer) from a cool and a warm environment of central Mexico. Can. J. Zool. 2023, 101, 1115–1123. [Google Scholar] [CrossRef]
- Scholander, P.F.; Hock, R.; Walters, V.; Johnson, F.; Irving, l. Heat regulation in some arctic and tropical mammals and birds. Biol. Bull. 1950, 99, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.J. Relationship between behavioral and autonomic thermoregulation in the guinea pig. Physiol. Behav. 1986, 38, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.J. Relationship between preferred ambient temperature and autonomic thermoregulatory function in rat. Am. J. Physiol. 1987, 252, R1130–R1137. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, N. Further analysis of the data by Akaike’s information criterion and the finite corrections. Commun. Stat. Theory Methods 1978, 7, 13–26. [Google Scholar] [CrossRef]
- Liu, J.S.; Wang, D.H.; Sun, R.Y. Metabolism and thermoregulation in three species of rodent from Northeastern China. J. Therm. Biol. 2004, 29, 177–183. [Google Scholar] [CrossRef]
- Holloway, J.; Geiser, F. Seasonal changes in the thermoenergetics of the marsupial sugar glider. Petaurus breviceps. J. Comp. Physiol. B 2001, 171, 643–650. [Google Scholar] [CrossRef]
- Johnstone, A.M.; Murison, S.D.; Duncan, J.S.; Rance, K.A.; Speakman, J.R. Factors influencing variation in basal metabolic rate include fat-free mass, fat mass, age, and circulating thyroxine but not sex, circulating leptin, or triiodothyronine. Am. J. Clin. Nutr. 2005, 82, 941–948. [Google Scholar] [CrossRef]
- Meyer, D. Naive time series forecasting methods. R News 2002, 2, 7–10. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Li, K.; Sommer, S.; Ozgul, A.; Zhang, Y.; Wang, D. Body-Size Change in a Rodent Is Affected by Environmental Warming and Population-Specific Thermoneutral Zone. Animals 2025, 15, 1112. https://doi.org/10.3390/ani15081112
Chen Y, Li K, Sommer S, Ozgul A, Zhang Y, Wang D. Body-Size Change in a Rodent Is Affected by Environmental Warming and Population-Specific Thermoneutral Zone. Animals. 2025; 15(8):1112. https://doi.org/10.3390/ani15081112
Chicago/Turabian StyleChen, Yan, Ke Li, Stefan Sommer, Arpat Ozgul, Yizhen Zhang, and Deng Wang. 2025. "Body-Size Change in a Rodent Is Affected by Environmental Warming and Population-Specific Thermoneutral Zone" Animals 15, no. 8: 1112. https://doi.org/10.3390/ani15081112
APA StyleChen, Y., Li, K., Sommer, S., Ozgul, A., Zhang, Y., & Wang, D. (2025). Body-Size Change in a Rodent Is Affected by Environmental Warming and Population-Specific Thermoneutral Zone. Animals, 15(8), 1112. https://doi.org/10.3390/ani15081112






