Environmental Gradients and Hen Spatial Distribution in a Cage-Free Aviary System: Internet of Things-Based Real-Time Monitoring for Proactive Management
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
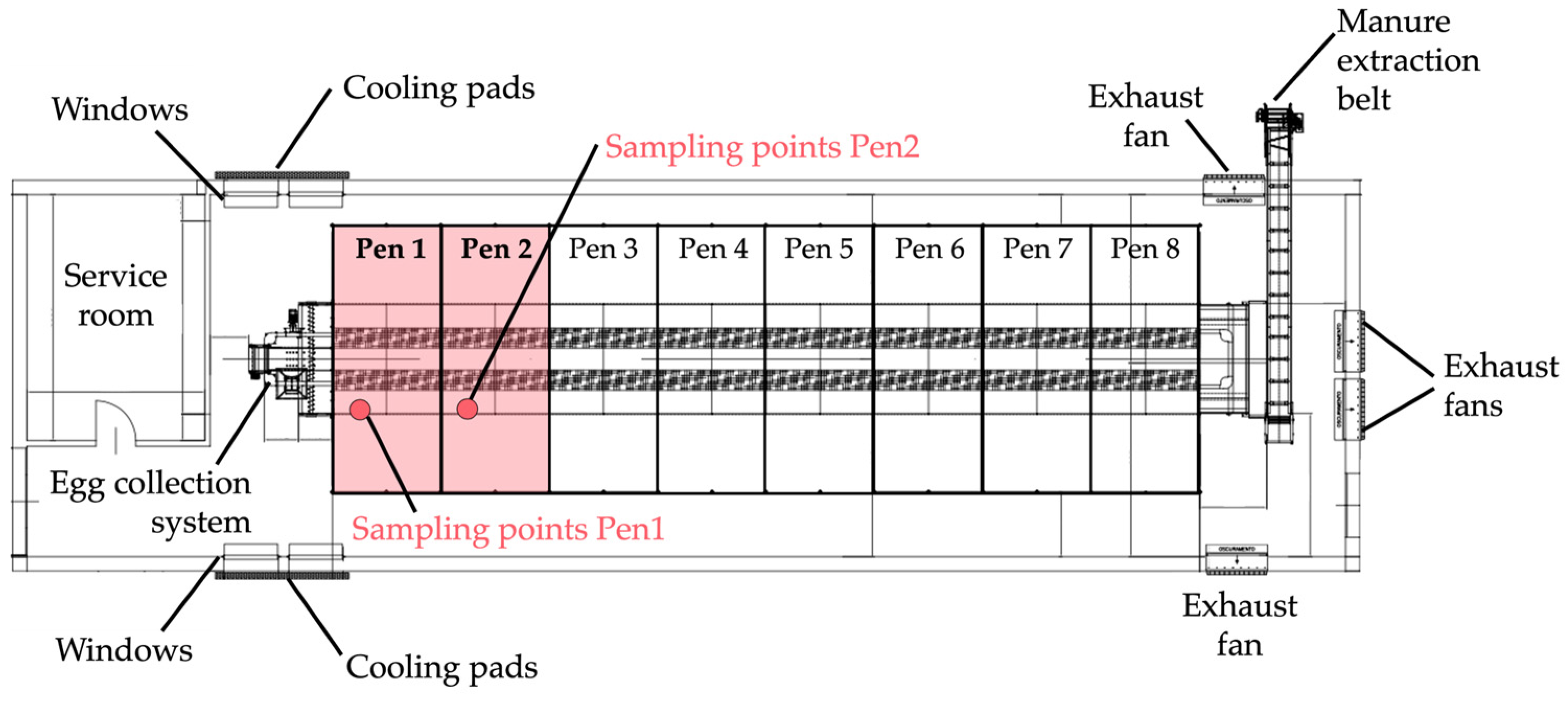
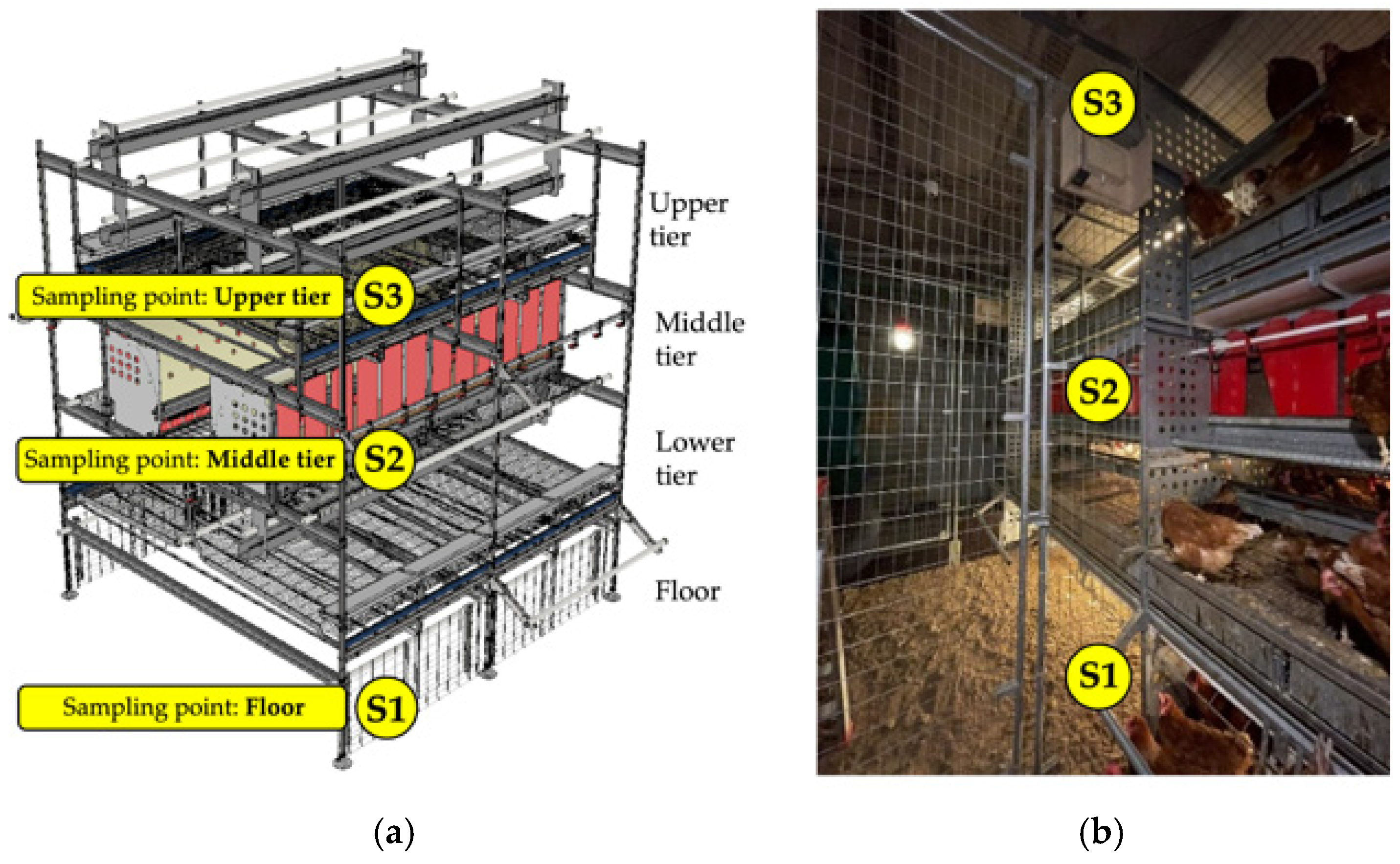
2.1. Experimental Facility, Animals, and Arrangement
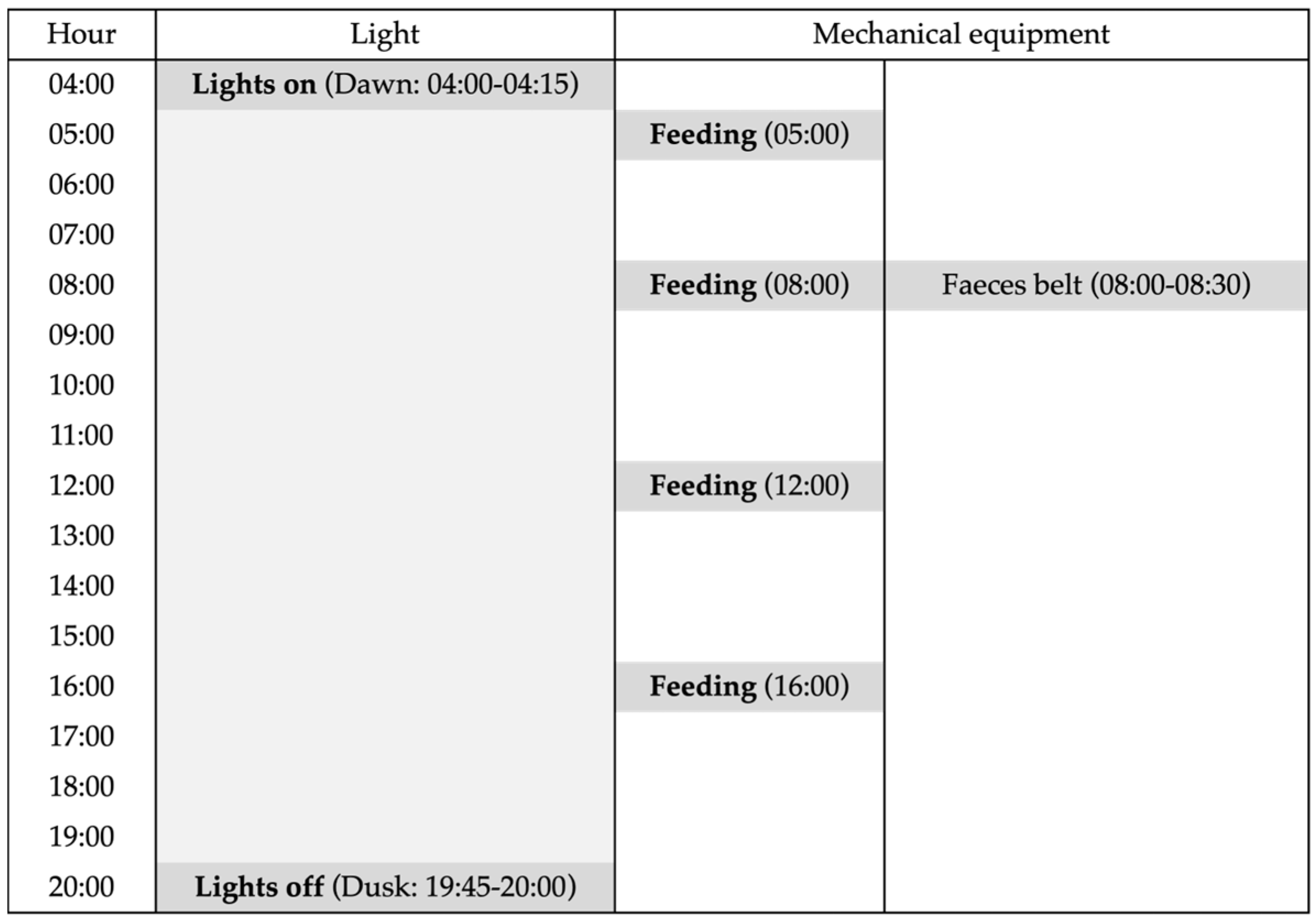
2.2. Aviary Management
2.3. Video Recording and Hen Distribution
2.4. Environmental Monitoring Through an Integrated Internet of Things System
- -
- Humidity and CO2 were measured at one-minute intervals; we collected the data and aggregated them by calculating their mean over each ten-minute window.
- -
- Ammonia (NH3) measurements were taken every 10 s, with values averaged across each corresponding 10 min segment.
- -
- Sound pressure: The sensor sampled data every 10 s. For each 10 min interval, both the maximum and average values were computed.
- -
- Particulate matter measurements were taken every 15 min, and the resulting data were transmitted at 10 min intervals. Although this strategy limited the detection of rapid fluctuations, the 15 min sampling rate was adopted to balance measurement fidelity with power efficiency, aiming to enable up to three weeks of uninterrupted operation under battery power.
2.5. Statistical Analysis
3. Results and Discussion
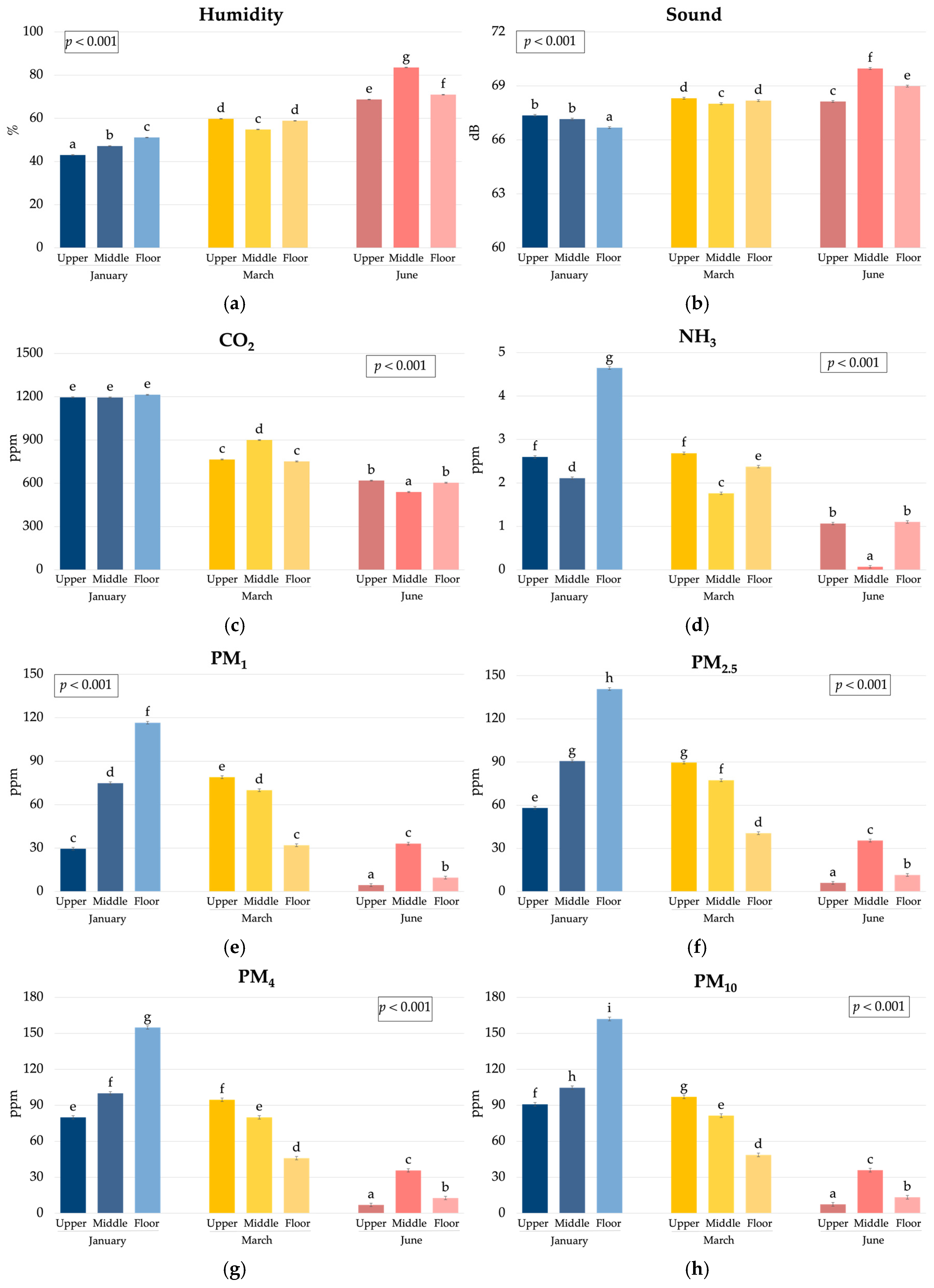
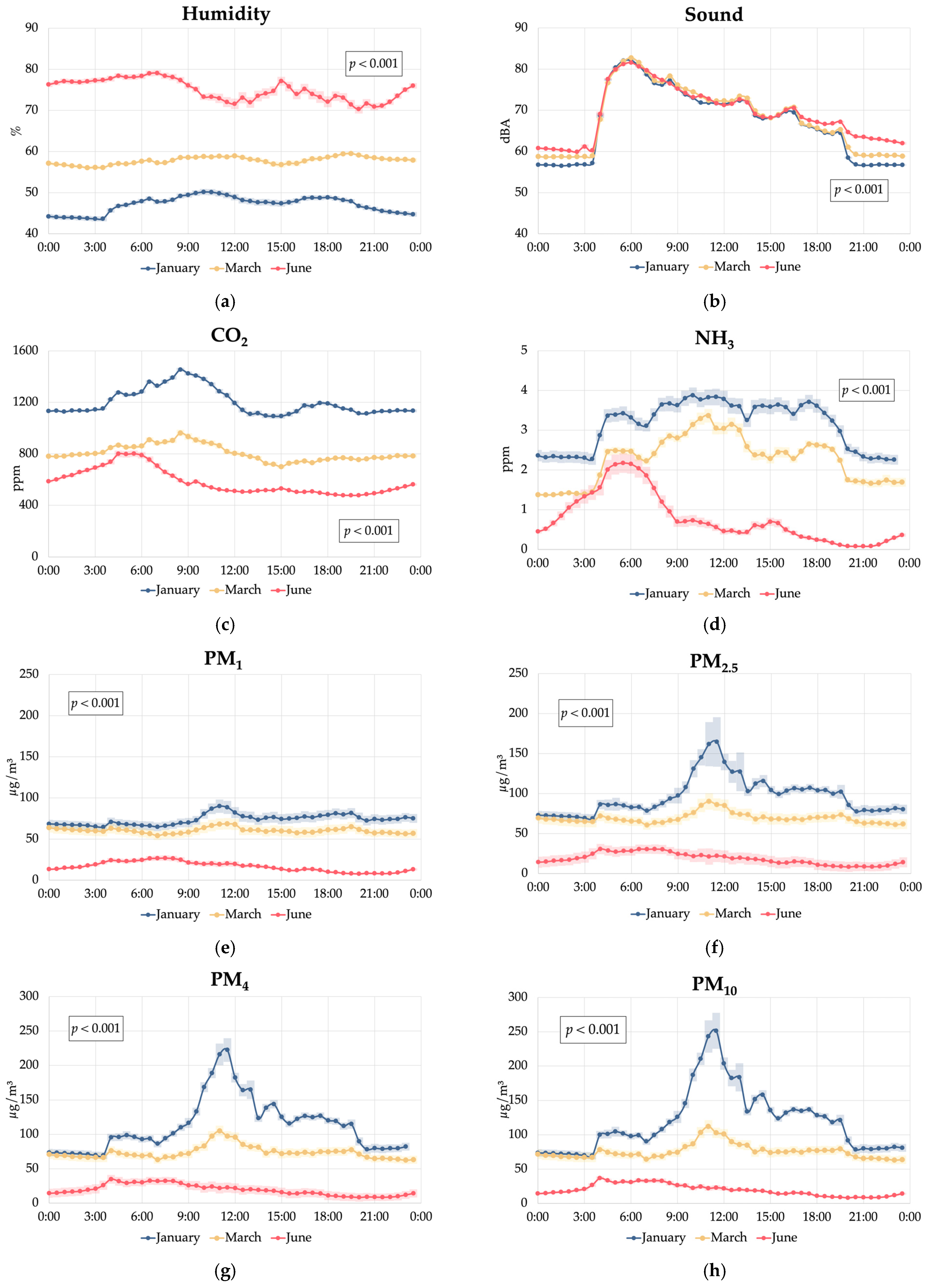
3.1. Environmental Conditions
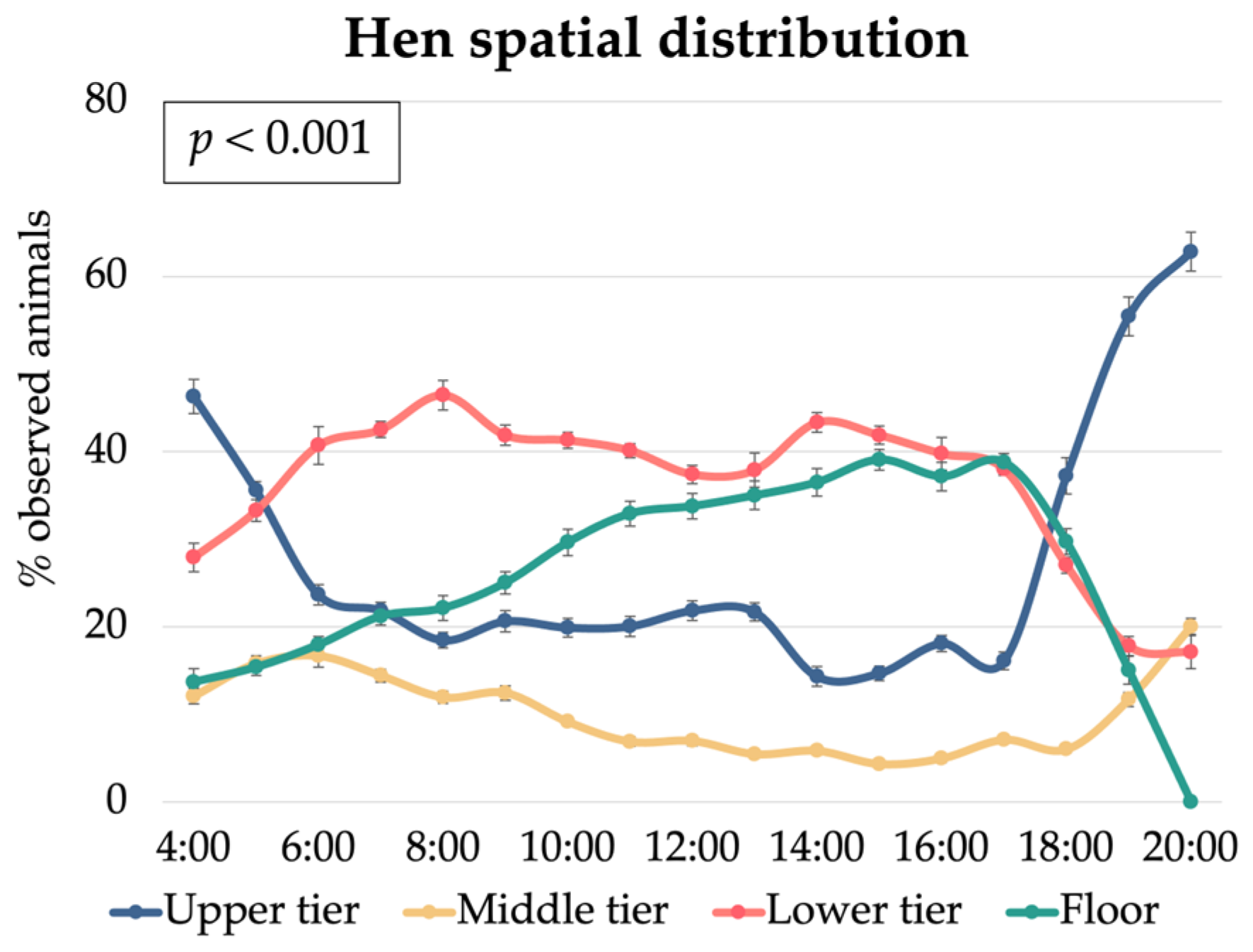
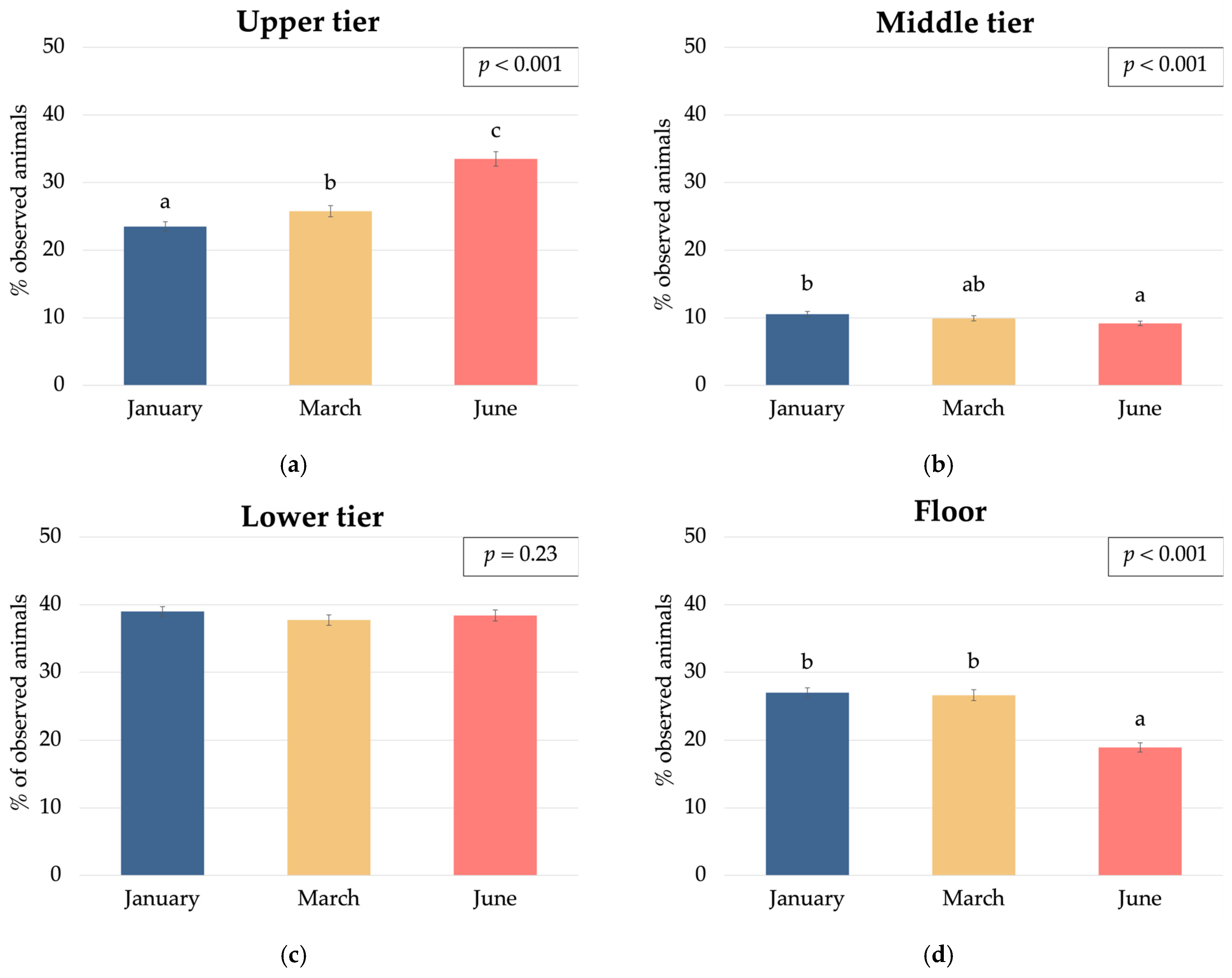
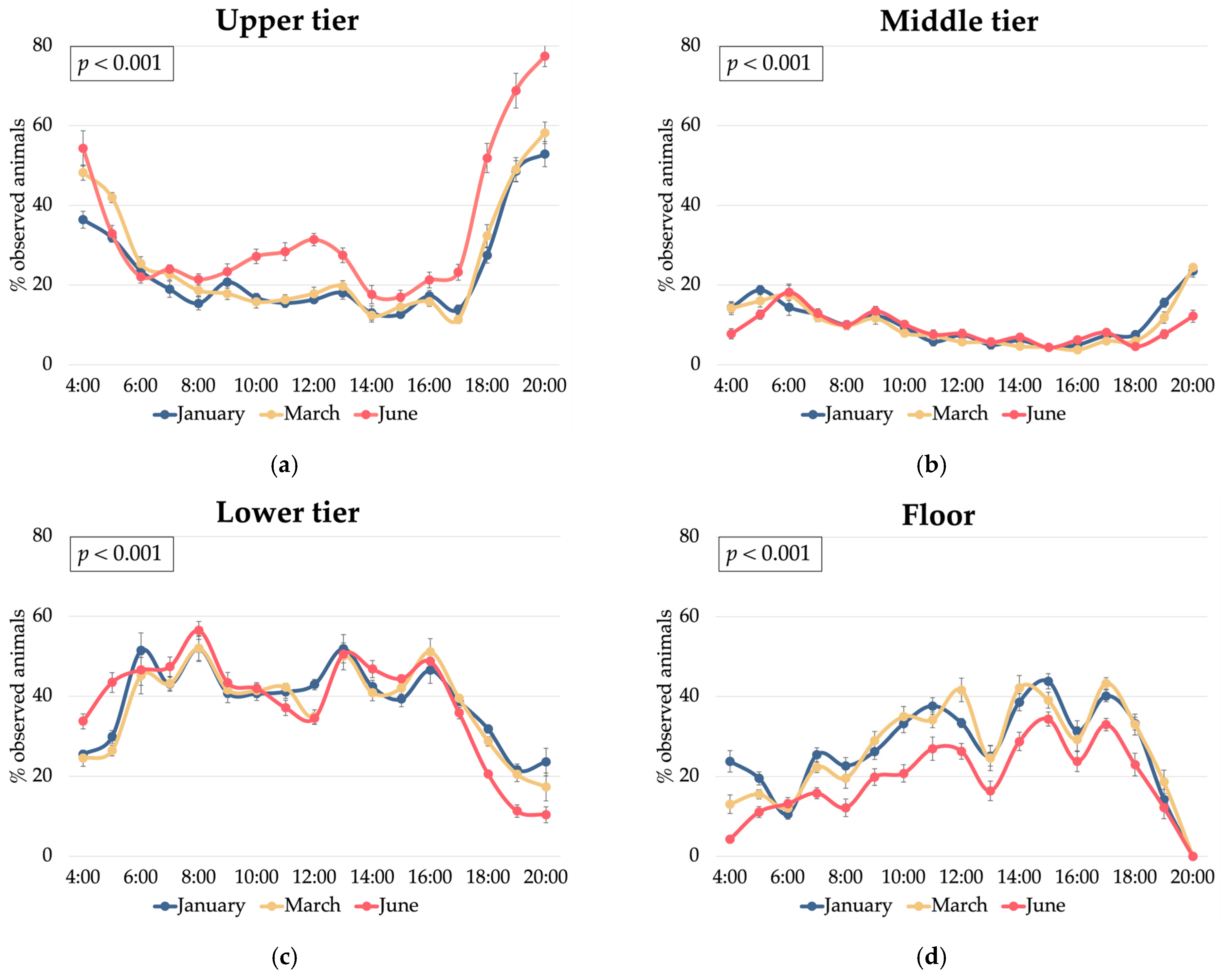
3.2. Hen Spatial Distribution
3.3. Relationships Between Hen Spatial Distribution and Environmental Conditions
3.4. Implications: Real-Time Monitoring for Proactive Interventions
3.5. Limitations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PM | Particulate matter |
| RMSE | Root mean square error |
Appendix A
References
- Campbell, D.L.M.; Makagon, M.M.; Swanson, J.C.; Siegford, J.M. Laying hen movement in a commercial aviary: Enclosure to floor and back again. Poult. Sci. 2016, 95, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Stratmann, A.; Fröhlich, E.K.F.; Gebhardt-Henrich, S.G.; Harlander-Matauschek, A.; Würbel, H.; Toscano, M.J. Modification of aviary design reduces incidence of falls, collisions and keel bone damage in laying hens. Appl. Anim. Behav. Sci. 2015, 165, 112–123. [Google Scholar] [CrossRef]
- Michel, V.; Berk, J.; Bozakova, N.; Van Der Eijk, J.; Estevez, I.; Mircheva, T.; Relic, R.; Rodenburg, T.B.; Sossidou, E.N.; Guinebretière, M. The relationships between damaging behaviours and health in laying hens. Animals 2022, 12, 986. [Google Scholar] [CrossRef]
- Ciarelli, C.; Pillan, G.; Bordignon, F.; Xiccato, G.; Birolo, M.; Trocino, A. Space use and navigation ability of hens at housing in the aviary for the laying phase: Effect of enrichment with additional perches and genotype. Poult. Sci. 2023, 102, 102962. [Google Scholar] [CrossRef]
- Van Veen, L.A.; Van Den Oever, A.C.M.; Kemp, B.; Van Den Brand, H. Perception of laying hen farmers, poultry veterinarians, and poultry experts regarding sensor-based continuous monitoring of laying hen health and welfare. Poult. Sci. 2023, 102, 102581. [Google Scholar] [CrossRef] [PubMed]
- Bordignon, F.; Provolo, G.; Riva, E.; Caria, M.; Todde, G.; Sara, G.; Cesarini, F.; Grossi, G.; Vitali, A.; Lacetera, N.; et al. Smart technologies to improve the management and resilience to climate change of livestock housing: A systematic and critical review. Ital. J. Anim. Sci. 2025, 24, 376–392. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, H.; Fang, C. Research on machine vision online monitoring system for egg production and quality in cage environment. Poult. Sci. 2025, 104, 104552. [Google Scholar] [CrossRef]
- Subedi, S.; Bist, R.B.; Yang, X.; Li, G.; Chai, L. Advanced deep learning methods for multiple behavior classification of cage-free laying hens. AgriEngineering 2025, 7, 24. [Google Scholar] [CrossRef]
- van Veen, L.A.; van den Bran, H.; van der Oever, A.C.M.; Kemp, B.; Youssef, A. An adaptive expert-in-the-loop algorithm for flock-specific anomaly detection in laying hen production. Comput. Electron. Agr. 2025, 229, 109755. [Google Scholar] [CrossRef]
- Carraro, A.; Pravato, M.; Marinello, F.; Bordignon, F.; Trocino, A.; Xiccato, G.; Pezzuolo, A. A new tool to improve the computation of animal kinetic activity indices in precision poultry farming. Artif. Intell. Agric. 2025, in press. [Google Scholar] [CrossRef]
- Zheng, W.; Xiong, Y.; Gates, R.S.; Wang, Y.; Koelkebeck, K.W. Air temperature, carbon dioxide, and ammonia assessment inside a commercial cage layer barn with manure-drying tunnels. Poult. Sci. 2020, 99, 3885–3896. [Google Scholar] [CrossRef]
- Ni, J.-Q.; Chai, L.; Chen, L.; Bogan, B.W.; Wang, K.; Cortus, E.L.; Heber, A.J.; Lim, T.-T.; Diehl, C.A. Characteristics of ammonia, hydrogen sulfide, carbon dioxide, and particulate matter concentrations in high-rise and manure-belt layer hen houses. Atmos. Environ. 2012, 57, 165–174. [Google Scholar] [CrossRef]
- Kilic, I.; Yaslioglu, E. Ammonia and carbon dioxide concentrations in a layer house. Asian Australas. J. Anim. Sci 2014, 27, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, R.; Qi, R.; Li, H.; Li, J.; Liu, W.; Wan, Y.; Liu, Z.; Li, S.; Chang, X.; et al. Study on the changing patterns of production performance of laying hens and their relationships with environmental factors in a large-scale henhouse. Poult. Sci. 2024, 103, 104185. [Google Scholar] [CrossRef] [PubMed]
- Bist, R.B.; Yang, X.; Subedi, S.; Sharma, M.K.; Singh, A.K.; Ritz, C.W.; Kim, W.K.; Chai, L. Temporal variations of air quality in cage-free experimental pullet houses. Poultry 2023, 2, 320–333. [Google Scholar] [CrossRef]
- Ellen, H.H.; Bottcher, R.W.; Von Wachenfelt, E.; Takai, H. Dust levels and control methods in poultry houses. J. Agric. Saf. Health 2000, 6, 275–282. [Google Scholar] [CrossRef]
- Liu, M.; Chen, H.; Zhou, Z.; Du, X.; Zhao, Y.; Ji, H.; Teng, G. Development of an intelligent service platform for a poultry house facility environment based on the internet of things. Agriculture 2024, 14, 1277. [Google Scholar] [CrossRef]
- Channing, C.E.; Hughes, B.O.; Walker, A.W. Spatial distribution and behaviour of laying hens housed in an alternative system. Appl. Anim. Behav. Sci. 2001, 72, 335–345. [Google Scholar] [CrossRef]
- Campbell, D.L.M.; Makagon, M.M.; Swanson, J.C.; Siegford, J.M. Litter use by laying hens in a commercial aviary: Dust bathing and piling. Poult. Sci. 2016, 95, 164–175. [Google Scholar] [CrossRef]
- Odén, K.; Keeling, L.J.; Algers, B. Behaviour of laying hens in two types of aviary systems on 25 commercial farms in Sweden. Br. Poult. Sci. 2002, 43, 169–181. [Google Scholar] [CrossRef]
- Provolo, G.; Brandolese, C.; Grotto, M.; Marinucci, A.; Fossati, N.; Ferrari, O.; Beretta, E.; Riva, E. An Internet of Things framework for monitoring environmental conditions in livestock housing to improve animal welfare and assess environmental impact. Animals 2025, 15, 644. [Google Scholar] [CrossRef] [PubMed]
- SAS (Statistical Analysis System Institute, Inc.). SAS/STAT(R) 9.3 User’s Guide, 3rd ed.; SAS Institute: Cary, NC, USA, 2013; Available online: https://support.sas.com/documentation/cdl/en/statug/63962/HTML/default/viewer.htm#titlepage.htm (accessed on 27 February 2025).
- EFSA Panel on Animal Health and Animal Welfare (AHAW); Nielsen, S.S.; Alvarez, J.; Bicout, D.J.; Calistri, P.; Canali, E.; Drewe, J.A.; Garin-Bastuji, B.; Gonzales Rojas, J.L.; Gortázar Schmidt, C.; et al. Welfare of laying hens on farm. EFSA J. 2023, 21, e07789. [Google Scholar] [CrossRef]
- CIGR—International Commission of Agricultural Engineering. Report of Working Group on Climatization of Animal Houses; Scottish Farm Building Investigation Unit: Aberdeen, UK, 1984; pp. 29–30. Available online: https://www.cigr.org/sites/default/files/documets/CIGR-Workinggroupreport1984.pdf (accessed on 17 March 2025).
- ASHRAE. Environmental control for animals and plants. In ASHRAE Handbook—HVAC Applications—SI Edition; American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE): Atlanta, GA, USA, 2019; pp. 25.1–25.25. Available online: https://www.ashrae.org/advertising/handbook-advertising/applications/environmental-control-for-animals-and-plants (accessed on 17 March 2025).
- Campo, J.L.; Gil, M.G.; Dávila, S.G. Effects of specific noise and music stimuli on stress and fear levels of laying hens of several breeds. Appl. Anim. Behav. Sci. 2005, 91, 75–84. [Google Scholar] [CrossRef]
- Gvaryahu, G.; Cunningham, D.L.; Van Tienhoven, A. Filial imprinting, environmental enrichment, and music application effects on behavior and performance of meat strain chicks. Poult. Sci. 1989, 68, 211–217. [Google Scholar] [CrossRef]
- Gross, W.B. Effect of exposure to a short-duration sound on the stress response of chickens. Avian Dis. 1990, 34, 759. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide; WHO European Centre for Environment and Health: Bonn, Germany, 2021; Available online: https://iris.who.int/bitstream/handle/10665/345329/9789240034228-eng.pdf?sequence=1 (accessed on 17 March 2025).
- Bist, R.B.; Yang, X.; Subedi, S.; Paneru, B.; Chai, L. An Integrated engineering method for improving air quality of cage-free hen housing. AgriEngineering 2024, 6, 2795–2810. [Google Scholar] [CrossRef]
- Zhao, Y.; Shepherd, T.A.; Li, H.; Xin, H. Environmental assessment of three egg production systems–Part I: Monitoring system and indoor air quality. Poult. Sci. 2015, 94, 518–533. [Google Scholar] [CrossRef]
- St-Germain, M.-W.; Létourneau, V.; Larios Martínez, A.D.; Godbout, S.; Boulianne, M.; Duchaine, C. Airborne dust and bioaerosols in canadian conventional and alternative houses for laying hens. J. Occup. Environ. Hyg. 2024, 21, 909–919. [Google Scholar] [CrossRef]
- Li, Z.; Xiong, Y.; Wang, S.; Wang, C.; Ji, B.; Liu, Y.; Liang, C.; Tong, Q. Assessing particulate matter concentration level and its limit exceedance based on year-round field measurements of different laying hen building systems. Biosyst. Eng. 2023, 226, 266–279. [Google Scholar] [CrossRef]
- Ahmadi Babadi, K.; Khorasanizadeh, H.; Aghaei, A. CFD Modeling of air flow, humidity, CO2 and NH3 distributions in a caged laying hen house with tunnel ventilation system. Comput. Electron. Agric. 2022, 193, 106677. [Google Scholar] [CrossRef]
- Kim, D.-H.; Kim, Y.B.; Lee, S.H.; Lee, K.-W. Effects of relative humidity on physiology and behavior of laying hens exposed to high ambient temperatures. Trop. Anim. Health Prod. 2024, 56, 275. [Google Scholar] [CrossRef] [PubMed]
- McAdie, T.M.; Foster, T.M.; Temple, W.; Matthews, L.R. A method for measuring the aversiveness of sounds to domestic hens. Appl. Anim. Behav. Sci. 1993, 37, 223–238. [Google Scholar] [CrossRef]
- Chloupek, P.; Voslářová, E.; Chloupek, J.; Bedáňová, I.; Pištěková, V.; Večerek, V. Stress in broiler chickens due to acute noise exposure. Acta Vet. Brno 2009, 78, 93–98. [Google Scholar] [CrossRef]
- David, B.; Mejdell, C.; Michel, V.; Lund, V.; Moe, R. Air quality in alternative housing systems may have an impact on laying hen welfare. Part II—Ammonia. Animals 2015, 5, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Brandstätter, R. Encoding time of day and time of year by the avian circadian system. J. Neuroendocrinology 2003, 15, 398–404. [Google Scholar] [CrossRef]
- Zhong, C.; Cao, Y.; Cao, M.; Gao, J.; Li, S.; Liu, M.; Teng, G.; Wang, C. Vocalization characteristics of laying hens under cage and perch systems. Trans. Chinese Soc. Agric. Eng. 2021, 37, 135–141. [Google Scholar] [CrossRef]
- Wathes, C.M.; Holden, M.R.; Sneath, R.W.; White, R.P.; Phillips, V.R. Concentrations and emission rates of aerial ammonia, nitrous oxide, methane, carbon dioxide, dust and endotoxin in UK broiler and layer houses. Br. Poult. Sci. 1997, 38, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.; Xin, H.; Wang, Y.; Oliveira, J.; Wang, K.; Zhao, Y. Mitigating particulate matter generation in a commercial cage-free hen house. Trans. ASABE 2019, 62, 877–886. [Google Scholar] [CrossRef]
- Fabbri, C.; Valli, L.; Guarino, M.; Costa, A.; Mazzotta, V. Ammonia, methane, nitrous oxide and particulate matter emissions from two different buildings for laying hens. Biosyst. Eng. 2007, 97, 441–455. [Google Scholar] [CrossRef]
- Green, A.R.; Wesley, I.; Trampel, D.W.; Xin, H. Air quality and bird health status in three types of commercial egg layer houses. J. Appl. Poult. Res. 2009, 18, 605–621. [Google Scholar] [CrossRef]
- Nimmermark, S.; Lund, V.; Gustafsson, G.; Eduard, W. Ammonia, dust and bacteria in welfare-oriented systems for laying hens. Ann. Agric. Environ. Med. 2009, 16, 103–113. [Google Scholar] [PubMed]
- Pillan, G.; Xiccato, G.; Pascual, A.; Bordignon, F.; Birolo, M.; Concollato, A.; Trocino, A. Changes in oviposition time of brown hens during the cycle in an aviary system. In 26th World’s Poultry Congress, Paris, France, 7–11 August 2022, Books of Abstracts 2022; Tixier-Boichard, M., Duclos, M., Eds.; French Branch of the World’s Poultry Science Association: Paris, France, 2022; ID: 1798; p. 418. [Google Scholar]
- Weeks, C.A.; Nicol, C.J. Behavioural needs, priorities and preferences of laying hens. J. World’s Poult. Sci. 2006, 62, 296–307. [Google Scholar] [CrossRef]
- Miles, D.M.; Rowe, D.E.; Cathcart, T.C. High litter moisture content suppresses litter ammonia volatilization. Poult. Sci. 2011, 90, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Cambra-López, M.; Aarnink, A.J.A.; Zhao, Y.; Calvet, S.; Torres, A.G. Airborne particulate matter from livestock production systems: A review of an air pollution problem. Environ. Pollut. 2010, 158, 1–17. [Google Scholar] [CrossRef]



| Node | Sensor | Technology | Measurement | Range | Accuracy |
|---|---|---|---|---|---|
| N1 | Sensirion SHT30 | CMOSens | Temperature | −10–+60 °C | ±0.2 °C |
| Sensirion SHT30 | CMOSens | Relative humidity | 0–100% | ±2% | |
| SPW2430 | MEMS | Sound intensity | 30–130 dBA | ±2% | |
| N2 | Sensirion SCD30 | Nondispersive infrared (NDIR) | CO2 concentration | 400–10,000 ppm | ±3% |
| GS +4NH3100 | Electrochemical cell | NH3 concentration | 0–100 ppm | ±10% | |
| N3 | - | Laser scattering | PM1, PM2.5, PM4, PM10 | 0–1000 μg/m3 | ±10 μg/m3 ±25 μg/m3 |
| Variables | Tier (T) | Month (M) | p-Value | RMSE | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Floor | Middle | Upper | January | March | June | Hour (H) | T | M | H × T | H × M | T × M | ||
| Humidity, % | 60.3 b | 61.9 c | 57.2 a | 47.1 a | 57.8 b | 74.4 c | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 5.12 |
| Sound, dB | 68.0 b | 68.4 c | 67.9 a | 67.1 a | 68.2 b | 69.0 c | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 2.01 |
| CO2, ppm | 856 a | 878 b | 860 a | 1201 c | 806 b | 587 a | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 130 |
| NH3, ppm | 2.71 c | 1.31 a | 2.12 b | 3.12 c | 2.27 b | 0.75 a | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 1.04 |
| PM1, μg/m3 | 52.1 b | 59.3 c | 38.3 a | 73.6 c | 60.3 b | 15.7 a | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 29.4 |
| PM2.5, μg/m3 | 63.6 b | 67.8 c | 51.9 a | 96.5 c | 69.2 b | 17.7 a | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 35.3 |
| PM4, μg/m3 | 70.5 b | 71.9 b | 61.2 a | 112 c | 73.5 b | 18.5 a | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 42.9 |
| PM10, μg/m3 | 74.0 b | 74.0 b | 65.8 a | 119 c | 75.7 b | 18.9 a | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 47.5 |
| Variable | Estimate | SE | Odds Ratio | 95% CI | p-Value | |
|---|---|---|---|---|---|---|
| Lower 95% | Upper 95% | |||||
| Intercept | −4.37 | 0.71 | - | - | - | <0.001 |
| Time period | ||||||
| Early (4:00–8:00) | - | - | - | - | - | - |
| Morning (8:00–12:00) | 2.82 | 0.74 | 16.8 | 3.92 | 71.7 | <0.001 |
| Afternoon (12:00–16:00) | 1.86 | 0.77 | 6.41 | 1.41 | 29.1 | <0.05 |
| Late (16:00–20:00) | −0.76 | 1.23 | 0.47 | 0.04 | 5.21 | 0.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bordignon, F.; Pravato, M.; Trocino, A.; Xiccato, G.; Marinello, F.; Pezzuolo, A. Environmental Gradients and Hen Spatial Distribution in a Cage-Free Aviary System: Internet of Things-Based Real-Time Monitoring for Proactive Management. Animals 2025, 15, 1225. https://doi.org/10.3390/ani15091225
Bordignon F, Pravato M, Trocino A, Xiccato G, Marinello F, Pezzuolo A. Environmental Gradients and Hen Spatial Distribution in a Cage-Free Aviary System: Internet of Things-Based Real-Time Monitoring for Proactive Management. Animals. 2025; 15(9):1225. https://doi.org/10.3390/ani15091225
Chicago/Turabian StyleBordignon, Francesco, Mattia Pravato, Angela Trocino, Gerolamo Xiccato, Francesco Marinello, and Andrea Pezzuolo. 2025. "Environmental Gradients and Hen Spatial Distribution in a Cage-Free Aviary System: Internet of Things-Based Real-Time Monitoring for Proactive Management" Animals 15, no. 9: 1225. https://doi.org/10.3390/ani15091225
APA StyleBordignon, F., Pravato, M., Trocino, A., Xiccato, G., Marinello, F., & Pezzuolo, A. (2025). Environmental Gradients and Hen Spatial Distribution in a Cage-Free Aviary System: Internet of Things-Based Real-Time Monitoring for Proactive Management. Animals, 15(9), 1225. https://doi.org/10.3390/ani15091225








