Metabolomics-Based Analysis of Adaptive Mechanism of Eleutheronema tetradactylum to Low-Temperature Stress
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. The Experimental Animals
2.2. Experimental Procedures and Samples Collection
2.3. Histological Observation
2.4. Determination of Antioxidant and Immune Enzyme Activities
2.5. Liver Sample Collection and Preparation for LC-MS
2.6. LC-MS/MS Analysis
2.7. LC–MS Data Processing
2.8. Metabolite Identification and Pathway Analysis
3. Results
3.1. Histopathological Changes in the Liver Following Hypothermic Stress
3.2. Changes in Antioxidant Enzyme Activity in E. tetradactylum Liver
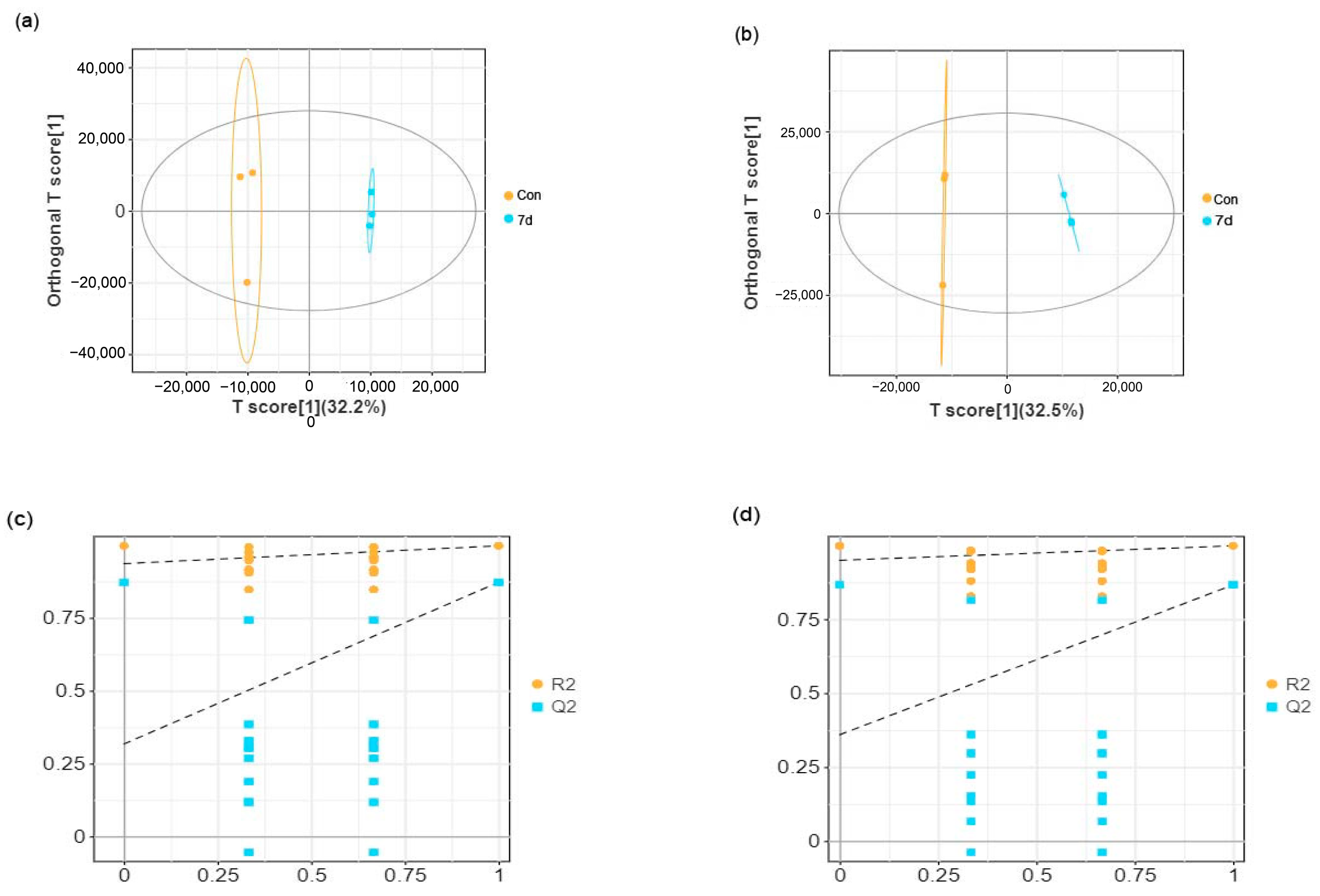
3.3. Effects of Low Temperature on Metabolomic Alterations in Liver Samples
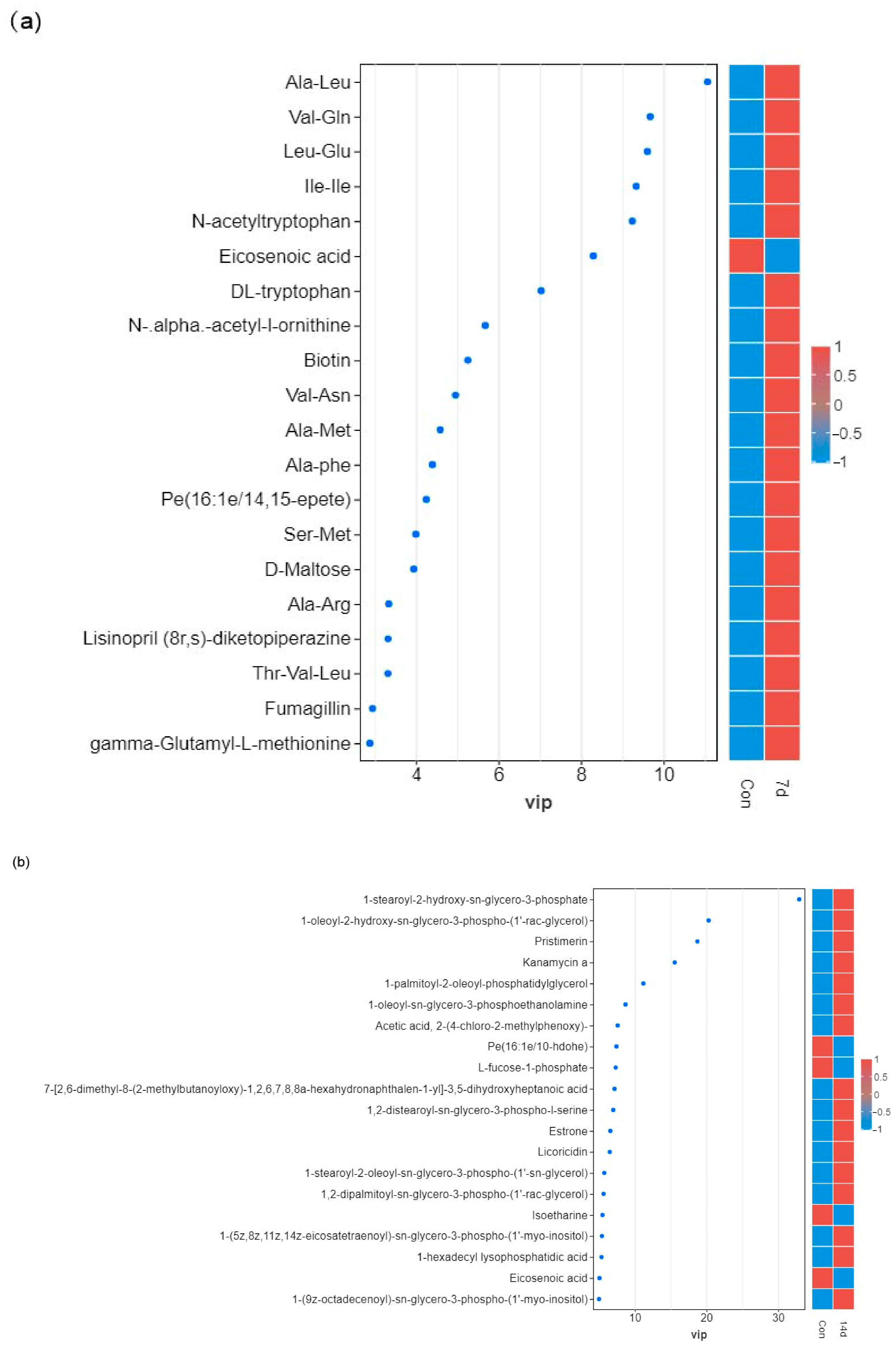
3.4. Identification of Differential Metabolites
3.5. Metabolic Pathway Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mahanty, A.; Mohanty, S.; Mohanty, B.P. Dietary Supplementation of Curcumin Augments Heat Stress Tolerance through Upregulation of Nrf-2-Mediated Antioxidative Enzymes and Hsps in Puntius sophore. Fish Physiol. Biochem. 2017, 43, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, S.; Gesto, M.; Sadoul, B. Temperature Increase and Its Effects on Fish Stress Physiology in the Context of Global Warming. J. Fish Biol. 2020, 98, 1496–1508. [Google Scholar] [CrossRef]
- Lazoglou, G.; Anagnostopoulou, C.; Tolika, K.; Kolyva-Machera, F. A Review of Statistical Methods to Analyze Extreme Precipitation and Temperature Events in the Mediterranean Region. Theor. Appl. Climatol. 2018, 136, 99–117. [Google Scholar] [CrossRef]
- Fadhlaoui, M.; Couture, P. Combined Effects of Temperature and Metal Exposure on the Fatty Acid Composition of Cell Membranes, Antioxidant Enzyme Activities and Lipid Peroxidation in Yellow Perch (Perca flavescens). Aquat. Toxicol. 2016, 180, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Neuheimer, A.B.; Thresher, R.E.; Lyle, J.M.; Semmens, J.M. Tolerance Limit for Fish Growth Exceeded by Warming Waters. Nat. Clim. Change 2011, 1, 110–113. [Google Scholar] [CrossRef]
- Wu, W.; Sun, J.; Ji, H.; Yu, H.; Zhou, J. Amp-Activated Protein Kinase in the Grass Carp Ctenopharyngodon idellus: Molecular Characterization, Tissue Distribution and Mrna Expression in Response to Overwinter Starvation Stress. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2020, 246–247, 110457. [Google Scholar] [CrossRef]
- Zhang, Y.-K.; Yang, B.-K.; Zhang, C.-N.; Xu, S.-X.; Sun, P. Effects of Polystyrene Microplastics Acute Exposure in the Liver of Swordtail Fish (Xiphophorus helleri) Revealed by Lc-Ms Metabolomics. Sci. Total Environ. 2022, 850, 157772. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, C.; Fan, K.; Zhang, L.; Liu, Y.; Liu, P.-f. Metabolomics Analysis of the Effects of Temperature on the Growth and Development of Juvenile European Seabass (Dicentrarchus labrax). Sci. Total Environ. 2021, 769, 145155. [Google Scholar] [CrossRef]
- Soengas, J.L.; Song, M.; Zhao, J.; Wen, H.-S.; Li, Y.; Li, J.-F.; Li, L.-M.; Tao, Y.-X. The Impact of Acute Thermal Stress on the Metabolome of the Black Rockfish (Sebastes schlegelii). PLoS ONE 2019, 14, e0217133. [Google Scholar]
- Xuan, Z.; Wang, W.-X. Diversity of Life History and Population Connectivity of Threadfin Fish Eleutheronema tetradactylum Along the Coastal Waters of Southern China. Sci. Rep. 2023, 13, 3976. [Google Scholar] [CrossRef]
- Guo, J.-J.; Chang, P.-Y.; Lai, C.-Y.; Lin, R.-C.; Chou, C.-Y.; Chou, R.-L.; Wu, F.-C. Screening of Nutritional Additives for Cold-Induced Stress Mitigation in Fourfinger Threadfin (Eleutheronema tetradactyla). J. Taiwan Fish. Res. 2022, 30, 31–39. [Google Scholar]
- Bechmann, L.P.; Hannivoort, R.A.; Gerken, G.; Hotamisligil, G.S.; Trauner, M.; Canbay, A. The Interaction of Hepatic Lipid and Glucose Metabolism in Liver Diseases. J. Hepatol. 2012, 56, 952–964. [Google Scholar] [CrossRef]
- Zheng, J.-L.; Zhu, Q.-L.; Shen, B.; Zeng, L.; Zhu, A.-Y.; Wu, C.-W. Effects of Starvation on Lipid Accumulation and Antioxidant Response in the Right and Left Lobes of Liver in Large Yellow Croaker Pseudosciaena crocea. Ecol. Indic. 2016, 66, 269–274. [Google Scholar] [CrossRef]
- Iona, A.; Theodorou, A.; Sofianos, S.; Watelet, S.; Troupin, C.; Beckers, J.-M. Mediterranean Sea Climatic Indices: Monitoring Long-Term Variability and Climate Changes. Earth Syst. Sci. Data 2018, 10, 1829–1842. [Google Scholar] [CrossRef]
- Phornphan, P.; Panase, P.; Saenphet, S.; Saenphet, K. Histopathology and Oxidative Stress Responses of Nile Tilapia Oreochromis niloticus Exposed to Temperature Shocks. Fish. Sci. 2021, 87, 491–502. [Google Scholar]
- Sun, S.; Wu, Y.; Yu, H.; Su, Y.; Ren, M.; Zhu, J.; Ge, X. Serum Biochemistry, Liver Histology and Transcriptome Profiling of Bighead Carp Aristichthys nobilis Following Different Dietary Protein Levels. Fish Shellfish. Immunol. 2019, 86, 832–839. [Google Scholar] [CrossRef]
- Lin, Y.; Miao, L.-H.; Pan, W.-J.; Huang, X.; Dengu, J.M.; Zhang, W.-X.; Ge, X.-P.; Liu, B.; Ren, M.-C.; Zhou, Q.-L.; et al. Effect of Nitrite Exposure on the Antioxidant Enzymes and Glutathione System in the Liver of Bighead Carp, Aristichthys nobilis. Fish Shellfish. Immunol. 2018, 76, 126–132. [Google Scholar] [CrossRef]
- Li, W.; Pan, X.; Cheng, W.; Cheng, Y.; Yin, Y.; Chen, J.; Xu, G.; Xie, L. Serum Biochemistry, Histology and Transcriptomic Profile Analysis Reflect Liver Inflammation and Damage Following Dietary Histamine Supplementation in Yellow Catfish (Pelteobagrus fulvidraco). Fish Shellfish. Immunol. 2018, 77, 83–90. [Google Scholar] [CrossRef]
- Sheriff, S.A.; Sundaram, B.; Ramamoorthy, B.; Ponnusamy, P. Synthesis and in Vitro Antioxidant Functions of Protein Hydrolysate from Backbones of Rastrelliger kanagurta by Proteolytic Enzymes. Saudi J. Biol. Sci. 2014, 21, 19–26. [Google Scholar] [CrossRef]
- Rossi, A.; Bacchetta, C.; Cazenave, J. Effect of Thermal Stress on Metabolic and Oxidative Stress Biomarkers of Hoplosternum littorale (Teleostei, Callichthyidae). Ecol. Indic. 2017, 79, 361–370. [Google Scholar] [CrossRef]
- Lushchak, V.I. Environmentally Induced Oxidative Stress in Aquatic Animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Man, Z.; Hu, J.; Zhu, J.; Wang, Y.; Zhang, Y.; Li, Y.; Xu, S.; Yan, X.; Zhang, D. Transcriptome, Antioxidant Enzymes and Histological Analysis Reveal Molecular Mechanisms Responsive to Long-Term Cold Stress in Silver Pomfret (Pampus argenteus). Fish Shellfish. Immunol. 2022, 121, 351–361. [Google Scholar]
- Marutani-Hert, M.; Hunter, W.B.; David, G. Hall Gene Response to Stress in the Asian Citrus Psyllid (Hemiptera: Psyllidae). Fla. Entomol. 2010, 93, 519–525. [Google Scholar] [CrossRef]
- Pandey, S.; Parvez, S.; Sayeed, I.; Haque, R.; Bin-Hafeez, B.; Raisuddin, S. Biomarkers of Oxidative Stress: A Comparative Study of River Yamuna Fish Wallago attu (Bl. & Schn.). Sci. Total Environ. 2003, 309, 105–115. [Google Scholar]
- Jun, Q.; Yang, H.; Wang, H.; Kpundeh, M.D.; Xu, P. Interacting Effects of Water Temperature and Dietary Protein Level on Hematological Parameters in Nile Tilapia Juveniles, Oreochromis niloticus (L.) and Mortality under Streptococcus iniae Infection. Fish Shellfish. Immunol. 2013, 34, 8–16. [Google Scholar]
- Bin, W.; Jin, S.-R.; Chen, Z.-Z.; Gao, J.-Z. Physiological Responses to Cold Stress in the Gills of Discus Fish (Symphysodon aequifasciatus) Revealed by Conventional Biochemical Assays and Gc-Tof-Ms Metabolomics. Sci. Total Environ 2018, 640–641, 1372–1381. [Google Scholar]
- Shuang, J.; Nie, M.; Song, H.; Xu, D.; You, F. Physiological Responses to Cold and Starvation Stresses in the Liver of Yellow Drum (Nibea albiflora) Revealed by Lc-Ms Metabolomics. Sci. Total Environ. 2020, 715, 136940. [Google Scholar]
- Candida, F.; Disciglio, V.; Bertora, S.; Signorile, M.L.; Simone, C. Foxo3a from the Nucleus to the Mitochondria: A Round Trip in Cellular Stress Response. Cells 2019, 8, 1110. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.-L.; Ma, Q.; Wang, J.; Li, L.-Y.; Han, S.-L.; Limbu, S.M.; Li, D.-L.; Chen, L.-Q.; Zhang, M.-L.; Du, Z.-Y. Fasting Enhances Cold Resistance in Fish through Stimulating Lipid Catabolism and Autophagy. J. Physiol. 2019, 597, 1585–1603. [Google Scholar] [CrossRef]
- Riccardo, M.; Sanna, R.; Braca, A.; Bonaglini, E.; Cappuccinelli, R.; Slawski, H.; Roggio, T.; Uzzau, S.; Anedda, R. Molecular Details on Gilthead Sea Bream (Sparus aurata) Sensitivity to Low Water Temperatures from 1h Nmr Metabolomics. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2017, 204, 129–136. [Google Scholar]
- Wen, X.; Hu, Y.; Zhang, X.; Wei, X.; Wang, T.; Yin, S. Integrated Application of Multi-Omics Provides Insights into Cold Stress Responses in Pufferfish Takifugu fasciatus. BMC Genom. 2019, 20, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gerd, S.; Stremmel, W.; Probst, M.; Moehle, C.; Zahn, A.; Mauerer, R.; Langmann, T. Real-Time Reverse Transcription-Pcr Expression Profiling of the Complete Human Atp-Binding Cassette Transporter Superfamily in Various Tissues. Clin. Chem. 2003, 49, 230–238. [Google Scholar]
- Chunfa, H.; Freter, C. Lipid Metabolism, Apoptosis and Cancer Therapy. Int. J. Mol. Sci. 2015, 16, 924–949. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, A.A.; Horrocks, L.A.; Farooqui, T. Glycerophospholipids in Brain: Their Metabolism, Incorporation into Membranes, Functions, and Involvement in Neurological Disorders. Chem. Phys. Lipids 2000, 106, 1–29. [Google Scholar] [CrossRef]
- Robert, E.; Ejsing, C.S.; Antonny, B. Homeoviscous Adaptation and the Regulation of Membrane Lipids. J. Mol. Biol. 2016, 428, 4776–4791. [Google Scholar]




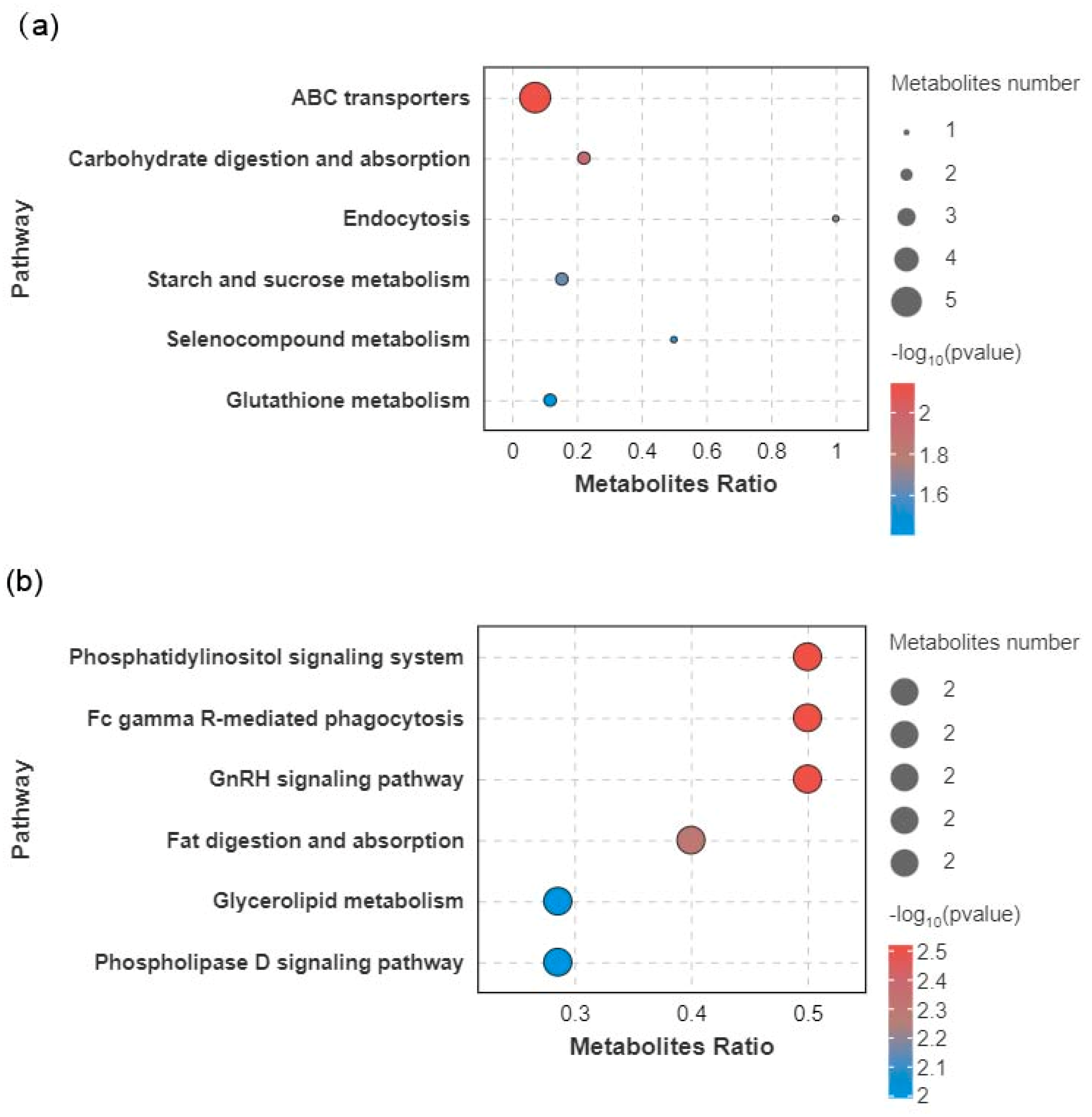
| Metabolic Pathway | In Set | In Background | p Value | SL | Metabolites |
|---|---|---|---|---|---|
| ABC transporters | 5 | 70 | 0.007104 | ** | Biotin ↑, D-Maltose ↑, Glutathione ↑, L-Valine ↑, Maltose ↑ |
| Carbohydrate digestion and absorption | 2 | 9 | 0.011049 | * | D-Maltose ↑, Maltose ↑ |
| Endocytosis | 1 | 1 | 0.018764 | * | Guanosine 5′-diphosphate ↑ |
| Starch and sucrose metabolism | 2 | 13 | 0.022904 | * | D-Maltose ↑, Maltose ↑ |
| Selenocompound metabolism | 1 | 2 | 0.037196 | * | Seleno-l-methionine ↓ |
| Glutathione metabolism | 2 | 17 | 0.03821 | * | gamma-L-Glutamyl-L-valine ↑, Glutathione ↑ |
| Metabolic Pathway | In Set | In Background | p Value | SL | Metabolites |
|---|---|---|---|---|---|
| Phosphatidylinositol signaling system | 2 | 4 | 0.002988 | ** | 1-Hexadecanoyl-2-(9Z-octadecenoyl)-sn-glycero-3-phosphoric acid ↓, 1-stearoyl-2-linoleoyl-sn-glycero-3-phosphate ↑ |
| Fc gamma R-mediated phagocytosis | 2 | 4 | 0.002988 | ** | 1-Hexadecanoyl-2-(9Z-octadecenoyl)-sn-glycero-3-phosphoric acid ↓, 1-stearoyl-2-linoleoyl-sn-glycero-3-phosphate ↑ |
| GnRH signaling pathway | 2 | 4 | 0.002988 | ** | 1-Hexadecanoyl-2-(9Z-octadecenoyl)-sn-glycero-3-phosphoric acid ↓, 1-stearoyl-2-linoleoyl-sn-glycero-3-phosphate ↑ |
| Fat digestion and absorption | 2 | 5 | 0.00491 | ** | 1-Hexadecanoyl-2-(9Z-octadecenoyl)-sn-glycero-3-phosphoric acid ↓, 1-stearoyl-2-linoleoyl-sn-glycero-3-phosphate ↑ |
| Glycerolipid metabolism | 2 | 7 | 0.010026 | * | 1-Hexadecanoyl-2-(9Z-octadecenoyl)-sn-glycero-3-phosphoric acid ↓, 1-stearoyl-2-linoleoyl-sn-glycero-3-phosphate ↑ |
| Phospholipase D signaling pathway | 2 | 7 | 0.010026 | * | 1-Hexadecanoyl-2-(9Z-octadecenoyl)-sn-glycero-3-phosphoric acid ↓, 1-stearoyl-2-linoleoyl-sn-glycero-3-phosphate ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, M.; Zheng, A.; Mkulo, E.M.; Wang, L.; Zhang, H.; Tang, B.; Zhou, H.; Wang, B.; Huang, J.; Wang, Z. Metabolomics-Based Analysis of Adaptive Mechanism of Eleutheronema tetradactylum to Low-Temperature Stress. Animals 2025, 15, 1174. https://doi.org/10.3390/ani15081174
Jin M, Zheng A, Mkulo EM, Wang L, Zhang H, Tang B, Zhou H, Wang B, Huang J, Wang Z. Metabolomics-Based Analysis of Adaptive Mechanism of Eleutheronema tetradactylum to Low-Temperature Stress. Animals. 2025; 15(8):1174. https://doi.org/10.3390/ani15081174
Chicago/Turabian StyleJin, Minxuan, Anna Zheng, Evodia Moses Mkulo, Linjuan Wang, Huijuan Zhang, Baogui Tang, Hui Zhou, Bei Wang, Jiansheng Huang, and Zhongliang Wang. 2025. "Metabolomics-Based Analysis of Adaptive Mechanism of Eleutheronema tetradactylum to Low-Temperature Stress" Animals 15, no. 8: 1174. https://doi.org/10.3390/ani15081174
APA StyleJin, M., Zheng, A., Mkulo, E. M., Wang, L., Zhang, H., Tang, B., Zhou, H., Wang, B., Huang, J., & Wang, Z. (2025). Metabolomics-Based Analysis of Adaptive Mechanism of Eleutheronema tetradactylum to Low-Temperature Stress. Animals, 15(8), 1174. https://doi.org/10.3390/ani15081174






