Fecal Glucocorticoid Metabolite Responses of Brown Kiwi (Apteryx mantelli) to Ambassador Program Participation and Translocation: Implications for Captive Management and Welfare
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Animals
2.2. Acclimatization Process for the Ambassador Kiwi Program
2.3. Ambassador Outreach Events
2.4. Gut Transit Time
2.5. Fecal Sample Collection
2.6. Fecal Hormone Metabolite Extraction
2.7. Hormone Analyses
3. Statistical Analyses
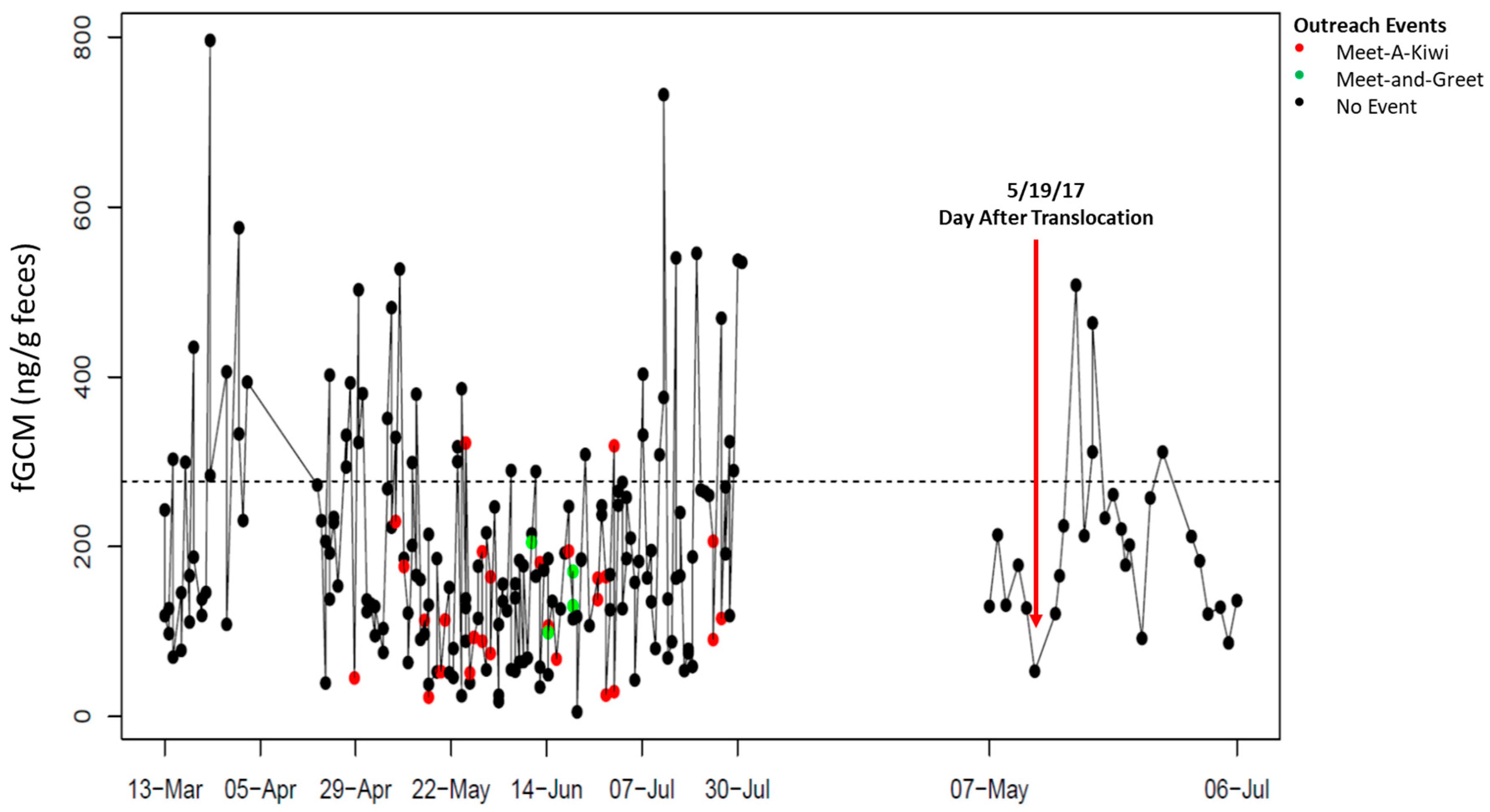
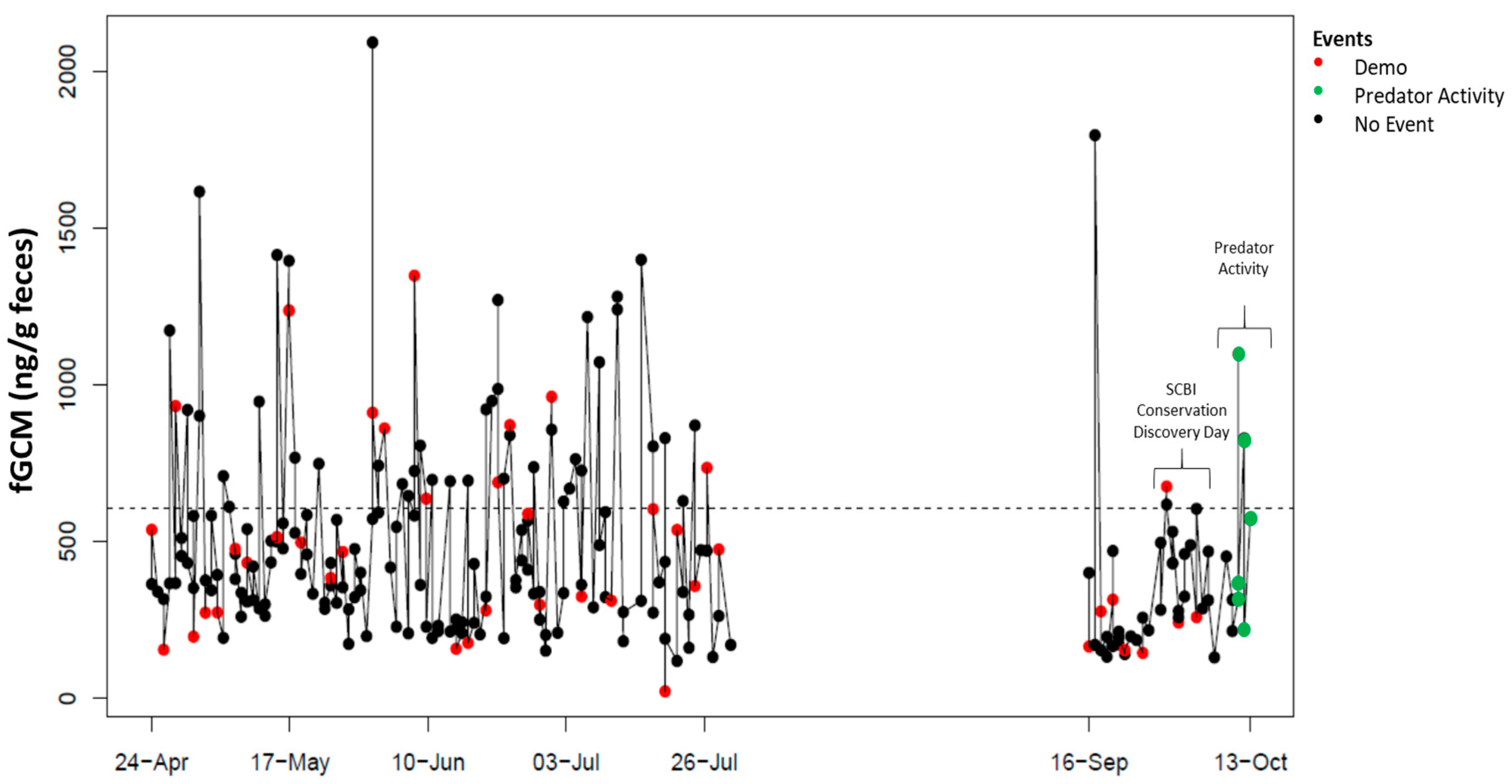
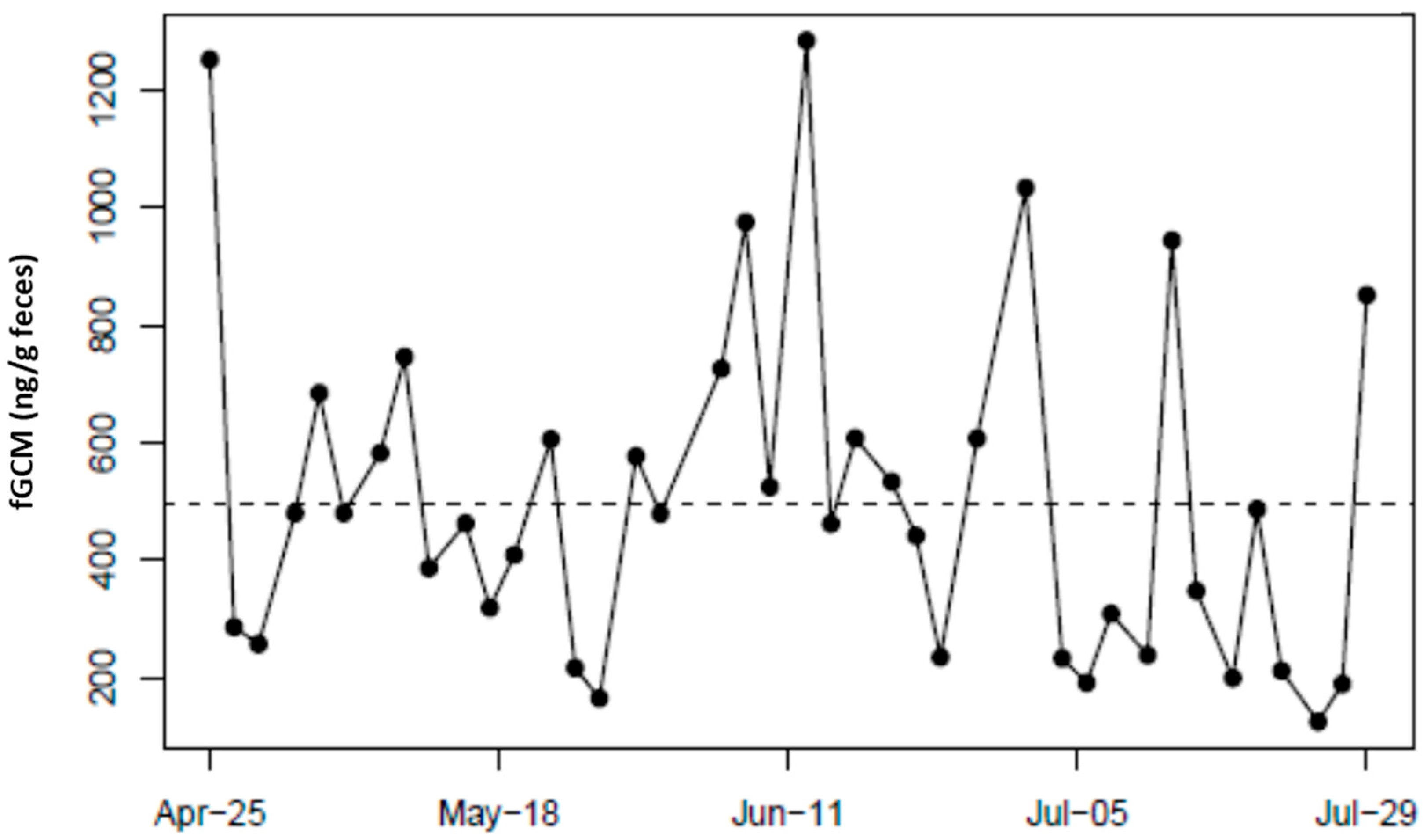
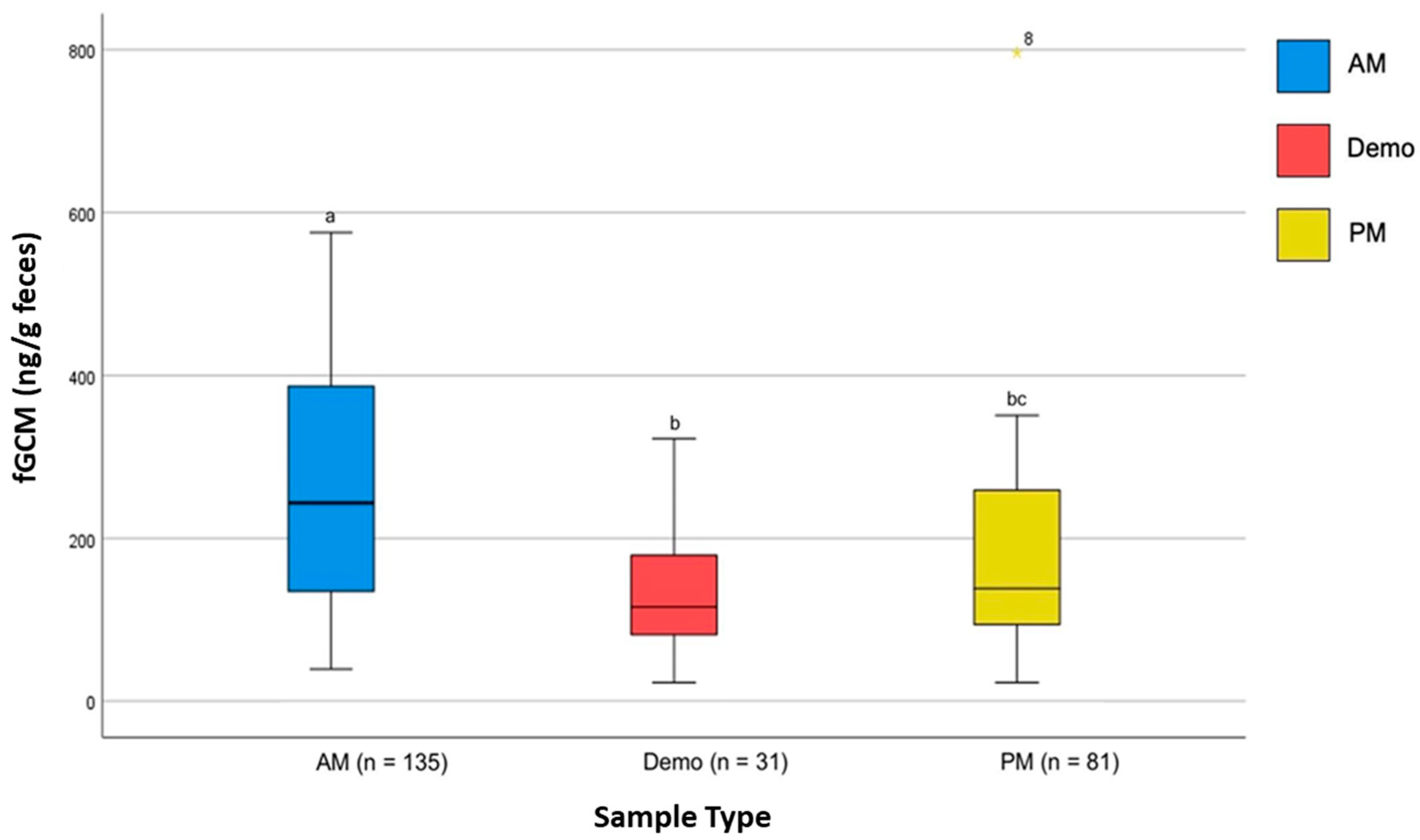
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Department of Conservation. Brown Kiwi. Kiwi. 2020. Available online: www.doc.govt.nz/nature/native-animals/birds/birds-a-z/kiwi/brown-kiwi/ (accessed on 15 May 2024).
- Holzapfel, S.; Robertson, H.A.; McLennan, J.A.; Sporle, W.; Hackwell, K.; Impey, M. Kiwi (Aptryx ssp.) Recovery Plan 2008-2018. Retrieved from Wellington, New Zealand. 2008. Available online: https://www.doc.govt.nz/Documents/science-and-technical/tsrp60entire.pdf (accessed on 15 May 2024).
- Robertson, H.A.; Baird, K.; Dowding, J.E.; Elliott, G.P.; Hitchmough, R.A.; Miskelly, C.M.; Taylor, G.A. Conservation Status of New Zealand Birds, 2016. Retrieved from Wellington, New Zealand. 2017. Available online: https://www.doc.govt.nz/documents/science-and-technical/nztcs19entire.pdf (accessed on 15 May 2024).
- Robertson, H.A.; Colbourne, R.M.; Graham, P.J.; Miller, P.J.; Pierce, R.J. Experimental management of Brown kiwi Apteryx mantelli in central Northland, New Zealand. Bird Conserv. Int. 2011, 21, 207–220. [Google Scholar] [CrossRef]
- Bryan, C.G.; Brader, K. North Island Brown Kiwi (Apteryx Australis Mantelli) AZA Species Survival Plan—Yellow Program; Population Management Center: Chicago, IL, USA, 2019. [Google Scholar]
- McLennan, J.A.; McCann, A.J. Short communication—Incubation temperatures of great spotted kiwi, Apteryx haastii. N. Z. J. Ecol. 1991, 15, 163–166. [Google Scholar]
- Kinsky, F.C. The consistent presence of paired ovaries in the kiwi (Apteryx) with some discussion of this condition in other birds. J. Ornithol. 1971, 112, 334–357. [Google Scholar] [CrossRef]
- Cunningham, S.J.; Castro, I. The secret life of wild brown kiwi: Studying behaviour of a cryptic species by direct observation. N. Z. J. Ecol. 2011, 35, 209–219. [Google Scholar]
- Colbourne, R. Incubation behaviour and egg physiology of kiwi (Apteryx spp.) in natural habitats. N. Z. J. Ecol. 2002, 26, 129–138. [Google Scholar]
- Calder, W.A. The Kiwi. Sci. Am. 1978, 239, 132–143. [Google Scholar] [CrossRef]
- Bailey, W. (Smithsonian National Zoo and Conservation Biology Institute, Front Royal, VA, USA). Personal communication, unpublished.
- French, F.; Bwye, P.; Carrigan, L.; Coe, J.C.; Kelly, R.; Leek, T.; Lynch, E.C.; Mahan, E.; Mingee, C. Welfare and Enrichment of Managed Nocturnal Species, Supported by Technology. Animals 2024, 14, 2378. [Google Scholar] [CrossRef]
- Fernandez, E.J.; Tamborski, M.A.; Pickens, S.R.; Timberlake, W. Animal-visitor interactions in the modern zoo: Conflicts and interventions. Appl. Anim. Behav. Sci. 2009, 120, 1–8. [Google Scholar] [CrossRef]
- Jensen, T.; Durrant, B. Assessment of reproductive status and ovulation in female brown kiwi (Apteryx mantelli) using fecal steroids and ovarian follicle size. Zoo Biol. 2006, 25, 25–34. [Google Scholar] [CrossRef]
- Colbourne, R.; Bassett, S.; Billing, T.; McCormick, H.; McLennan, J.; Nelson, A.; Robertson, H. The Development of Operation Nest Egg as a Tool in the Conservation Management of Kiwi; Department of Conservation: Wellington, New Zealand, 2005. [Google Scholar]
- Brader, K. (Smithsonian National Zoo and Conservation Biology Institute, Washington, DC, USA). Personal communication, unpublished.
- Smulders, T.V. Telencephalic regulation of the HPA axis in birds. Neurobiol. Stress 2021, 15, 100351. [Google Scholar] [CrossRef]
- Cockrem, J.F. Stress, corticosterone responses and avian personalities. J. Ornithol. 2007, 148 (Suppl. S2), 169–178. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar]
- Wingfield, J.C.; Breuner, C.W.; Jacobs, J. Corticosterone and behavioral responses to unpredictable events. In Perspectives in Avian Endocrinology; Harvey, S., Etches, R.J., Eds.; J. Endocrinology Press: Bristol, UK, 1997; pp. 267–278. [Google Scholar]
- Wingfield, J.C.; Ramenofsky, R. Hormones and the behavioral ecology of stress. In Stress Physiology in Animals; Balm, P.H.M., Ed.; CRC Press: Boca Raton, FL, USA, 1999; pp. 1–51. [Google Scholar]
- Landys, M.M.; Ramenofsky, M.; Wingfield, J.C. Actions of glucocorticoids at a seasonal baseline as compared to stress-related levels in the regulation of periodic life processes. Gen. Comp. Endocrinol. 2006, 148, 132–149. [Google Scholar] [CrossRef] [PubMed]
- Munck, A.; Guyre, P.M.; Holbrook, N.J. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocrinol. Rev. Winter 1984, 5, 25–44. [Google Scholar] [CrossRef] [PubMed]
- Wingfield, J.C.; Kitaysky, A.S. Endocrine Responses to Unpredictable Environmental Events: Stress or Anti-Stress Hormones? Integr. Comp. Biol. 2002, 42, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Silverin, B. Corticosterone-binding proteins and behavioral effects of high plasma levels of corticosterone during the breeding period in the pied flycatcher. Gen. Comp. Endocrinol. 1986, 64, 67–74. [Google Scholar] [CrossRef]
- Cockrem, J.; Silverin, B. Variant within and between birds in corticosterone responses of great tits (Parus major). Gen. Comp. Endocrinol. 2002, 125, 197–205. [Google Scholar] [CrossRef]
- Fraisse, F.; Cockrem, J.F. Corticosterone and the measurement of stress and fear in cage housed laying chickens. Br. Poult. Sci. 2006, 47, 1–10. [Google Scholar]
- Satterlee, D.G.; Johnson, W.A. Selection of Japanese quail for contrasting blood corticosterone response to immobilization. Poult. Sci. 1986, 67, 25–32. [Google Scholar] [CrossRef]
- Goymann, W. Noninvasive monitoring of hormones in bird droppings: Physiological validation, sampling, extraction, sex differences, and the influence of diet on hormone metabolite levels. Ann. N. Y. Acad. Sci. 2005, 1046, 35–53. [Google Scholar] [CrossRef]
- Palme, R.; Rettenbacker, S.; Touma, C.; El-Bahr, S.M.; Mostl, E. Stress hormones in mammals and birds: Comparative aspects regarding metabolism, excretion, and non-invasive measurement in fecal samples. Ann. N. Y. Acad. Sci. 2006, 1040, 162–171. [Google Scholar] [CrossRef]
- Carere, C.; Groothuis, T.G.G.; Möstl, E.; Daan, S.; Koolhaas, J.M. Fecal corticosteroids in a territorial bird selected for different personalities: Daily rhythm and the response to social stress. Horm. Behav. 2003, 43, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Hirschenhauser, K.; Kotrschal, K.; Möstl, E. Synthesis of measuring steroid metabolites in goose feces. Ann. N. Y. Acad. Sci. 2005, 1046, 138–153. [Google Scholar] [CrossRef]
- Morgan, K.J. Kiwi First Aid and Veterinary Care. Department of Conservation. 2008; p. 44. Available online: https://www.doc.govt.nz/documents/science-and-technical/sap245entire.pdf (accessed on 15 February 2025).
- Sales, J. Digestive physiology and nutrition of ratites. Avian Poult. Biol. Rev. 2006, 17, 41–55. [Google Scholar] [CrossRef]
- Herd, R.M.; Dawson, T.J. Fibre digestion in the emu, Dromaius novaehollandiae, a large bird with a simple gut and high rate of passage. Physiol. Zool. 1984, 57, 70–84. [Google Scholar] [CrossRef]
- Wilson, M.F. Gut retention times of experimental pseudoseeds by emus. Biotropica 1989, 21, 210–213. [Google Scholar] [CrossRef]
- Westcott, D.A.; Bentrupperbäumer, J.; Bradford, M.G.; McKeown, A. Incorporating patterns of disperser behaviour into models of seed dispersal and its effects on estimated dispersal curves. Oecologia 2005, 146, 57–67. [Google Scholar] [CrossRef]
- Leche, A.; Vera Cortez, M.; Della Costa, N.S.; Navarro, J.L.; Marin, R.H.; Martella, M.B. Stress response assessment during translocation of captive-bred Greater Rheas into the wild. J. Ornithololgy 2016, 157, 599–607. [Google Scholar] [CrossRef]
- Stewart, J. Ratites. In Avian Medicine: Principles and Applications; Ritchie, B.W., Harrison, G.J., Harrison, L.R., Eds.; Wingers Publishing: Lake Worth, FL, USA, 1994; pp. 1284–1326. [Google Scholar]
- Watson, R.; Munro, C.; Edwards, K.L.; Norton, V.; Brown, J.L.; Walker, S.L. Development of a versatile enzyme immunoassay for non-invasive assessment of glucocorticoid metabolites in a diversity of taxonomic species. Gen. Comp. Endocrinol. 2013, 186, 16–24. [Google Scholar] [CrossRef]
- Wasser, S.K.; Hunt, K.E.; Brown, J.L.; Cooper, K.; Crockett, C.M.; Bechert, U.; Millspaugh, J.J.; Larson, S.; Monfort, S.L. A generalized fecal glucocorticoid assay for use in a diverse array of nondomestic mammalian and avian species. Gen. Comp. Endocrinol. 2000, 120, 260–275. [Google Scholar] [CrossRef]
- Donelan, E.M.; Philpott, M.P.; MacKinnon, K.M.; Klosterman, K.A.; Roth, T.L. Fecal glucocorticoid metabolite concentrations associated with illness, sex, age, and season in a kea Nestor notabiliis population at the Cincinnati Zoo and Botanical Garden. J. Zoo Aquar. Res. 2022, 10, 107–114. [Google Scholar]
- Brown, J.L.; Wildt, D.E.; Wielebnowski, N.; Goodrowe, K.L.; Graham, L.H.; Wells SHoward, J.G. Reproductive activity in captive female cheetahs (Acinonyx jubatus) assessed by faecal steroids. J. Reprod. Fertil. 1996, 106, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Fanson, B.; Fanson, K. hormlong: Longitudinal Analysis of Hormone Data. R Package, Version 2.13.2. 2014. Available online: https://www.researchgate.net/publication/308965751_hormLong_An_R_package_for_longitudinal_data_analysis_in_wildlife_endocrinology_studies (accessed on 15 May 2024).
- Greenhouse, S.W.; Geisser, S. On the methods in the analysis of profile data. Psychometrika 1959, 24, 95–112. [Google Scholar] [CrossRef]
- Dunn, O.J. Multiple comparisons using rank sums. Technometrics 1964, 6, 241–252. [Google Scholar] [CrossRef]
- Cockrem, J.; Adams, D.; Bennett, E.; Candy, E.; Henare, S.; Hawke, E.; Potter, M. Endocrinology and the conservation of New Zealand birds. In Experimental Approaches to Conservation Biology; Gordon, M.S., Bartol, S.M., Eds.; University of California Press: Los Angeles, CA, USA, 2004; pp. 101–121. [Google Scholar]
- Hartell-DeNardo, J.; Kozlowski, C.; Baskir, E.; Macek, M.; Dorsey, C.; Powell, D.M. Behavior and adrenal physiology of Magellanic penguins (Spheniscus magellanicus) serving as ambassador animals. Zoo Biol. 2023, 42, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.F. Habituation: A history. Neurobiol. Learn. Mem. 2009, 2, 127–134. [Google Scholar] [CrossRef]
- Dissegna, A.; Turatto, M.; Chiandetti, C. Context-specific habituation: A review. Animals 2021, 11, 1767. [Google Scholar] [CrossRef]
- McDiarmid, T.A.; Yu, A.J.; Rankin, C.H. Habituation Is More Than Learning to Ignore: Multiple Mechanisms Serve to Facilitate Shifts in Behavioral Strategy. Bioessays 2019, 41, 1900077. [Google Scholar] [CrossRef]
- Chiandetti, C.; Turatto, M. Context-specific habituation of the freezing response in newborn chicks. Behav. Neurosci. 2017, 131, 437. [Google Scholar] [CrossRef]
- Scheun, J.; Miller, R.J.; Ganswindt, A.; Waller, L.J.; Pichegru, L.; Sherley, R.B.; Maneveldt, G.W. Urofaecal glucocorticoid metabolite concentrations in African penguin (Sheniscus demersus) chick populations experiencing different levels of human disturbance. Conserv. Physiol. 2021, 9, coab078. [Google Scholar] [CrossRef]
- Oster, H.; Challet, E.; Ott, V.; Arvat, E.; de Kloet, E.R.; Dijk, D.; Lightman, S.; Vgontzas, A.; Van Cauter, E. The functional and clinical significance of the 24-h rhythm of circulating glucocorticoids. Endocr. Rev. 2017, 38, 3–45. [Google Scholar] [CrossRef] [PubMed]
- Spiga, F.; Walker, J.J.; Terry, J.R.; Lightman, S.L. HPA axis-rhythms. Compr. Physiol. 2014, 4, 1273–1298. [Google Scholar] [CrossRef]
- Barbosa-Moyano, H.; Sobral, G.; de Oliveira, C.A. Glucocorticoid metabolites in an ex-situ nocturnal bird, the tropical screech owl Megascops choliba: Effects of sex, activity period, and inter-individual variation. Conserv. Physiol. 2023, 11, coad016. [Google Scholar] [CrossRef] [PubMed]
- Dickens, M.J.; Delehanty, D.J.; Romero, L.M. Stress and translocation: Alterations in the stress physiology of translocated birds. Proceedings. Biol. Sci. 2009, 276, 2051–2056. [Google Scholar] [CrossRef]
- Williams-Kelly, K.S.; Berry, L.; Branch, K.; Cowen, S.; Garretson, S.; Holland, G.J.; Ladd, R.; Olds, L.; Rayner, K.; Sims, C.; et al. Physiological response after translocation differs between source populations in a threatened mammal. R. Soc. Open Sci. 2023, 10, 230836. [Google Scholar] [CrossRef]
- Injaian, A.S.; Francis, C.D.; Ouyang, J.Q.; Dominoni, D.M.; Donald, J.W.; Fuxjager, M.J.; Goymann, W.; Hau, M.; Husak, J.F.; Johnson, M.A.; et al. Baseline and stress-induced corticosterone levels across birds and reptiles do not reflect urbanization levels. Conserv. Physiol. 2020, 8, coz110. [Google Scholar] [CrossRef] [PubMed]
- Jakob-Hoff, R.; Kingan, M.; Chiaki, F.; Schmid, G.; Cockrem, J.F.; Crackle, A.; Van Bemmel, E.; Connor, R.; Descovich, K. Potential Impact of Construction Noise on Selected Zoo Animals. Hum. Influ. Behav. Welf. Zoo Anim. 2019, 9, 504. [Google Scholar] [CrossRef]
- Kleist, N.J.; Guralnick, R.P.; Cruz, A.; Lowry, C.A.; Francis, C.D. Chronic anthropogenic noise disrupts glucocorticoid signaling and has multiple effects on fitness in an avian community. Proc. Natl. Acad. Sci. USA 2018, 115, E648–E657. [Google Scholar] [CrossRef]
- Adams, D.C. Corticosterone Responses of Captive and Wild Brown Kiwi (Apteryx Mantelli): A Thesis Presented in Partial Fulfillment of the Requirements for the Degree of Master of Science in Zoology. Master’s of Science, Massey University, Palmerston North, New Zealand, 2000. [Google Scholar]
- Crossin, G.T.; Trathan, P.N.; Phillips, R.A.; Gorman, K.B.; Dawson, A.; Sakamoto, K.Q.; Williams, T.D. Corticosterone predicts foraging behavior and parental care in Macaroni Penguins. Am. Nat. 2012, 180, E31–E41. [Google Scholar] [CrossRef]
- Angelier, F.; Shaffer, S.A.; Weimerskirch, H.; Trouve, C.; Chastel, O. Corticosterone and foraging behavior in a pelagic seabird. Physiol. Biochem. Zool. 2007, 80, 257–344. [Google Scholar] [CrossRef]
- Rose, P.; Freeman, M.; Hickey, I.; Kelly, R.; Greenwell, P. Considering what animals “need to do” in enclosure design: Questions for bird flight and aviaries. Birds 2024, 5, 586–603. [Google Scholar] [CrossRef]


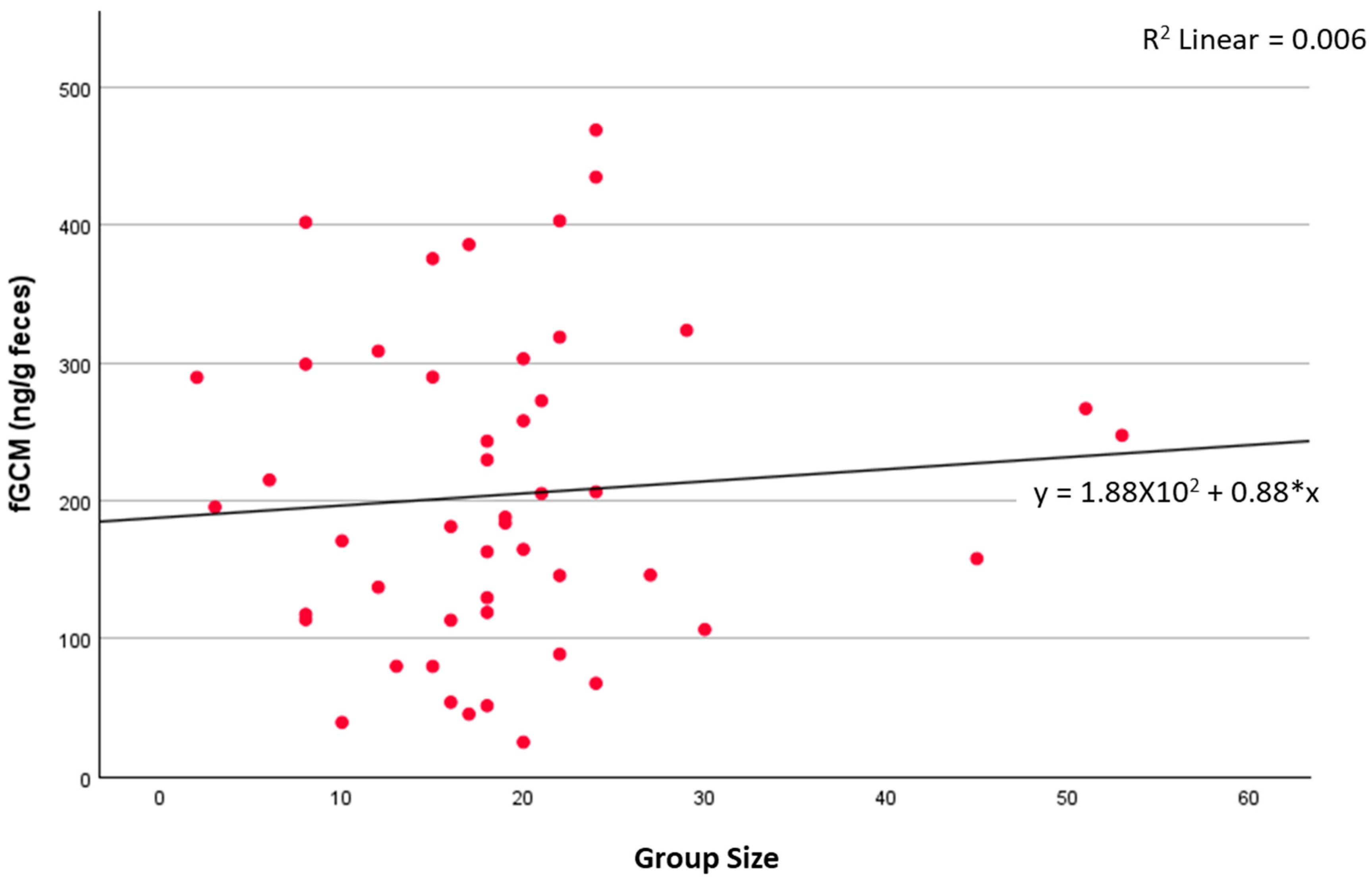

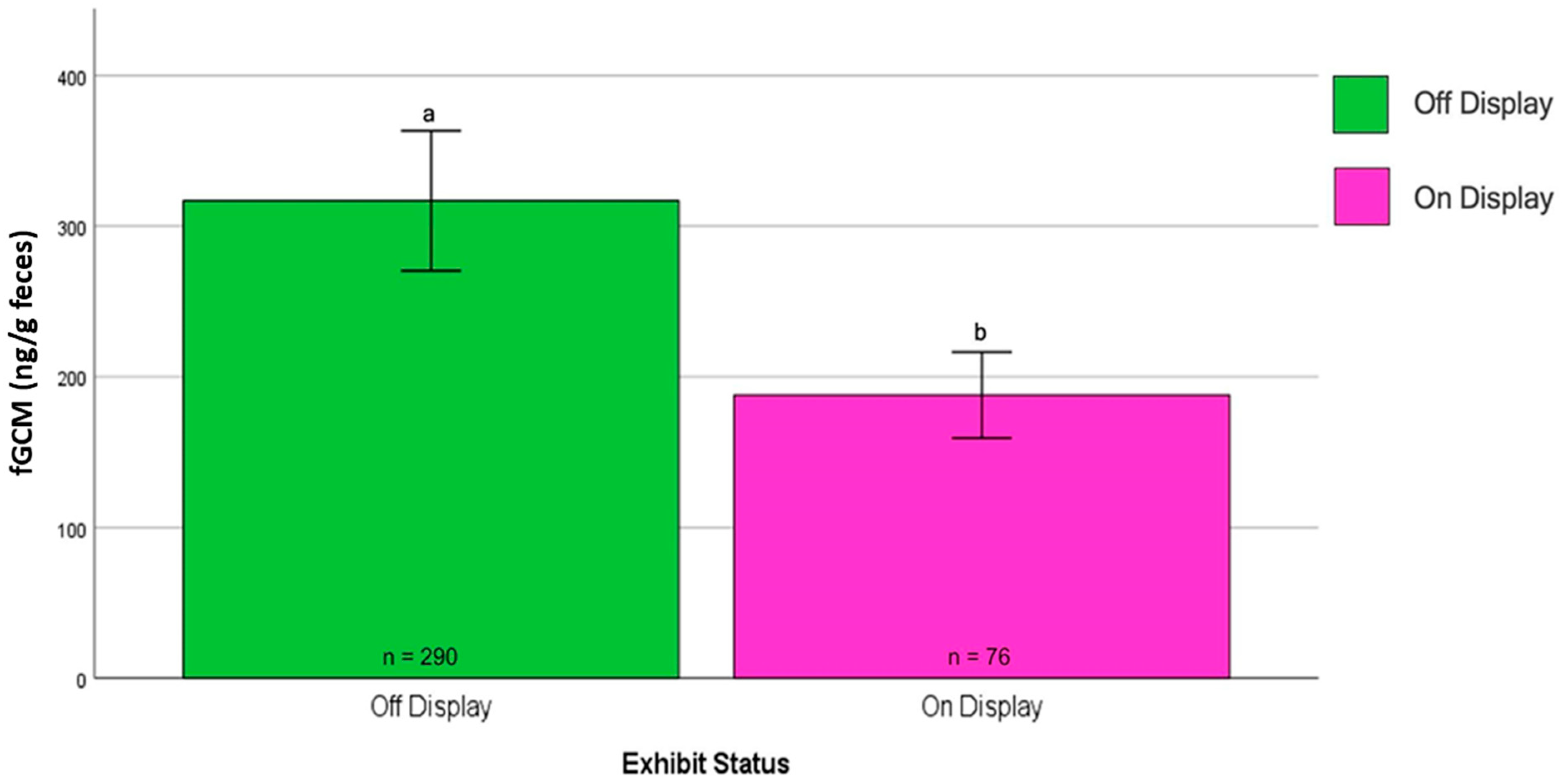


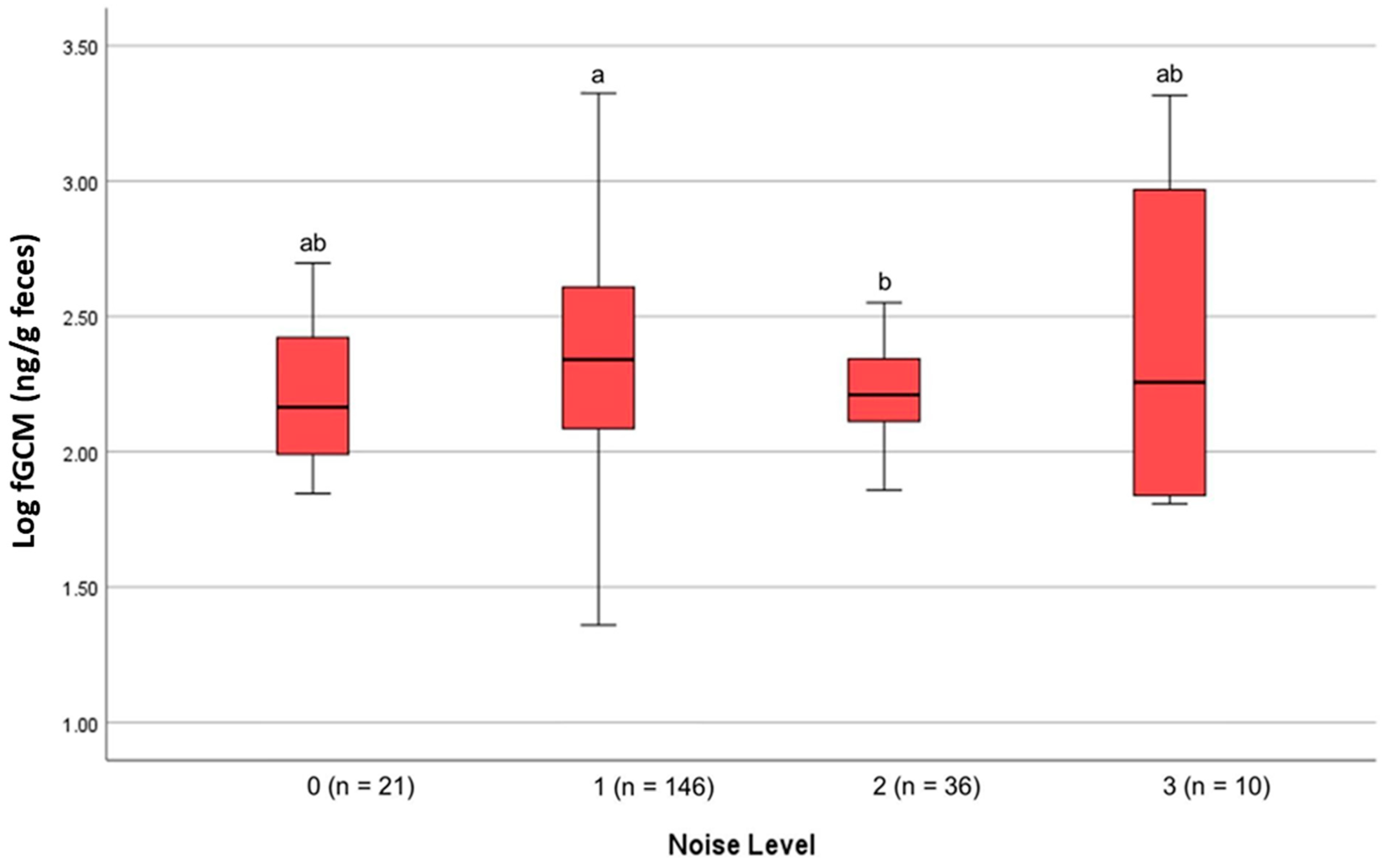
| Bird ID | Bird Type | Age | Sex | Hatch | Housing | Exhibit Status | Collection Dates | Translocated in 2017 | Sample Total |
|---|---|---|---|---|---|---|---|---|---|
| NZP#1 | Ambassador | 5 | M | Captive | Outdoors | Off | 14 March 2016–1 August 2016, 8 May 2017–7 July 2017 | No | 221 |
| NZP#2 | Control | 32 | M | Wild | Outdoors | Off | 14 March 2016–29 July 2016, 8 May 2017–16 June 2017 | Yes | 49 |
| NZP#3 | Control | 42 | M | Captive | Indoors | On | 14 March 2016–29 July 2016, 8 May 2017–30 June 2017 | Yes | 76 |
| SCBI#1 | Ambassador | 46 | M | Wild | Outdoors | Off | 25 April 2016–31 July 2016, 17 September 2016–14 October 2016 | No | 229 |
| SCBI#2 | Control | 4 | F | Captive | Outdoors | Off | 25 April 2016–29 July 2016 | No | 40 |
| Noise Level | Definition | Number of Samples |
|---|---|---|
| 0 | No discernable noise | 21 |
| 1 | Low noise, such as talking or traffic noise from a road | 146 |
| 2 | Loud noise, such as yelling, clapping, or music | 36 |
| 3 | Very loud noise, such as noisy equipment, mowing, leaf blowing, banging on glass, electric hedge trimmers, or generators | 10 |
| Bird ID | Age | Sample Type | Bird Type | n | Mean (ng/mg Dry Feces) | Median (ng/mg Dry Feces) | SEM | Percent CV | Min (ng/mg Dry Feces) | Max (ng/mg Dry Feces) | Baseline (ng/mg Dry Feces) | Base Mean (ng/mg Dry Feces) | Peak Mean (ng/mg Dry Feces) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NZP#1 | AM | Ambassador | 108 | 205.46 | 184.19 b | 12.15 | 61.48 | 17.72 | 575.52 | 171.52 | 104.54 | 299.17 | |
| NZP#1 | 5 | PM | 82 | 179.52 | 139.06 | 15.65 | 78.93 | 5.48 | 796.17 | 144.34 | 87.4 | 276.25 | |
| NZP#1 | Demo | 31 | 133.92 | 115.53 | 13.81 | 57.43 | 22.89 | 322.12 | 123.72 | 74.44 | 197.36 | ||
| NZP#2 | 32 | AM | Control | 49 | 880.86 | 734.95 a | 99.56 | 79.12 | 125.88 | 3650.29 | 618.85 | 380.74 | 1288.37 |
| NZP#3 | 42 | AM | Control | 76 | 187.85 | 155.71 b | 14.35 | 66.60 | 35.36 | 884.21 | 167.97 | 116.02 | 281.44 |
| SCBI#1 | AM | Ambassador | 108 | 434.30 | 347.87 | 25.54 | 61.10 | 118.34 | 1413.83 | 377.08 | 266.75 | 678.01 | |
| SCBI#1 | 46 | PM | 76 | 566.53 | 463.97 | 46.34 | 71.30 | 130.92 | 2092.01 | 283.47 | 213.49 | 701.33 | |
| SCBI#1 | Demo | 45 | 486.12 | 459.94 | 43.28 | 59.72 | 21.51 | 1347.93 | 542.22 | 331.74 | 827.94 | ||
| SCBI#2 | 4 | AM | Control | 40 | 503.87 | 471.01 | 46.22 | 58.01 | 125.75 | 1283.57 | 497.51 | 317.58 | 783.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brader, K.; Prado, N.A.; Brown, J.L.; Kearney, M.; Boisseau, N.; Ware, L.; Delaski, K.M.; Bailey, W. Fecal Glucocorticoid Metabolite Responses of Brown Kiwi (Apteryx mantelli) to Ambassador Program Participation and Translocation: Implications for Captive Management and Welfare. Animals 2025, 15, 1156. https://doi.org/10.3390/ani15081156
Brader K, Prado NA, Brown JL, Kearney M, Boisseau N, Ware L, Delaski KM, Bailey W. Fecal Glucocorticoid Metabolite Responses of Brown Kiwi (Apteryx mantelli) to Ambassador Program Participation and Translocation: Implications for Captive Management and Welfare. Animals. 2025; 15(8):1156. https://doi.org/10.3390/ani15081156
Chicago/Turabian StyleBrader, Kathleen, Natalia A. Prado, Janine L. Brown, Mary Kearney, Nicole Boisseau, Lisa Ware, Kristina M. Delaski, and Wesley Bailey. 2025. "Fecal Glucocorticoid Metabolite Responses of Brown Kiwi (Apteryx mantelli) to Ambassador Program Participation and Translocation: Implications for Captive Management and Welfare" Animals 15, no. 8: 1156. https://doi.org/10.3390/ani15081156
APA StyleBrader, K., Prado, N. A., Brown, J. L., Kearney, M., Boisseau, N., Ware, L., Delaski, K. M., & Bailey, W. (2025). Fecal Glucocorticoid Metabolite Responses of Brown Kiwi (Apteryx mantelli) to Ambassador Program Participation and Translocation: Implications for Captive Management and Welfare. Animals, 15(8), 1156. https://doi.org/10.3390/ani15081156






