UFBP1 Ameliorates Heat Stress-Induced Apoptosis via Mitochondria-Mediated Pathway in Bovine Mammary Epithelial Cells
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sample Collection
2.2. Histology and Immunostaining
2.3. Cell Culture, Transfection, and Treatments
2.4. Flow Cytometry to Detect Cell Apoptosis
2.5. Detection of Intracellular Reactive Oxygen Species
2.6. Detection of Cellular ATP Levels and the NAD+/NADH Ratio
2.7. Mitochondrial Membrane Potential Assays
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
3.1. Expression of UFBP1 in Heat-Stressed Mammary Gland and BMECs
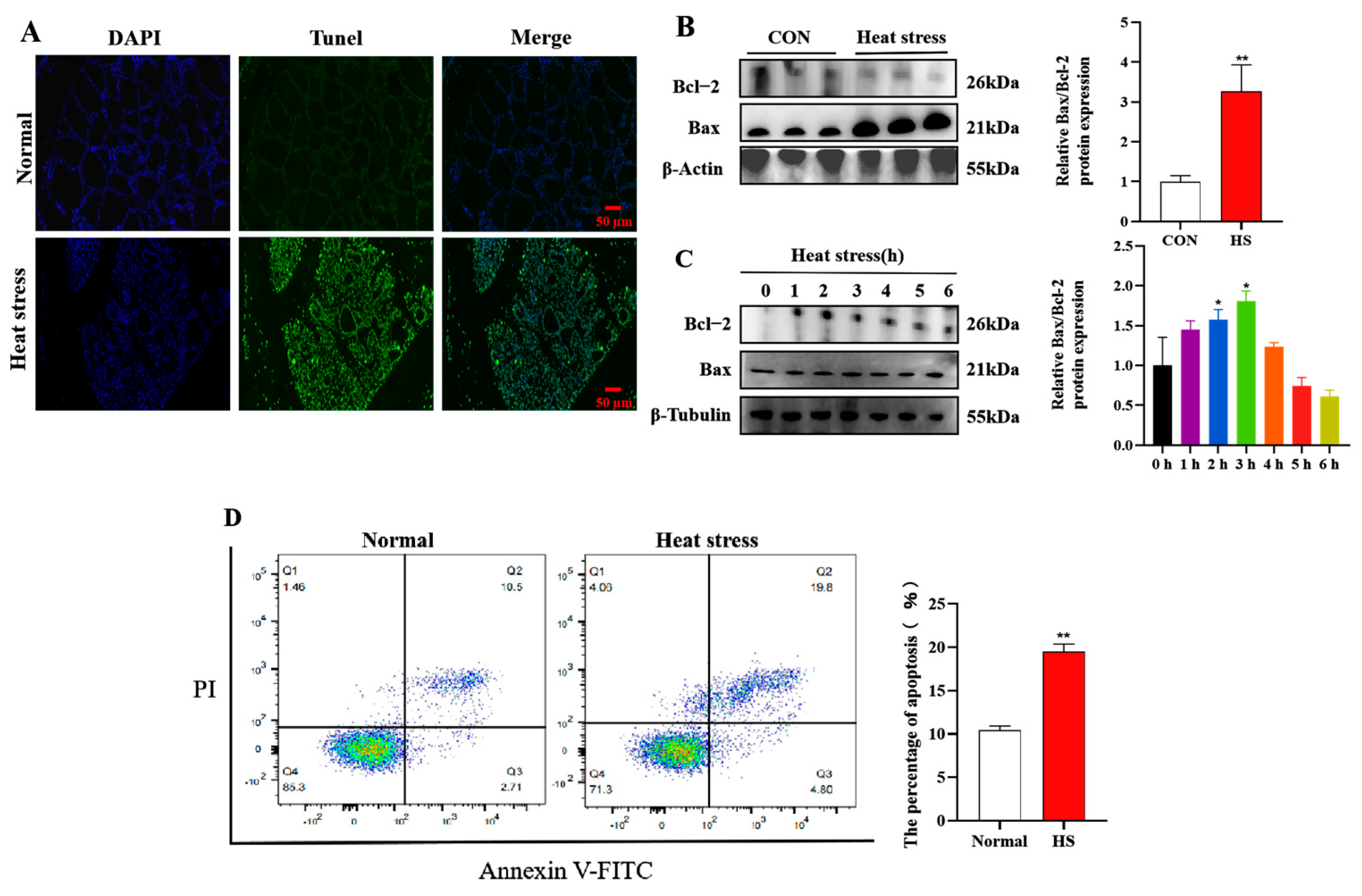
3.2. Heat Stress Induces Apoptosis in Heat-Stressed Mammary Gland and BMECs
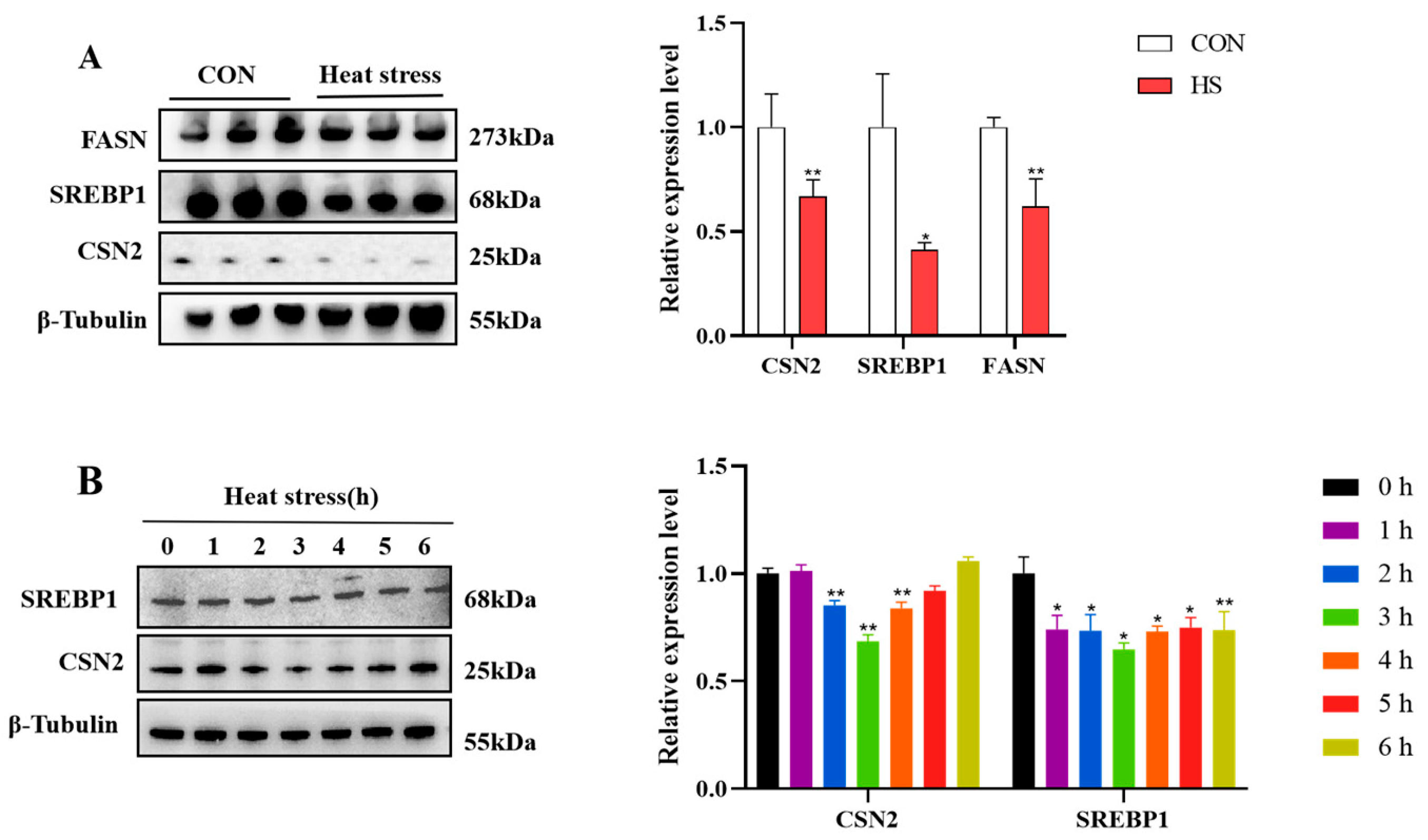
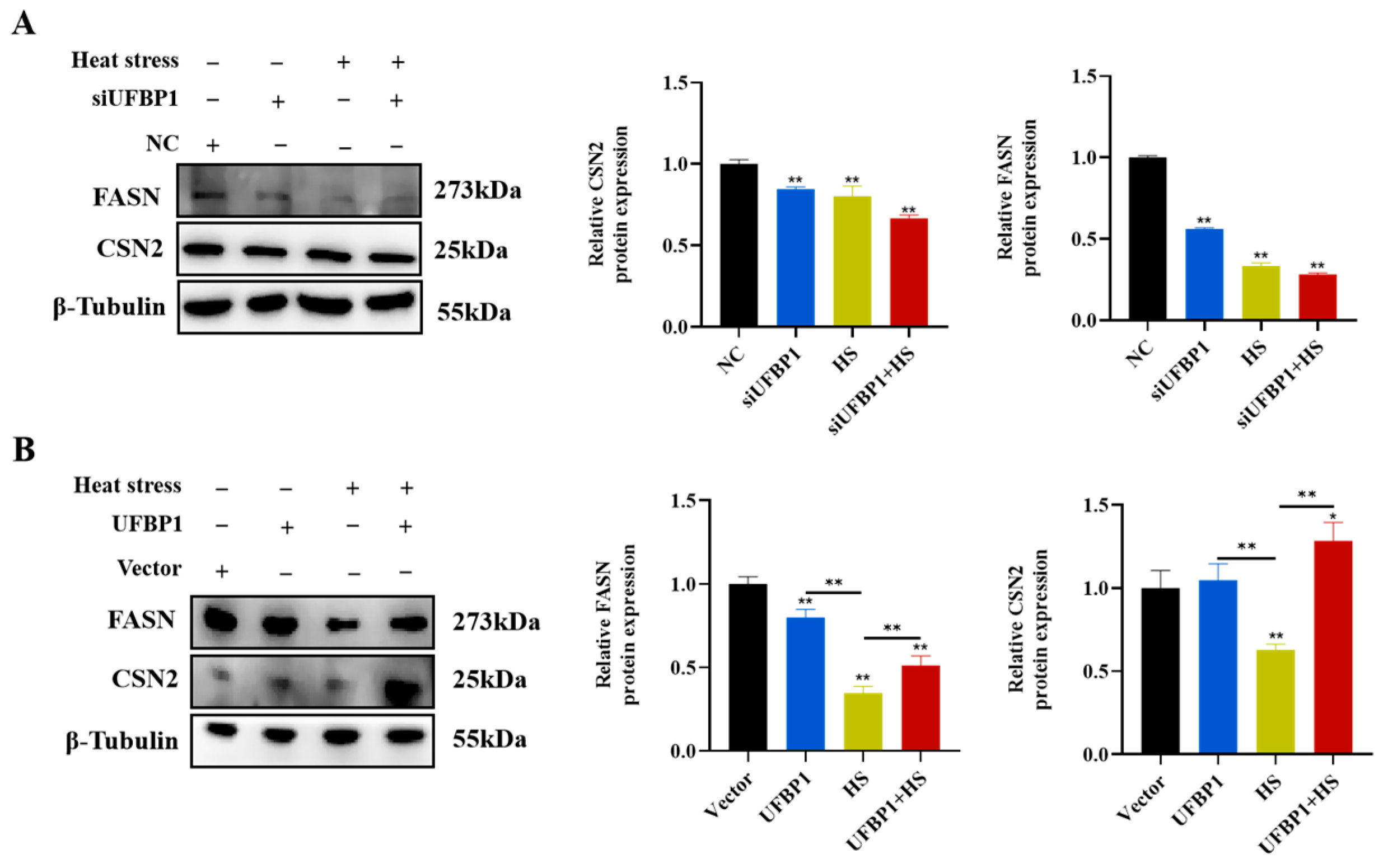
3.3. Heat Stress Inhibits Milk Fat and Protein Synthesis-Related Gene Expression
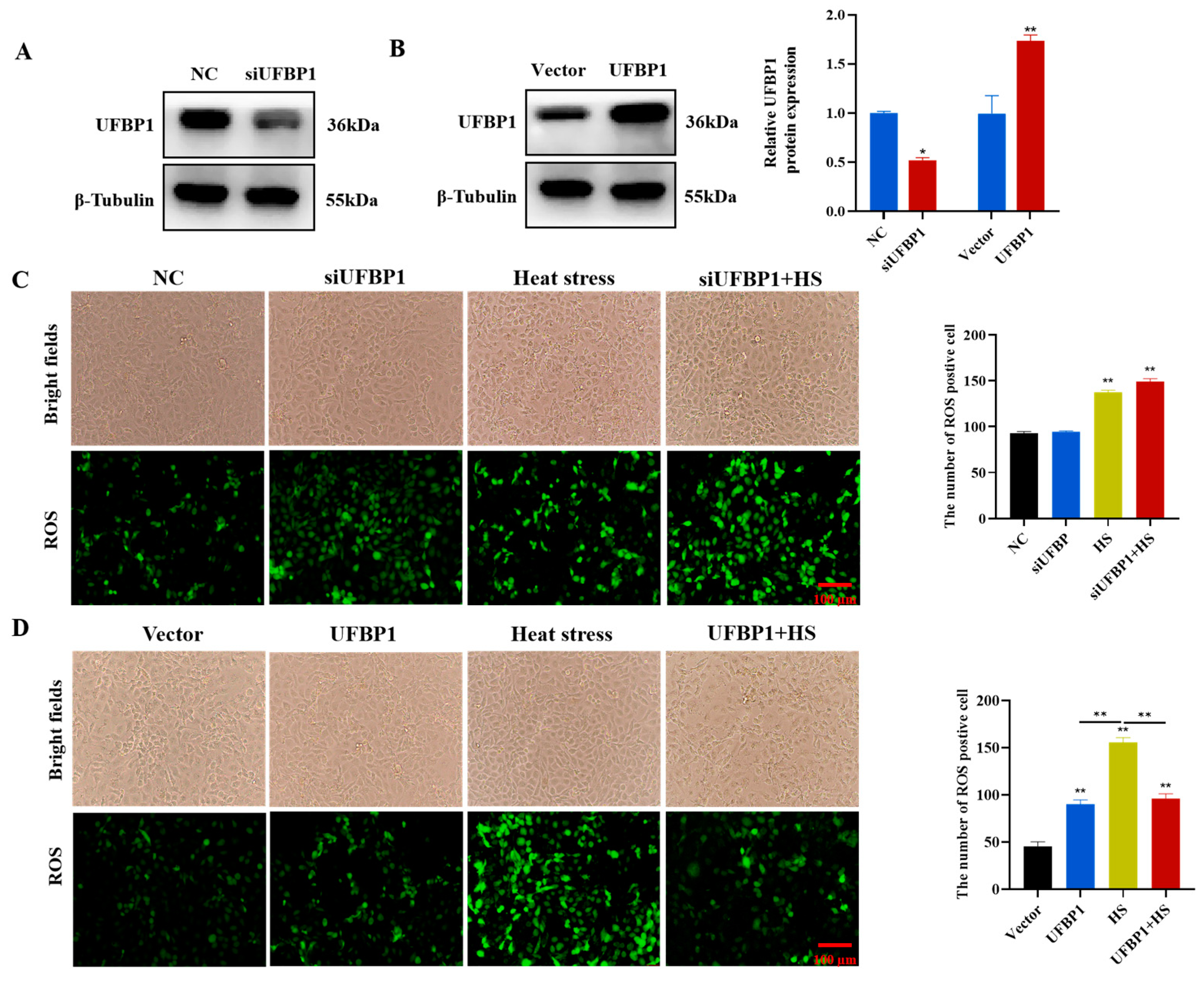
3.4. Intracellular Reactive Oxygen Species Measurement
3.5. UFBP1 Improves Heat Stress-Induced Mitochondrial Dysfunction in BMECs
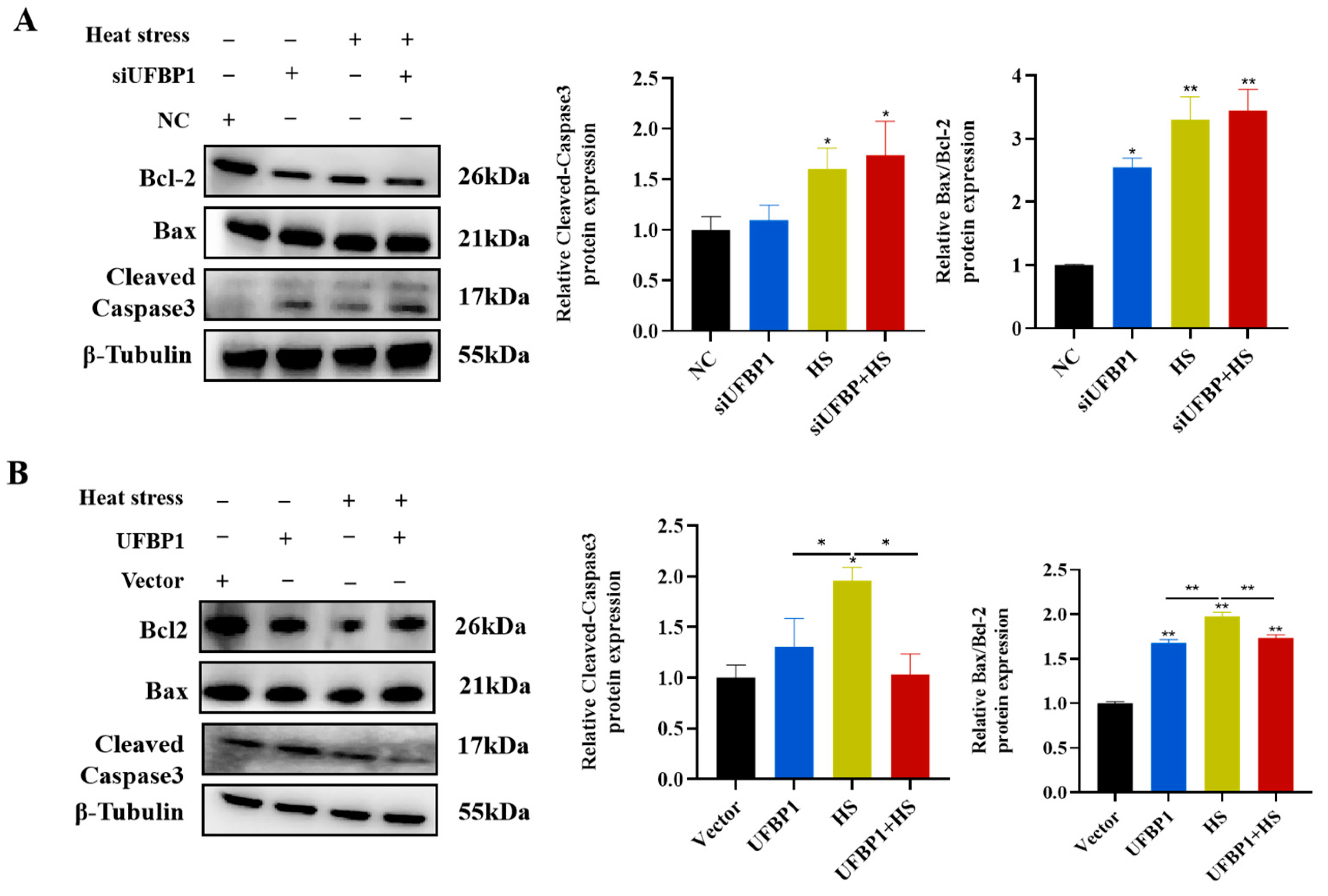
3.6. UFBP1 Regulates Heat Stress-Induced Apoptosis in BMECS
3.7. UFBP1 Regulates Milk Fat and Protein Synthesis-Related Gene Expression in Heat-Stressed BMECs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Becker, C.A.; Collier, R.J.; Stone, A.E. Invited review: Physiological and behavioral effects of heat stress in dairy cows. J. Dairy Sci. 2020, 103, 6751–6770. [Google Scholar] [CrossRef]
- Polsky, L.; von Keyserlingk, M. Invited review: Effects of heat stress on dairy cattle welfare. J. Dairy Sci. 2017, 100, 8645–8657. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Orellana, R.R.; Marins, T.N.; Chen, Y.C.; Gao, J.; Bernard, J.K. Impact of heat stress on lactational performance of dairy cows. Theriogenology 2020, 150, 437–444. [Google Scholar] [CrossRef]
- Tao, S.; Orellana, R.M.; Weng, X.; Marins, T.N.; Dahl, G.E.; Bernard, J.K. Symposium review: The influences of heat stress on bovine mammary gland function. J. Dairy Sci. 2018, 101, 5642–5654. [Google Scholar] [CrossRef]
- Capuco, A.V.; Ellis, S.E.; Hale, S.A.; Long, E.; Erdman, R.A.; Zhao, X.; Paape, M.J. Lactation persistency: Insights from mammary cell proliferation studies. J. Anim. Sci. 2003, 81 (Suppl. S3), 18–31. [Google Scholar] [CrossRef] [PubMed]
- Collier, R.J.; Stiening, C.M.; Pollard, B.C.; VanBaale, M.J.; Baumgard, L.H.; Gentry, P.C.; Coussens, P.M. Use of gene expression microarrays for evaluating environmental stress tolerance at the cellular level in cattle. J. Anim. Sci. 2006, 84 (Suppl. S13), E1–E13. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sun, Y.; Wu, J.; Li, X.; Luo, M.; Wang, G. The global effect of heat on gene expression in cultured bovine mammary epithelial cells. Cell Stress Chaperones 2015, 20, 381–389. [Google Scholar] [CrossRef]
- Wang, H.; Hao, W.; Yang, L.; Li, T.; Zhao, C.; Yan, P.; Wei, S. Procyanidin B2 Alleviates Heat-Induced Oxidative Stress through the Nrf2 Pathway in Bovine Mammary Epithelial Cells. Int. J. Mol. Sci. 2022, 23, 7769. [Google Scholar] [CrossRef]
- Zeng, H.; Li, S.; Chang, H.; Zhai, Y.; Wang, H.; Weng, H.; Han, Z. Circ_002033 Regulates Proliferation, Apoptosis, and Oxidative Damage of Bovine Mammary Epithelial Cells via the miR-199a-5p-MAP3K11 Axis in Heat Stress. J. Agric. Food. Chem. 2024, 72, 14386–14401. [Google Scholar] [CrossRef]
- Wang, H.; Hao, W.; Yang, L.; Yan, P.; Wei, S. Preconditioning with procyanidin B2 protects MAC-T cells against heat exposure-induced mitochondrial dysfunction and inflammation. Mol. Immunol. 2022, 147, 126–135. [Google Scholar] [CrossRef]
- Yamazaki, S.; Satoh, H.; Watanabe, T. Liraglutide enhances insulin sensitivity by activating AMP-activated protein kinase in male Wistar rats. Endocrinology 2014, 155, 3288–3301. [Google Scholar] [CrossRef]
- Bernabucci, U.; Basirico, L.; Morera, P.; Dipasquale, D.; Vitali, A.; Piccioli, C.F.; Calamari, L. Effect of summer season on milk protein fractions in Holstein cows. J. Dairy Sci. 2015, 98, 1815–1827. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, Y.; Zheng, N.; Cheng, J.; Wang, J. The effect of heat stress on gene expression and synthesis of heat-shock and milk proteins in bovine mammary epithelial cells. Anim. Sci. J. 2016, 87, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Franklin, J.L. Redox regulation of the intrinsic pathway in neuronal apoptosis. Antioxid. Redox Signal. 2011, 14, 1437–1448. [Google Scholar] [CrossRef]
- Fan, H.; Ding, R.; Liu, W.; Zhang, X.; Li, R.; Wei, B.; Su, S.; Jin, F.; Wei, C.; He, X.; et al. Heat shock protein 22 modulates NRF1/TFAM-dependent mitochondrial biogenesis and DRP1-sparked mitochondrial apoptosis through AMPK-PGC1alpha signaling pathway to alleviate the early brain injury of subarachnoid hemorrhage in rats. Redox Biol. 2021, 40, 101856. [Google Scholar] [CrossRef]
- Cong, X.; Zhang, Q.; Li, H.; Jiang, Z.; Cao, R.; Gao, S.; Tian, W. Puerarin ameliorates heat stress-induced oxidative damage and apoptosis in bovine Sertoli cells by suppressing ROS production and upregulating Hsp72 expression. Theriogenology 2017, 88, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Ma, Y.; Han, X.; Ergu, A.; Zhang, C.; Chen, P.; Liu, W.; Luo, Q.; Shi, Z.; Xu, H.; et al. Sun-simulated-driven production of high-purity methanol from carbon dioxide. Nat. Commun. 2025, 16, 857. [Google Scholar] [CrossRef] [PubMed]
- Gerakis, Y.; Quintero, M.; Li, H.; Hetz, C. The UFMylation System in Proteostasis and Beyond. Trends Cell Biol. 2019, 29, 974–986. [Google Scholar] [CrossRef]
- Millrine, D.; Peter, J.J.; Kulathu, Y. A guide to UFMylation, an emerging posttranslational modification. FEBS J. 2023, 290, 5040–5056. [Google Scholar] [CrossRef]
- Zhou, X.; Mahdizadeh, S.J.; Le Gallo, M.; Eriksson, L.A.; Chevet, E.; Lafont, E. UFMylation: A ubiquitin-like modification. Trends Biochem. Sci. 2024, 49, 52–67. [Google Scholar] [CrossRef]
- Tatsumi, K.; Sou, Y.S.; Tada, N.; Nakamura, E.; Iemura, S.; Natsume, T.; Kang, S.H.; Chung, C.H.; Kasahara, M.; Kominami, E.; et al. A novel type of E3 ligase for the Ufm1 conjugation system. J. Biol. Chem. 2010, 285, 5417–5427. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Song, L.; Zeng, L.; Yi, W.; Liu, T.; Chen, H.; Wang, M.; Ju, Z.; Cong, Y. A critical role of DDRGK1 in endoplasmic reticulum homoeostasis via regulation of IRE1alpha stability. Nat. Commun. 2017, 8, 14186. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhou, T.; Wang, X.; Xia, Y.; Cao, X.; Cheng, X.; Cao, Y.; Ma, P.; Ma, H.; Qin, A.; et al. Loss of DDRGK1 impairs IRE1alpha UFMylation in spondyloepiphyseal dysplasia. Int. J. Biol. Sci. 2023, 19, 4709–4725. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, K.; Moura, R.F.; Granvik, M.; Igoillo-Esteve, M.; Hohmeier, H.E.; Hendrickx, N.; Newgard, C.B.; Waelkens, E.; Cnop, M.; Schuit, F. Ubiquitin fold modifier 1 (UFM1) and its target UFBP1 protect pancreatic beta cells from ER stress-induced apoptosis. PLoS ONE 2011, 6, e18517. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhu, G.; Liu, S.; Pan, Z.; Quintero, M.; Poole, C.J.; Lu, C.; Zhu, H.; Islam, B.; Riggelen, J.V.; et al. Indispensable role of the Ubiquitin-fold modifier 1-specific E3 ligase in maintaining intestinal homeostasis and controlling gut inflammation. Cell Discov. 2019, 5, 7. [Google Scholar] [CrossRef]
- Cai, Y.; Pi, W.; Sivaprakasam, S.; Zhu, X.; Zhang, M.; Chen, J.; Makala, L.; Lu, C.; Wu, J.; Teng, Y.; et al. UFBP1, a Key Component of the Ufm1 Conjugation System, Is Essential for Ufmylation-Mediated Regulation of Erythroid Development. PLoS Genet. 2015, 11, e1005643. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, T.; Guo, X.; Zhang, R.; Jia, Y.; Shang, C.; Wang, A.; Jin, Y.; Lin, P. Ufmylation regulates granulosa cell apoptosis via ER stress but not oxidative stress during goat follicular atresia. Theriogenology 2021, 169, 47–55. [Google Scholar] [CrossRef]
- Li, C.; Li, L.; Chen, K.; Wang, Y.; Yang, F.; Wang, G. UFL1 Alleviates Lipopolysaccharide-Induced Cell Damage and Inflammation via Regulation of the TLR4/NF-kappaB Pathway in Bovine Mammary Epithelial Cells. Oxidative Med. Cell. Longev. 2019, 2019, 6505373. [Google Scholar]
- Valero, T. Mitochondrial biogenesis: Pharmacological approaches. Curr. Pharm. Des. 2014, 20, 5507–5509. [Google Scholar] [CrossRef]
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative stress: The mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch. Toxicol. 2013, 87, 1157–1180. [Google Scholar] [CrossRef]
- Guo, Z.; Gao, S.; Ouyang, J.; Ma, L.; Bu, D. Impacts of Heat Stress-Induced Oxidative Stress on the Milk Protein Biosynthesis of Dairy Cows. Animals 2021, 11, 726. [Google Scholar] [CrossRef]
- Schapira, A.H. Mitochondrial diseases. Lancet 2012, 379, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, H.; Pal, S.; Sabnam, S.; Pal, A. High glucose augments ROS generation regulates mitochondrial dysfunction and apoptosis via stress signalling cascades in keratinocytes. Life Sci. 2020, 241, 117148. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, X.; Li, D.; Cao, L.; Cui, H.; Xu, G. UFBP1, a key component in ufmylation, enhances drug sensitivity by promoting proteasomal degradation of oxidative stress-response transcription factor Nrf2. Oncogene 2021, 40, 647–662. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.Y.; Seol, D.W. The role of mitochondria in apoptosis. BMB Rep. 2008, 41, 11–22. [Google Scholar] [CrossRef]
- Guo, X.; Chi, S.; Cong, X.; Li, H.; Jiang, Z.; Cao, R.; Tian, W. Baicalin protects sertoli cells from heat stress-induced apoptosis via activation of the Fas/FasL pathway and Hsp72 expression. Reprod. Toxicol. 2015, 57, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zou, Y.; Li, J.; Chen, F.; Li, H.; Cai, Y. CDK5RAP3, a Novel Nucleoplasmic Shuttle, Deeply Regulates HSF1-Mediated Heat Stress Response and Protects Mammary Epithelial Cells from Heat Injury. Int. J. Mol. Sci. 2020, 21, 8400. [Google Scholar] [CrossRef]
- Yan, Y.; Huang, J.; Chen, X.; Li, Y.; Zhao, W.; Li, C. UFL1 regulates cellular homeostasis by targeting endoplasmic reticulum and mitochondria in NEFA-stimulated bovine mammary epithelial cells via the IRE1alpha/XBP1 pathway. Free Radic. Biol. Med. 2024, 222, 16–26. [Google Scholar] [CrossRef]
- Gai, Z.; Wang, Y.; Wang, J.; Fu, J.; Tian, L.; Li, X.; Zhao, J.; Gong, G. Downregulation of CASTOR1 Inhibits Heat-Stress-Induced Apoptosis and Promotes Casein and Lipid Synthesis in Mammary Epithelial Cells. J. Agric. Food. Chem. 2022, 70, 5386–5395. [Google Scholar] [CrossRef]
- Liossis, S.N.; Ding, X.Z.; Kiang, J.G.; Tsokos, G.C. Overexpression of the heat shock protein 70 enhances the TCR/CD3- and Fas/Apo-1/CD95-mediated apoptotic cell death in Jurkat T cells. J. Immunol. 1997, 158, 5668–5675. [Google Scholar] [CrossRef]
- Li, C.; Li, L.; Ali, I.; Kuang, M.; Wang, X.; Wang, G. UFL1 regulates milk protein and fat synthesis-related gene expression of bovine mammary epithelial cells probably via the mTOR signaling pathway. In Vitro Cell. Dev. Biol.-Anim. 2021, 57, 550–559. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Yu, R.; Tan, S.; Jiang, Y.; Sun, L.; Shen, M.; Zhang, C.; Chen, K.; Li, C. UFBP1 Ameliorates Heat Stress-Induced Apoptosis via Mitochondria-Mediated Pathway in Bovine Mammary Epithelial Cells. Animals 2025, 15, 1233. https://doi.org/10.3390/ani15091233
Li Y, Yu R, Tan S, Jiang Y, Sun L, Shen M, Zhang C, Chen K, Li C. UFBP1 Ameliorates Heat Stress-Induced Apoptosis via Mitochondria-Mediated Pathway in Bovine Mammary Epithelial Cells. Animals. 2025; 15(9):1233. https://doi.org/10.3390/ani15091233
Chicago/Turabian StyleLi, Yuan, Ran Yu, Shujing Tan, Yunlong Jiang, Longwei Sun, Manman Shen, Chuanjian Zhang, Kunlin Chen, and Chengmin Li. 2025. "UFBP1 Ameliorates Heat Stress-Induced Apoptosis via Mitochondria-Mediated Pathway in Bovine Mammary Epithelial Cells" Animals 15, no. 9: 1233. https://doi.org/10.3390/ani15091233
APA StyleLi, Y., Yu, R., Tan, S., Jiang, Y., Sun, L., Shen, M., Zhang, C., Chen, K., & Li, C. (2025). UFBP1 Ameliorates Heat Stress-Induced Apoptosis via Mitochondria-Mediated Pathway in Bovine Mammary Epithelial Cells. Animals, 15(9), 1233. https://doi.org/10.3390/ani15091233





