Dysmagnesemia Incidence in Hospitalized Dogs and Cats: A Retrospective Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Quality Control Procedures
2.2. Statistical Analysis
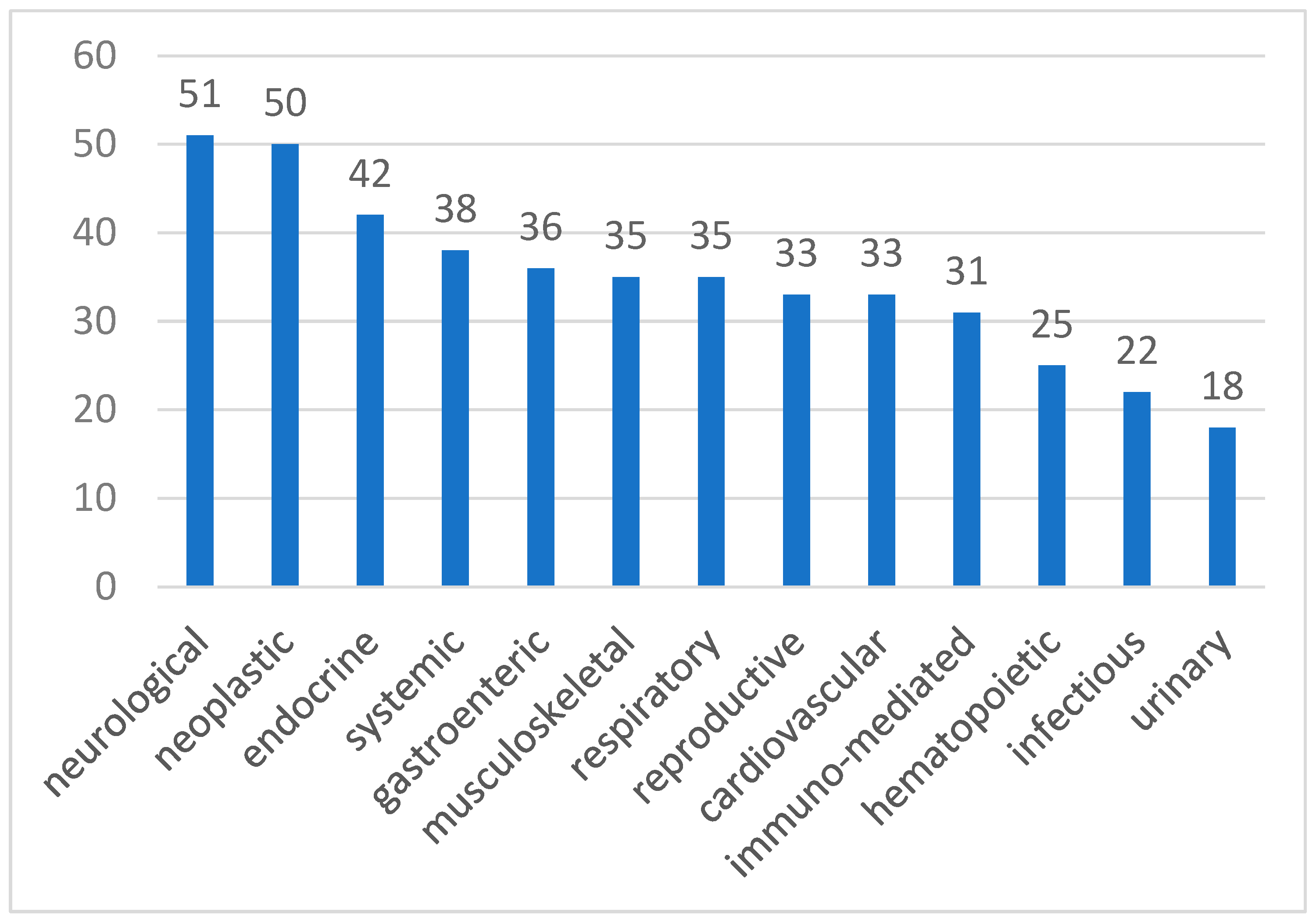
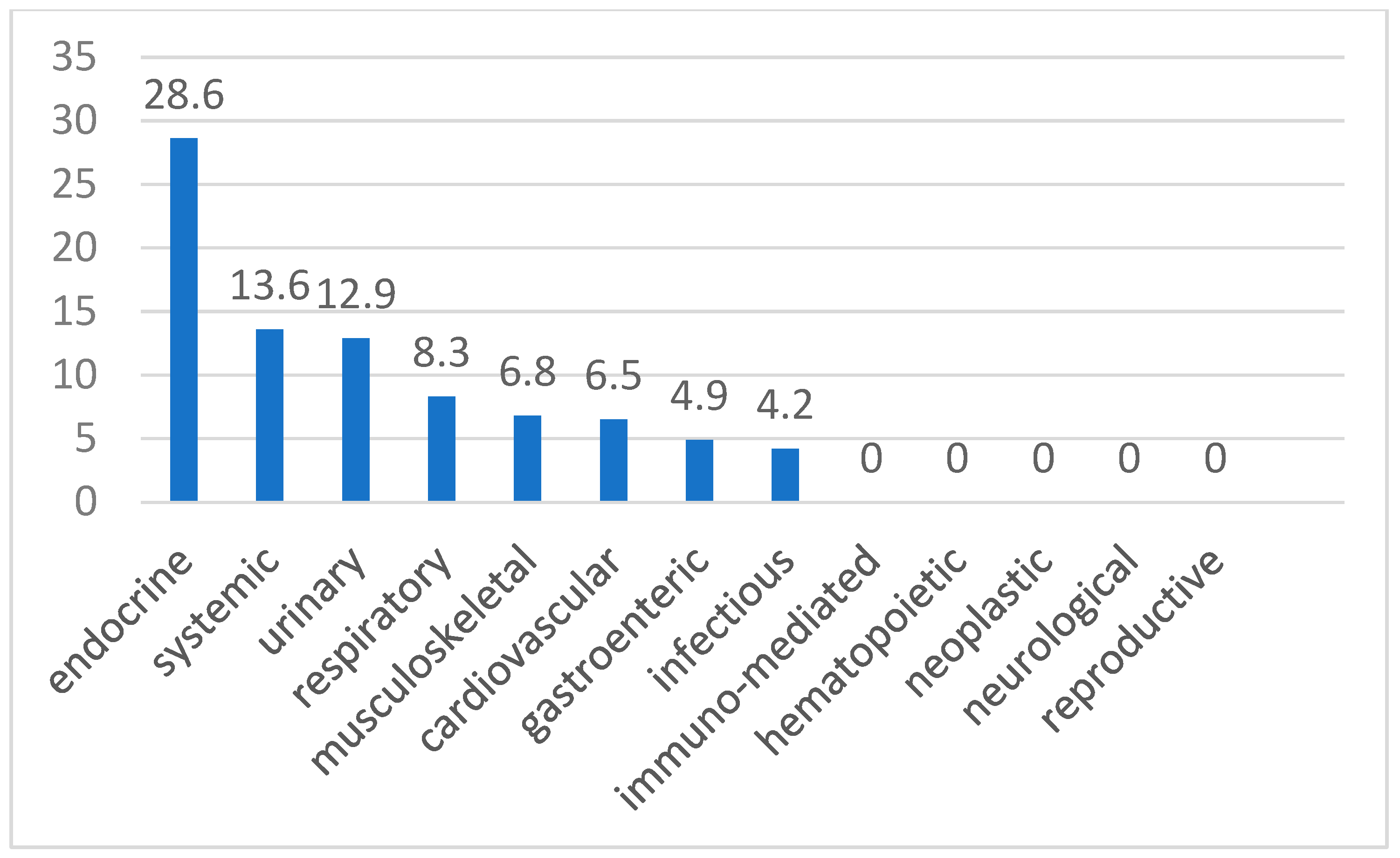
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bateman, S.W. Disorders of magnesium: Magnesium deficit and excess. In Fluid, Electrolyte and Acid-Base Disorders in Small Animal Practice, 4th ed.; DiBartola, S.P., Ed.; Elsevier: St. Louis, MO, USA, 2012; pp. 212–229. [Google Scholar]
- Toll, J.; Erb, H.; Birnbaum, N.; Schermerhorn, T. Prevalence and incidence of serum magnesium abnormalities in hospitalized cats. J. Vet. Intern. Med. 2002, 16, 217–221. [Google Scholar] [CrossRef]
- Khanna, C.; Lund, E.M.; Raffe, M.; Armstrong, P.J. Hypomagnesemia in 188 dogs: A hospital population-based prevalence study. J. Vet. Intern. Med. 1998, 12, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.G.; Matteson, V.L.; Wingfield, W.E.; van Pelt, D.R.; Hackett, T.B. Abnormalities of serum magnesium in critically ill dogs: Incidence and implications. J. Vet. Emerg. Crit. Care 1994, 4, 15–20. [Google Scholar] [CrossRef]
- Murray, M.E.; Boiron, L.; Buriko, Y.; Drobatz, K.; Waddell, L.S. Total serum and ionized magnesium concentrations in healthy and hospitalized dogs. J. Vet. Emerg. Crit. Care 2023, 33, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Huijgen, H.J.; Soesan, M.; Sanders, R.; Mairuhu, W.M.; Kesecioglu, J.; Sanders, G.T. Magnesium levels in critically ill patients. What should we measure? Am. J. Clin. Pathol. 2000, 114, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Yeh, D.D.; Chokengarmwong, N.; Chang, Y.; Yu, L.; Arsenault, C.; Rudolf, J.; Lee-Lewandrowski, E.; Lewandrowski, K. Total and ionized magnesium testing in the surgical intensive care unit—Opportunities for improved laboratory and pharmacy utilization. J. Crit. Care 2017, 42, 147–151. [Google Scholar] [CrossRef]
- Scarpati, G.; Baldassarre, D.; Oliva, F.; Pascale, G.; Piazza, O. Ionized or Total Magnesium levels, what should we measure in critical ill patients? Transl. Med. UniSa 2020, 23, 68–76. [Google Scholar] [CrossRef]
- Norris, C.R.; Nelson, R.W.; Christopher, M.M. Serum total and ionized magnesium concentrations and urinary fractional excretion of magnesium in cats with diabetes mellitus and diabetic ketoacidosis. J. Am. Vet. Med. Assoc. 1999, 215, 1455–1459. [Google Scholar] [CrossRef]
- Kumar, S.; Jain, S.; Agrawal, S.; Honmode, A. Impact of serum magnesium levels in critically ill elderly patients-a study in a rural teaching hospital. J. Clin. Geront and Ger. 2016, 7, 104–108. [Google Scholar] [CrossRef]
- Thongprayoon, C.; Sy-Go, J.P.T.; Nissaisorakarn, V.; Dumancas, C.Y.; Keddis, M.T.; Kattah, A.G.; Pattharanitima, P.; Vallabhajosyula, S.; Mao, M.A.; Qureshi, F.; et al. Machine Learning Consensus Clustering Approach for Hospitalized Patients with Dysmagnesemia. Diagnostics 2021, 11, 2119. [Google Scholar] [CrossRef]
- Al Alawi, A.M.; Berhane, T.; Majoni, S.W.; Falhammar, H. Characteristics and health outcomes of patients hospitalised with hypomagnesaemia: A retrospective study from a single centre in the Northern Territory of Australia. Intern. Med. J. 2022, 52, 1544–1553. [Google Scholar] [CrossRef]
- Al Shukri, Z.; Al-Maqbali, J.S.; Al Alawi, A.M.; Al Riyami, N.; Al Riyami, S.; Al Alawi, H.; Al Farai, Q.; Falhammar, H. Incidence of Dysmagnesemia among Medically Hospitalized Patients and Associated Clinical Characteristics: A Prospective Cohort Study. Int. J. Endocrinol. 2023, 4, 6650620. [Google Scholar] [CrossRef] [PubMed]
- Upala, S.; Jaruvongvanich, V.; Wijarnpreecha, K.; Sanguankeo, A. Hypomagnesemia and mortality in patients admitted to intensive care unit: A systematic review and meta-analysis. QJM 2016, 109, 453–459. [Google Scholar] [CrossRef]
- Shankar, P.; Reddy, V.M.; Devaraj, S. Hypomagnesemia as a predictor of mortality in critically ill pediatric patients in picu using prism score. Intern. J. Cont. Ped 2019, 6, 1294. [Google Scholar] [CrossRef]
- Gautam, S.; Khapunj, A. Prevalence of hypomagnesemia among elderly patients attending a tertiary care centre: A descriptive cross-sectional study. JNMA 2021, 59, 35–38. [Google Scholar] [CrossRef]
- Schwalfenberg, G.K.; Genuis, S.J. The Importance of Magnesium in Clinical Healthcare. Scientifica 2017, 2017, 4179326. [Google Scholar] [CrossRef]
- Solomon, R. The relationship between disorders of K+ and Mg2+ homeostasis. Semin. Nephrol. 1987, 7, 253–262. [Google Scholar]
- Lu, Z.; MacKinnon, R. Electrostatic tuning of Mg2+ affinity in an inward-rectifier K+ channel. Nature 1994, 371, 243–246. [Google Scholar] [CrossRef]
- Huang, C.L.; Kuo, E. Mechanism of hypokalemia in magnesium deficiency. J. Am. Soc. Nephrol. 2007, 18, 2649–2652. [Google Scholar] [CrossRef]
- Laurant, P.; Touyz, R.M. Physiological and pathophysiological role of magnesium in the cardiovascular system: Implications in hypertension. J. Hypertens. 2000, 18, 1177–1191. [Google Scholar] [CrossRef]
- DiBartola, S.P.; De Morais, H.A. Disorders of potassium: Hypokalemia and hyperkalemia. In Fluid, Electrolyte and Acid-Base Disorders in Small Animal Practice, 4th ed.; DiBartola, S.P., Ed.; Elsevier: St. Louis, MO, USA, 2012; pp. 92–119. [Google Scholar]
- Whang, R.; Whang, D.D.; Ryan, M.P. Refractory potassium repletion. A consequence of magnesium deficiency. Arch. Intern. Med. 1992, 152, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Rude, R.K. Renal cortical adenylate cyclase: Characterization of magnesium activation. Endocrinology 1983, 113, 1348–1355. [Google Scholar] [CrossRef]
- Gilman, A.G. Guanine nucleotide-binding regulatory proteins and dual control of adenylate cyclase. J. Clin. Investig. 1984, 73, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Hermans, C.; Lefebvre, C.; Devogelaer, J.P.; Lambert, M. Hypocalcaemia and chronic alcohol intoxication: Transient hypoparathyroidism secondary to magnesium deficiency. Clin. Rheumatol. 1996, 15, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Rude, R.K.; Oldham, S.B.; Singer, F.R. Functional hypoparathyroidism and parathyroid hormone end-organ resistance in human magnesium deficiency. Clin. Endocrinol. 1976, 5, 209–224. [Google Scholar] [CrossRef]
- Dai, L.J.; Ritchie, G.; Kerstan, D.; Kang, H.S.; Cole, D.E.; Quamme, G.A. Magnesium transport in the renal distal convoluted tubule. Physiol. Rev. 2001, 81, 51–84. [Google Scholar] [CrossRef]
- Gamba, G.; Friedman, P.A. Thick ascending limb: The Na+:K+:2Cl− co-transporter, NKCC2, and the calcium-sensing receptor, CaSR. Pflugers Arch. 2009, 458, 61–76. [Google Scholar] [CrossRef]
- Vink, R.; Nechifor, M. Magnesium in the Central Nervous System [Internet]; Vink, R., Nechifor, M., Eds.; University of Adelaide Press: Adelaide, AU, Australia, 2011. [Google Scholar]
- Gröber, U.; Schmidt, J.; Kisters, K. Magnesium in Prevention and Therapy. Nutrients 2015, 7, 8199–8226. [Google Scholar] [CrossRef]
- Castilho, R.F.; Ward, M.W.; Nicholls, D.G. Oxidative stress, mitochondrial function, and acute glutamate excitotoxicity in cultured cerebellar granule cells. J. Neurochem. 1999, 72, 1394–1401. [Google Scholar] [CrossRef]
- Humphrey, S.; Kirby, R.; Rudloff, E. Magnesium physiology and clinical therapy in veterinary critical care. J. Vet. Emerg. Crit. Care 2015, 25, 210–225. [Google Scholar] [CrossRef]
- Olloquequi, J.; Cornejo-Córdova, E.; Verdaguer, E.; Soriano, F.X.; Binvignat, O.; Auladell, C.; Camins, A. Excitotoxicity in the pathogenesis of neurological and psychiatric disorders: Therapeutic implications. J. Psychopharmacol. 2018, 32, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Obrenovitch, T.P.; Urenjak, J. Is high extracellular glutamate the key to excitotoxicity in traumatic brain injury? J. Neurotrauma 1997, 14, 677–698. [Google Scholar] [CrossRef] [PubMed]
- van den Heuvel, C.; Vink, R. The role of magnesium in traumatic brain injury. Clin. Calcium 2004, 14, 9–14. [Google Scholar] [PubMed]
- McIntosh, T.K.; Juhler, M.; Wieloch, T. Novel pharmacologic strategies in the treatment of experimental traumatic brain injury: 1998. J. Neurotrauma 1998, 15, 731–769. [Google Scholar] [CrossRef]
- Nayak, R.; Attry, S.; Ghosh, S.N. Serum Magnesium as a Marker of Neurological Outcome in Severe Traumatic Brain Injury Patients. Asian J. Neurosurg. 2018, 13, 685–688. [Google Scholar] [CrossRef]
- Prebble, J.J. Primary infantile hypomagnesaemia: Report of two cases. J. Paediatr. Child. Health 1995, 31, 54–56. [Google Scholar] [CrossRef]
- Unachak, K.; Louthrenoo, O.; Katanyuwong, K. Primary hypomagnesemia in Thai infants: A case report with 7 years follow-up and review of literature. J. Med. Assoc. Thai 2002, 85, 1226–1231. [Google Scholar]
- Visudhiphan, P.; Visudtibhan, A.; Chiemchanya, S.; Khongkhatithum, C. Neonatal seizures and familial hypomagnesemia with secondary hypocalcemia. Pediatr. Neurol. 2005, 33, 202–205. [Google Scholar] [CrossRef]
- Temkin, N.R.; Anderson, G.D.; Winn, H.R.; Ellenbogen, R.G.; Britz, G.W.; Schuster, J.; Lucas, T.; Newell, D.W.; Mansfield, P.N.; Machamer, J.E.; et al. Magnesium sulfate for neuroprotection after traumatic brain injury: A randomised controlled trial. Lancet Neurol. 2007, 6, 29–38. [Google Scholar] [CrossRef]
- Dhandapani, S.S.; Gupta, A.; Vivekanandhan, S.; Sharma, B.S.; Mahapatra, A.K. Randomized controlled trial of magnesium sulphate in severe closed traumatic brain injury. Ind. J. Neurotrauma 2008, 5, 27W33. [Google Scholar] [CrossRef]
- Elin, R.J. Magnesium: The fifth but forgotten electrolyte. Am. J. Clin. Pathol. 1994, 102, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, W.J.; Haxby, E.J.; Male, D.A. Magnesium: Physiology and pharmacology. Br. J. Anaesth. 1999, 83, 302–320. [Google Scholar] [CrossRef] [PubMed]
- Wolf, F.I.; Trapani, V. Cell (patho)physiology of magnesium. Clin. Sci. 2008, 114, 27–35. [Google Scholar] [CrossRef]
- Wolf, F.I.; Cittadini, A.R.; Maier, J.A. Magnesium and tumors: Ally or foe? Cancer Treat Rev. 2009, 35, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Guilbert, A.; Gautier, M.; Dhennin-Duthille, I.; Haren, N.; Sevestre, H.; Ouadid-Ahidouch, H. Evidence that TRPM7 is required for breast cancer cell proliferation. Am. J. Physiol. Cell Physiol. 2009, 297, C493–C502. [Google Scholar] [CrossRef]
- Günther, T. Comment on the number of Mg2+-activated enzymes. Magnes. Res. 2008, 21, 185–187. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Castiglioni, S.; Maier, J.A. Magnesium and cancer: A dangerous liason. Magnes. Res. 2011, 24, S92–S100. [Google Scholar] [CrossRef]
- Lajer, H.; Daugaard, G. Cisplatin and hypomagnesemia. Cancer Treat. Rev. 1999, 25, 47–58. [Google Scholar] [CrossRef]
- Gaughran, G.; Qayyum, K.; Smyth, L.; Davis, A. Carboplatin and hypomagnesemia: Is it really a problem? Asia Pac. J. Clin. Oncol. 2021, 17, 478–485. [Google Scholar] [CrossRef]
- Weglicki, W.B.; Stafford, R.E.; Dickens, B.F.; Mak, I.T.; Cassidy, M.M.; Phillips, T.M. Inhibition of tumor necrosis factor-alpha by thalidomide in magnesium deficiency. Mol. Cell Biochem. 1993, 129, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Breitschwerdt, E.B.; Meuten, D.J.; Greenfield, C.L.; Anson, L.W.; Cook, C.S.; Fulghum, R.E. Idiopathic hyperaldosteronism in a dog. J. Am. Vet. Med. Assoc. 1985, 187, 841–845. [Google Scholar] [CrossRef]
- Feldman, E.C.; Nelson, R.W. Primary mineralocorticoid excess–primary hyperaldosteronism. In Canine and Feline Endocrinology and Reproduction, 3rd ed.; Feldman, E.C., Nelson, R.W., Eds.; WB Saunders: Philadelphia, PA, USA, 2004; pp. 351–357. [Google Scholar]
- Horton, R.; Biglieri, E.G. Effect of aldosterone on the metabolism of magnesium. J. Clin. Endocrinol. Metab. 1962, 22, 1187–1192. [Google Scholar] [CrossRef]
- Chhokar, V.S.; Sun, Y.; Bhattacharya, S.K.; Ahokas, R.A.; Myers, L.K.; Xing, Z.; Smith, R.A.; Gerling, I.C.; Weber, K.T. Hyperparathyroidism and the calcium paradox of aldosteronism. Circulation 2005, 111, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, M.; Dominguez, L.J.; Galioto, A.; Ferlisi, A.; Cani, C.; Malfa, L.; Pineo, A.; Busardo’, A.; Paolisso, G. Role of magnesium in insulin action, diabetes and cardio-metabolic syndrome X. Mol. Aspects Med. 2003, 24, 39–52. [Google Scholar] [CrossRef]
- Wälti, M.K.; Zimmermann, M.B.; Walczyk, T.; Spinas, G.A.; Hurrell, R.F. Measurement of magnesium absorption and retention in type 2 diabetic patients with the use of stable isotopes. Am. J. Clin. Nutr. 2003, 78, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Gommers, L.M.; Hoenderop, J.G.; Bindels, R.J.; de Baaij, J.H. Hypomagnesemia in Type 2 Diabetes: A Vicious Circle? Diabetes 2016, 65, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Fincham, S.C.; Drobatz, K.J.; Gillespie, T.N.; Hess, R.S. Evaluation of plasma-ionized magnesium concentration in 122 dogs with diabetes mellitus: A retrospective study. J. Vet. Intern. Med. 2004, 18, 612–617. [Google Scholar] [CrossRef]
- Zofková, I.; Kancheva, R.L. The relationship between magnesium and calciotropic hormones. Magnes. Res. 1995, 8, 77–84. [Google Scholar]
- Cole, D.E.C.; Quamme, G.A. Inherited disorders of renal magnesium handling. J. Am. Soc. Nephrol. 2000, 11, 1937–1947. [Google Scholar] [CrossRef]
- Chandar, J.; Zilleruelo, G. Hypertensive crisis in children. Pediatr. Nephrol. 2012, 27, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.P.; Mitsnefes, M. Advances in the pathogenesis and management of hypertensive crisis. Curr. Opin. Pediatr. 2005, 17, 210–214. [Google Scholar] [CrossRef]
- Baracco, R.; Matoo, T.K. Pediatric hypertensive emergencies. Curr. Hypertens. Rep. 2014, 16, 456–463. [Google Scholar] [CrossRef] [PubMed]
- van den Broek, D.H.N.; Chang, Y.M.; Elliott, J.; Jepson, R.E. Prognostic importance of plasma total magnesium in a cohort of cats with azotemic chronic kidney disease. J. Vet. Intern. Med. 2018, 32, 1359–1371. [Google Scholar] [CrossRef] [PubMed]
- Al Harasi, S.; Al-Maqbali, J.S.; Falhammar, H.; Al-Mamari, A.; Al Futisi, A.; Al-Farqani, A.; Kumar, S.; Osman, A.; Al Riyami, S.; Al Riyami, N.; et al. Prevalence of Dysmagnesemia among Patients with Diabetes Mellitus and the Associated Health Outcomes: A Cross-Sectional Study. Biomedicines 2024, 12, 1068. [Google Scholar] [CrossRef]
- Swaminathan, R. Magnesium metabolism and its disorders. Clin. Biochem. Rev. 2003, 24, 47–66. [Google Scholar]
- Cascella, M.; Vaqar, S. Hypermagnesemia. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Shibutani, Y.; Yokota, T.; Iijima, S.; Fujioka, A.; Katsuno, S.; Sakamoto, K. Plasma and erythrocyte magnesium concentrations in thyroid disease: Relation to thyroid function and the duration of illness. Jpn. J. Med. 1989, 28, 496–502. [Google Scholar] [CrossRef]
- Hammond, H.K.; White, F.C.; Buxton, I.L.; Saltzstein, P.; Brunton, L.L.; Longhurst, J.C. Increased myocardial beta-receptors and adrenergic responses in hyperthyroid pigs. Am. J. Physiol. 1987, 252, H283–H290. [Google Scholar] [CrossRef]
- Walker, J.D.; Crawford, F.A.; Kato, S.; Spinale, F.G. The novel effects of 3,5,3’-triiodo-L-thyronine on myocyte contractile function and beta-adrenergic responsiveness in dilated cardiomyopathy. J. Thorac. Cardiovasc. Surg. 1994, 108, 672–679. [Google Scholar] [CrossRef]
- Celsing, F.; Blomstrand, E.; Melichna, J.; Terrados, N.; Clausen, N.; Lins, P.E.; Jansson, E. Effect of hyperthyroidism on fibre-type composition, fibre area, glycogen content and enzyme activity in human skeletal muscle. Clin. Physiol. 1986, 6, 171–181. [Google Scholar] [CrossRef]
- Quesada, A.; Sainz, J.; Wangensteen, R.; Rodriguez-Gomez, I.; Vargas, F.; Osuna, A. Nitric oxide synthase activity in hyperthyroid and hypothyroid rats. Eur. J. Endocrinol. 2002, 147, 117–122. [Google Scholar] [CrossRef] [PubMed]
- den Hollander, J.G.; Wulkan, R.W.; Mantel, M.J.; Berghout, A. Correlation between severity of thyroid dysfunction and renal function. Clin. Endocrinol. 2005, 62, 423–427. [Google Scholar] [CrossRef]
- Haro, J.M.; Sabio, J.M.; Vargas, F. Renal beta-adrenoceptors in thyroxine-treated rats. J. Endocrinol. Investig. 1992, 15, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Asmah, B.J.; Wan Nazaimoon, W.M.; Norazmi, K.; Tan, T.T.; Khalid, B.A. Plasma renin and aldosterone in thyroid diseases. Horm. Metab. Res. 1997, 29, 580–583. [Google Scholar] [CrossRef]
- Disashi, T.; Iwaoka, T.; Inoue, J.; Naomi, S.; Fujimoto, Y.; Umeda, T.; Tomita, K. Magnesium metabolism in hyperthyroidism. Endocr. J. 1996, 43, 397–402. [Google Scholar] [CrossRef]
- Geddes, R.; Aguiar, J. Feline Comorbidities: Balancing hyperthyroidism and concurrent chronic kidney disease. J. Feline Med. Surg. 2022, 24, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, R. Hypo—Hypermagnesaemia. In Oxford Textbook of Nephrology, 2nd ed.; Davison, A.M., Cameron, J.S., Gunfield, J.-P., Kerr, D.N.S., Ritz, E., Winearls, C., Eds.; Oxford University Press: Oxford, UK, 1998; pp. 271–310. [Google Scholar]
- Troìa, R.; Mascalzoni, G.; Calipa, S.; Magagnoli, I.; Dondi, F.; Giunti, M. Multiorgan dysfunction syndrome in feline sepsis: Prevalence and prognostic implication. J. Feline Med. Surg. 2019, 21, 559–565. [Google Scholar] [CrossRef]
- Wang, H.; Huang, J.; Jin, X.; Chen, C.; Zhang, A.; Wu, Y.; Chen, C. Hypermagnesaemia, but Not Hypomagnesaemia, Is a Predictor of Inpatient Mortality in Critically Ill Children with Sepsis. Dis. Markers 2022, 27, 3893653. [Google Scholar] [CrossRef]
- Clark, B.A.; Brown, R.S. Unsuspected morbid hypermagnesemia in elderly patients. Am. J. Nephrol. 1992, 12, 336–343. [Google Scholar] [CrossRef]
- Wang, M.; Tashiro, M.; Berlin, J.R. Regulation of L-type calcium current by intracellular magnesium in rat cardiac myocytes. J. Physiol. 2004, 555, 383–396. [Google Scholar] [CrossRef]
- Stippler, M.; Crago, E.; Levy, E.I.; Kerr, M.E.; Yonas, H.; Horowitz, M.B.; Kassam, A. Magnesium infusion for vasospasm prophylaxis after subarachnoid hemorrhage. J. Neurosurg. 2006, 105, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Tangvoraphonkchai, K.; Davenport, A. Magnesium and Cardiovascular Disease. Adv. Chronic Kidney Dis. 2018, 25, 251–260. [Google Scholar] [CrossRef]
- Hutter, O.F.; Kostial, K. Effect of magnesium and calcium ions on the release of acetylcholine. J. Physiol. 1954, 124, 234–241. [Google Scholar] [CrossRef]
- Mordes, J.P.; Wacker, W.E. Excess magnesium. Pharmacol. Rev. 1977, 29, 273–300. [Google Scholar] [CrossRef]
- Oren, S.; Rapoport, J.; Zlotnik, M.; Brami, J.L.; Heimer, D.; Chaimovitz, C. Extreme hypermagnesemia due to ingestion of Dead Sea water. Nephron 1987, 47, 199–201. [Google Scholar] [CrossRef] [PubMed]
- DiBartola, S.P. Disorders of sodium and water: Hypernatremia and hyponatremia. In Fluid, Electrolyte and Acid-Base Disorders in Small Animal Practice, 4th ed.; DiBartola, S.P., Ed.; Elsevier: St. Louis, MO, USA, 2012; pp. 45–79. [Google Scholar]
- Segev, G.; Livne, H.; Ranen, E.; Lavy, E. Urethral obstruction in cats: Predisposing factors, clinical, clinicopathological characteristics and prognosis. J. Feline Med. Surg. 2011, 13, 101–108. [Google Scholar] [CrossRef]
- Finco, D.R.; Cornelius, L.M. Characterization and treatment of water, electrolyte, and acid-base imbalances of induced urethral obstruction in the cat. Am. J. Vet. Res. 1977, 38, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Muller, K.M.; Burkitt-Creedon, J.M.; Epstein, S.E. Presentation Variables Associated with the Development of Severe Post-obstructive Diuresis in Male Cats Following Relief of Urethral Obstruction. Front. Vet. Sci. 2022, 9, 783874. [Google Scholar] [CrossRef]
- Nijenhuis, T.; Renkema, K.Y.; Hoenderop, J.G.; Bindels, R.J. Acid-base status determines the renal expression of Ca2+ and Mg2+ transport proteins. J. Am. Soc. Nephrol. 2006, 17, 617–626. [Google Scholar] [CrossRef]
- Paepe, D.; Daminet, S. Feline CKD: Diagnosis, staging and screening—What is recommended? J. Feline Med. Surg. 2013, 15, 15–27. [Google Scholar] [CrossRef]
- Schermerhorn, T. Understanding Diabetic Ketoacidosis. In Proceedings of the World Small Animal Veterinary Association World Congress Proceedings, Mexico City, Mexico, 11–14 May 2005. [Google Scholar]
- D’Elia, J.A.; Weinrauch, L.A. Calcium Ion Channels: Roles in Infection and Sepsis Mechanisms of Calcium Channel Blocker Benefits in Immunocompromised Patients at Risk for Infection. Int. J. Mol. Sci. 2018, 19, 2465. [Google Scholar] [CrossRef]
- Bruskiewicz, K.A.; Nelson, R.W.; Feldman, E.C.; Griffey, S.M. Diabetic ketosis and ketoacidosis in cats: 42 cases (1980–1995). J. Am. Vet Med. Assoc. 1997, 211, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Stiles, J.; Polzin, D.J.; Bistner, S.I. The prevalence of retinopathy in cats with systemic hypertension and chronic renal failure or hyperthyroidism. J. Am. Vet. Med. Assoc. 1994, 30, 564–572. [Google Scholar]
- Wong, H.; Mylrea, K.; Feber, J.; Drukker, A.; Filler, G. Prevalence of complications in children with chronic kidney disease according to KDOQI. Kidney Int. 2006, 70, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.J.; Foley, R.N.; Chavers, B.; Gilbertson, D.; Herzog, C.; Ishani, A.; Johansen, K.; Kasiske, B.L.; Kutner, N.; Liu, J.; et al. US Renal Data System 2013 Annual Data Report. Am. J. Kidney Dis. 2014, 63, A7. [Google Scholar] [CrossRef]
- Freedman, B.I.; Cohen, A.H. Hypertension-attributed nephropathy: What’s in a name? Nat. Rev. Nephrol. 2016, 12, 27–36. [Google Scholar] [CrossRef]
- Kobayashi, D.L.; Peterson, M.E.; Graves, T.K.; Lesser, M.; Nicols, C.E. Hypertension in cats with chronic renal failure or hyperthyroidism. J. Vet. Intern. Med. 1990, 4, 58–62. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Cortellini, S.; DeClue, A.E.; Giunti, M.; Goggs, R.; Hopper, K.; Menard, J.M.; Rabelo, R.C.; Rozanski, E.A.; Sharp, C.R.; Silverstein, D.C.; et al. Defining sepsis in small animals. J. Vet Emerg. Crit. Care 2024, 34, 97–109. [Google Scholar] [CrossRef]
- Mazzaferro, E. Magnesium Disorders. In Five Minute Veterinary Consult Clinical Companion Small Animal Emergency and Critical Care, 2nd ed.; Wiley Blackwell, Ed.; Wiley Blackwell: Ames, IA, USA, 2010; pp. 453–458. [Google Scholar]
| Parameter | Mean Value |
|---|---|
| Mg2+ | 0.44 ± 0.05 mmol/L |
| pH | 7.39 ± 0.06 |
| Na+ | 141.72 ± 6.4 mmol/L |
| Ca2+ | 1.27 ± 0.12 mmol/L |
| K+ | 3.99 ± 0.54 mmol/L |
| BUN | 18.15 ± 9.79 mg/dL |
| Creatinine | 1.39 ± 1.58 mg/dL |
| SAP | 149 ± 22 mmHg |
| MAP | 113 ± 17 mmHg |
| Parameter | Mean Value |
|---|---|
| Mg2+ | 1.2 ± 0.5 mmol/L |
| pH | 7.18 ± 0.22 |
| Na+ | 147.17 ± 9.27 mmol/L |
| Ca2+ | 1.19 ± 1.44 mmol/L |
| K+ | 4.85 ± 2 mmol/L |
| BUN | 89.52 ± 79.7 mg/dL |
| Creatinine | 3.54 ± 3.52 mg/dL |
| SAP | 160 ± 31 mmHg |
| MAP | 123 ± 30 mmHg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perini, F.; Di Franco, C.; Briganti, A. Dysmagnesemia Incidence in Hospitalized Dogs and Cats: A Retrospective Study. Animals 2025, 15, 1169. https://doi.org/10.3390/ani15081169
Perini F, Di Franco C, Briganti A. Dysmagnesemia Incidence in Hospitalized Dogs and Cats: A Retrospective Study. Animals. 2025; 15(8):1169. https://doi.org/10.3390/ani15081169
Chicago/Turabian StylePerini, Francesca, Chiara Di Franco, and Angela Briganti. 2025. "Dysmagnesemia Incidence in Hospitalized Dogs and Cats: A Retrospective Study" Animals 15, no. 8: 1169. https://doi.org/10.3390/ani15081169
APA StylePerini, F., Di Franco, C., & Briganti, A. (2025). Dysmagnesemia Incidence in Hospitalized Dogs and Cats: A Retrospective Study. Animals, 15(8), 1169. https://doi.org/10.3390/ani15081169






