Acetate Affects the Process of Lipid Metabolism in Rabbit Liver, Skeletal Muscle and Adipose Tissue
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Protocol and Sample Collection
2.2. Measurements
2.3. Statistical Analysis
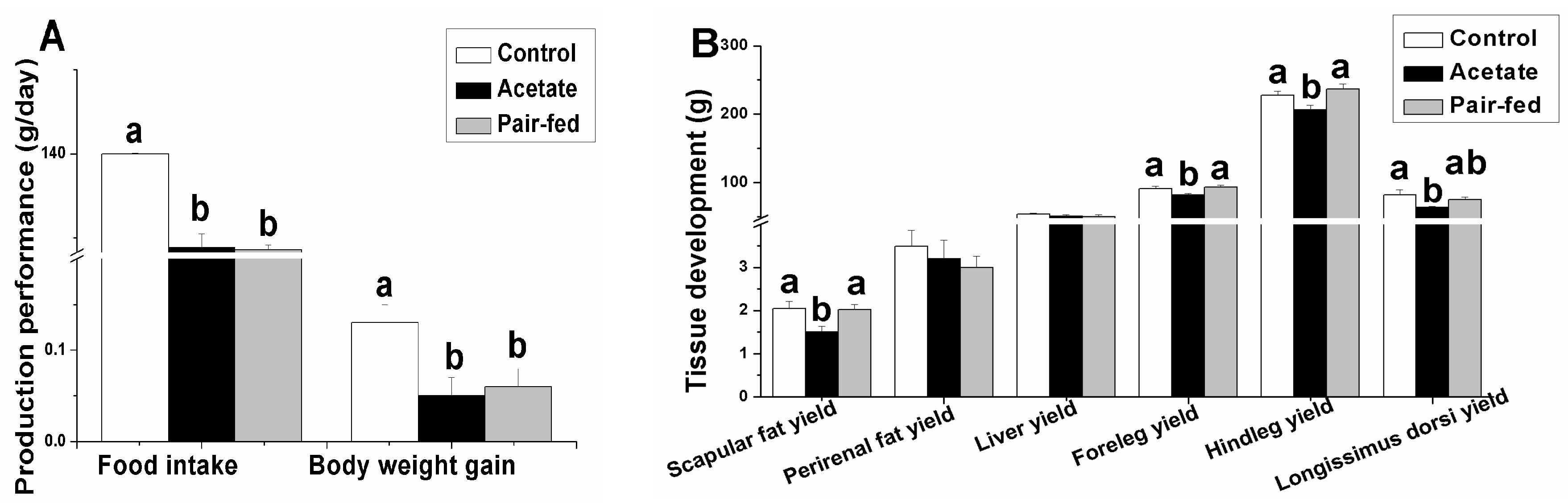
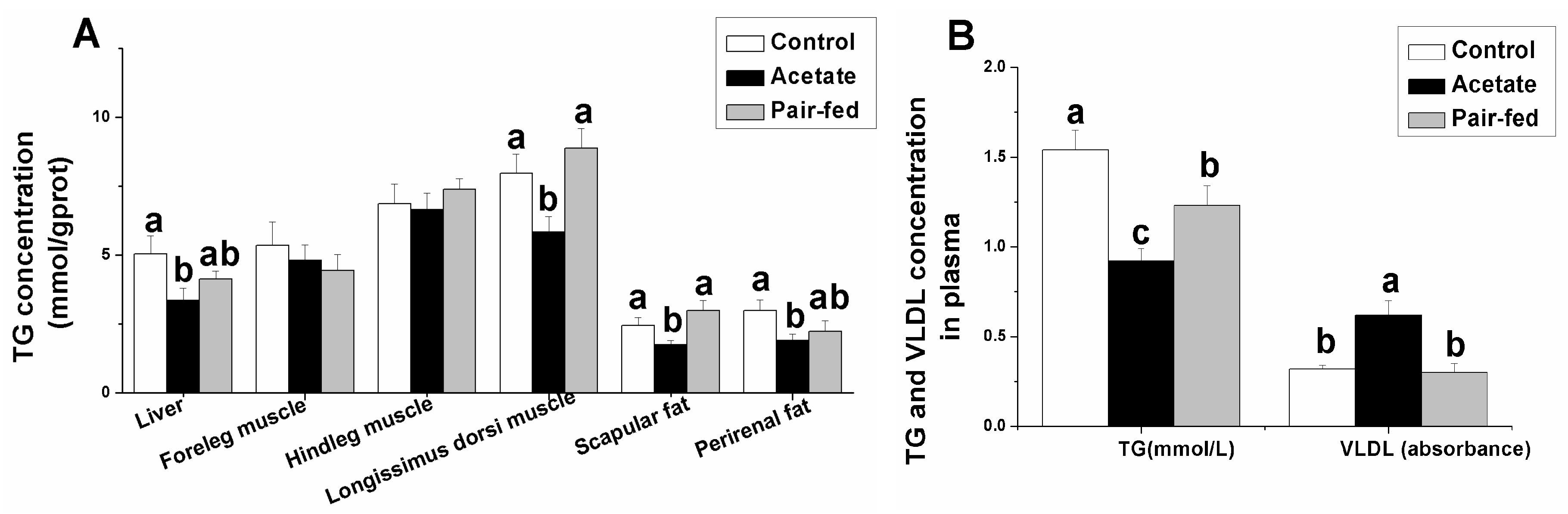
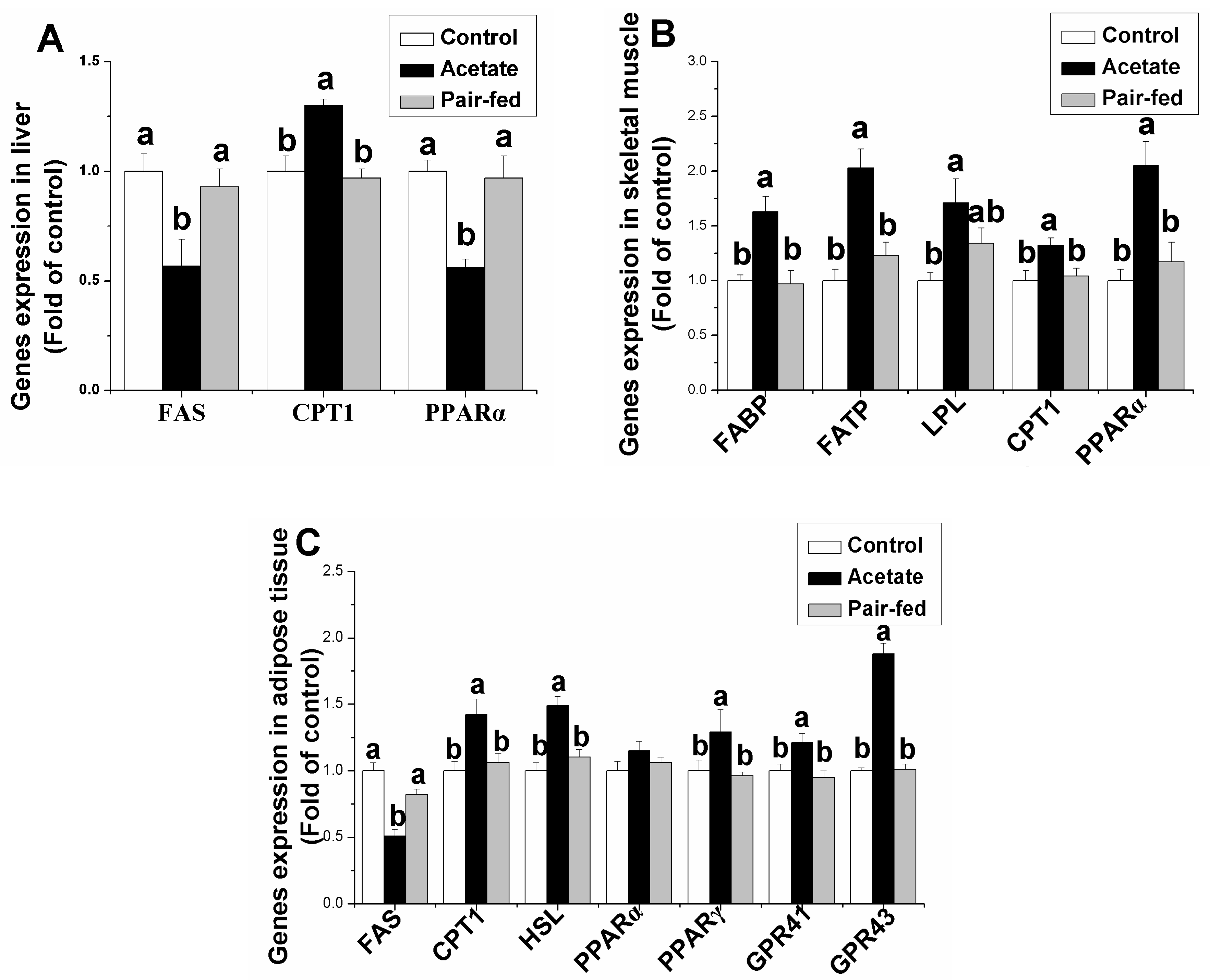
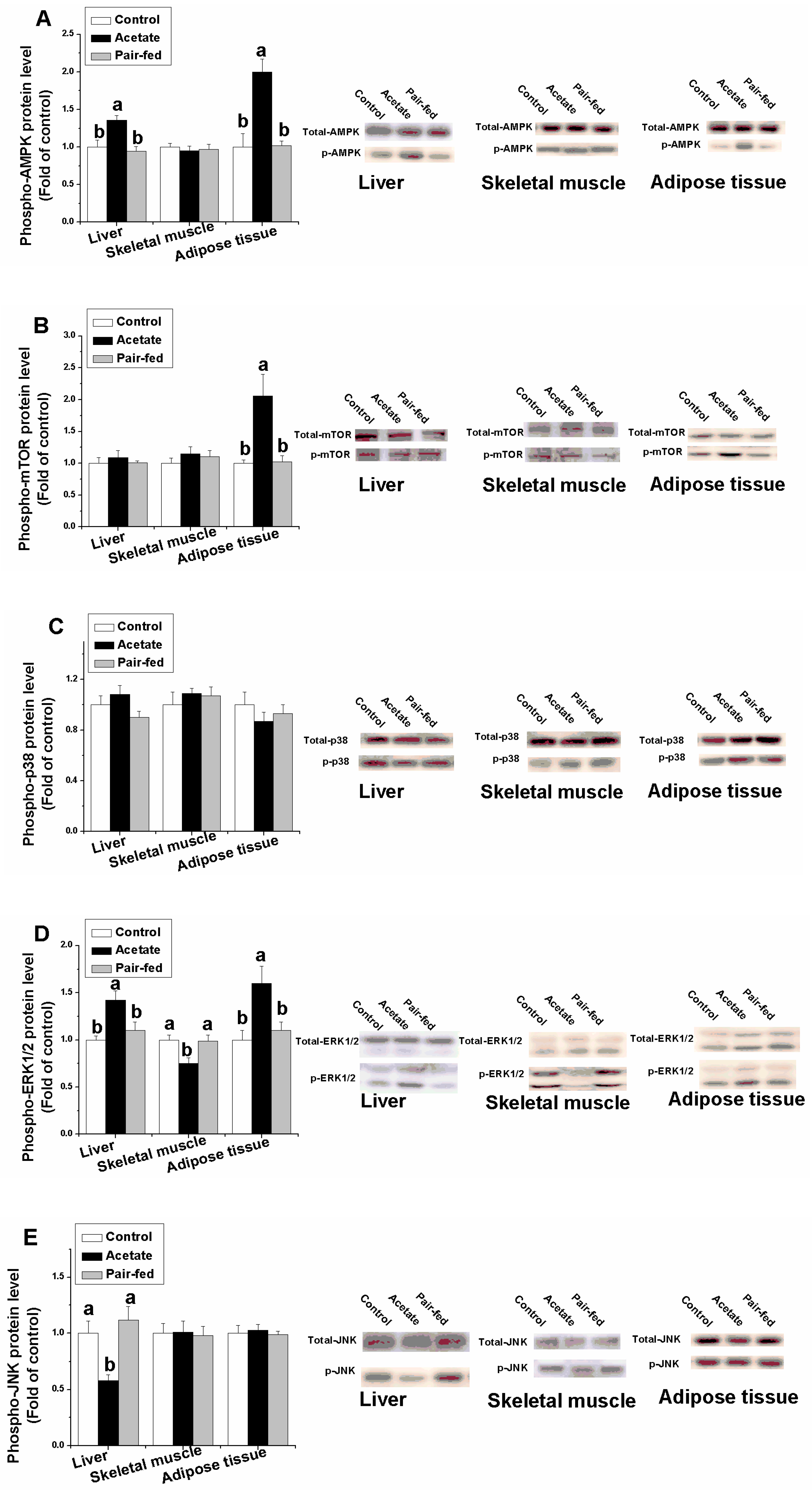
3. Results
3.1. Effect of Acetate on Rabbit Performance
3.2. Effect of Acetate on TG and VLDL Concentrations in Tissue or Plasma
3.3. Effect of Acetate on Genes Expression Related Lipid Metabolism in Liver, Skeletal Muscle and Adipose Tissue
3.4. Effect of Acetate on Protein Expression Related Lipid Metabolism in Liver, Skeletal Muscle and Adipose Tissue
4. Discussion
4.1. Acetate-Inhibited Lipid Accumulation
4.2. Acetate Reduced the Hepatic Lipid Content
4.3. Acetate Decreased the Intramuscular Lipid Content
4.4. PPAR Signaling Was Associated with the Acetate-Regulated Lipid Metabolism
4.5. MAPK Signaling Was Associated with the Acetate-Regulated Lipid Metabolism
4.6. GPR41/43 Signaling Pathway Was Associated with the Acetate-Regulated Lipid Metabolism in Adipose Tissue
4.7. AMPK Signaling Pathway Was Associated with the Acetate-Regulated Lipid Metabolism in Adipose Tissue
4.8. mTOR Signaling Was Associated with the Acetate-Regulated Lipid Metabolism in Adipose Tissue
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Frayn, K.N.; Arner, P.; Yki-Järvinen, H. Fatty acid metabolism in adipose tissue, muscle and liver in health and disease. Essays Biochem. 2006, 42, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, C.; Fu, C. Dietary Niacin supplementation suppressed hepatic lipid accumulation in rabbits. Asian-Australas J. Anim. Sci. 2016, 9, 1748–1755. [Google Scholar] [CrossRef] [PubMed]
- Thupari, J.N.; Pinn, M.L.; Kuhajda, F.P. Fatty acid synthase inhibition in human breast cancer cells leads to malonyl-CoA-induced inhibition of fatty acid oxidation and cytotoxicity. Biochem. Biophys. Res. Commun. 2001, 285, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.; Shen, W.J.; Nelson, B.D.; Patel, S.; Veerkamp, J.H.; Selwood, S.P.; Murphy, G.M., Jr.; Reaven, E.; Kraemer, F.B. Absence of cardiac lipid accumulation in transgenic mice with heart-specific HSL overexpression. Am. J. Physiol. Endocrinol. Metab. 2001, 281, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Pollare, T.; Vessby, B.; Lithell, H. Lipoprotein lipase activity in skeletal muscle is related to insulin sensitivity. Arter. Thromb. 1991, 11, 1192–1203. [Google Scholar] [CrossRef]
- Urban, T.; Mikolásová, R.; Kuciel, J.; Ernst, M.; Ingr, I. A study of associations of the H-FABP genotypes with fat and meat production of pigs. J. Appl. Genet. 2002, 43, 505–509. [Google Scholar]
- Lage, R.; Diéguez, C.; Vidal-Puig, A.; López, M. AMPK: A metabolic gauge regulating whole-body energy homeostasis. Trends Mol. Med. 2008, 14, 539–549. [Google Scholar] [CrossRef]
- Chinetti, G.; Fruchart, J.C.; Staels, B. Peroxisome proliferator-activated receptors (PPARs): Nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm. Res. 2000, 49, 497–505. [Google Scholar] [CrossRef]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef]
- Kersten, S. Mechanisms of nutritional and hormonal regulation of lipogenesis. EMBO Rep. 2001, 2, 282–286. [Google Scholar] [CrossRef]
- Camp, H.S.; Tafuri, S.R. Regulation of peroxisome proliferator-activated receptor gamma activity by mitogen-activated protein kinase. J. Biol. Chem. 1997, 272, 10811–10816. [Google Scholar] [CrossRef] [PubMed]
- Engelman, J.A.; Lisanti, M.P.; Scherer, P.E. Specific inhibitors of p38 mitogen-activated protein kinase block 3T3-L1 adipogenesis. J. Biol. Chem. 1998, 273, 32111–32120. [Google Scholar] [CrossRef]
- Hirosumi, J.; Tuncman, G.; Chang, L.; Görgün, C.Z.; Uysal, K.T.; Maeda, K.; Karin, M.; Hotamisligil, G.S. A central role for JNK in obesity and insulin resistance. Nature 2002, 420, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, A.; Czech, M.P.; Davis, R.J. An essential role of the JIP1 scaffold protein for JNK activation in adipose tissue. Genes Dev. 2004, 18, 1976–1980. [Google Scholar] [CrossRef]
- Polak, P.; Cybulski, N.; Feige, J.N.; Auwerx, J.; Rüegg, M.A.; Hall, M.N. Adipose-specific knockout of raptor results in lean mice with enhanced mitochondrial respiration. Cell Metab. 2008, 8, 399–410. [Google Scholar] [CrossRef]
- Yeh, W.C.; Bierer, B.E.; McKnight, S.L. Rapamycin inhibits clonal expansion and adipogenic differentiation of 3T3-L1 cells. Proc. Natl. Acad. Sci. USA 1995, 92, 11086–11090. [Google Scholar] [CrossRef]
- Rosen, E.D.; MacDougald, O.A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006, 7, 885–896. [Google Scholar] [CrossRef]
- Rabbani, G.H.; Albert, M.J.; Rahman, H.; Chowdhury, A.K. Short-chain fatty acids inhibit fluid and electrolyte loss induced by cholera toxin in proximal colon of rabbit in vivo. Dig. Dis. Sci. 1999, 44, 1547–1553. [Google Scholar] [CrossRef]
- Mineo, H.; Hashizume, Y.; Hanaki, Y.; Murata, K.; Maeda, H.; Onaga, T.; Kato, S.; Yanaihara, N. Chemical specificity of short-chain fatty acids in stimulating insulin and glucagon secretion in sheep. Am. J. Physiol.-Endocrinol. Metab. 1994, 267, 234–241. [Google Scholar] [CrossRef]
- Scheppach, W. Effects of short chain fatty acids on gut morphology and function. Gut 1994, 35, 35–38. [Google Scholar] [CrossRef]
- Sakata, T. Stimulatory effect of short-chain fatty acids on epithelial cell proliferation in the rat intestine: A possible explanation for trophic effects of fermentable fibre, gut microbes and luminal trophic factors. Br. J. Nutr. 1987, 58, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Heerdt, B.G.; Houston, M.A.; Augenlicht, L.H. Potentiation by specific short-chain fatty acids of differentiation and apoptosis in human colonic carcinoma cell lines. Cancer Res. 1994, 54, 3288–3294. [Google Scholar] [PubMed]
- Den Besten, G.; Bleeker, A.; Gerding, A.; van Eunen, K.; Havinga, R.; van Dijk, T.H.; Oosterveer, M.H.; Jonker, J.W.; Groen, A.K.; Reijngoud, D.J.; et al. Short-chain fatty acids protect against high-fat diet–induced obesity via a PPARγ-dependent switch from lipogenesis to fat oxidation. Diabetes 2015, 64, 2398–2408. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Liu, L.; Li, F. Acetate alters the process of lipid metabolism in rabbits. Animal 2017, 4, 1–8. [Google Scholar] [CrossRef]
- De Blas, C.; Mateos, G.G. Feed formulation. In Nutrition of the Rabbit; CAB International: Wallingford, UK, 1998; pp. 222–232. [Google Scholar]
- Barter, P.J.; Lally, J.I. Metabolism of esterified cholesterol in the plasma very low density lipoproteins of the rabbit. Atherosclerosis 1978, 31, 355–364. [Google Scholar] [CrossRef]
- Liu, L.; Liu, H.; Fu, C.; Li, C.; Li, F. Acetate induces anorexia via up-regulating the hypothalamic pro-opiomelanocortin (POMC) gene expression in rabbits. J. Anim. Feed Sci. 2017, 26, 266–273. [Google Scholar] [CrossRef]
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T.; Terasawa, K.; Kashihara, D.; Hirano, K.; Tani, T.; et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013, 4, 1829. [Google Scholar] [CrossRef]
- Kraemer, F.B.; Shen, W.J. Hormone-sensitive lipase knockouts. Nutr. Metab. 2006, 3, 1. [Google Scholar] [CrossRef]
- Milgraum, L.Z.; Witters, L.A.; Pasternack, G.R.; Kuhajda, F.P. Enzymes of the fatty acid synthesis pathway are highly expressed in situ breast carcinoma. Clin. Cancer Res. 1997, 3, 2115–2120. [Google Scholar]
- Bonnefont, J.P.; Demaugre, F.; Prip-Buus, C.; Saudubray, J.M.; Brivet, M.; Abadi, N.; Thuilliera, L. Carnitine palmitoyltransferase deficiencies. Mol. Genet. Metab. 1999, 68, 424–440. [Google Scholar] [CrossRef]
- Asai, A.; Miyazawa, T. Dietary curcuminoids prevent high-fat diet-induced lipid accumulation in rat liver and epididymal adipose tissue. J. Nutr. 2001, 131, 2932–2935. [Google Scholar] [CrossRef] [PubMed]
- Hara, H.; Haga, S.; Aoyama, Y.; Kiriyama, S. Short-chain fatty acids suppress cholesterol synthesis in rat liver and intestine. J. Nutr. 1999, 129, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Coppack, S.W.; Jensen, M.D.; Miles, J.M. In vivo regulation of lipolysis in humans. J. Lipid Res. 1994, 35, 177–193. [Google Scholar] [PubMed]
- Corcoran, M.P.; Lamon-Fava, S.; Fielding, R.A. Skeletal muscle lipid deposition and insulin resistance. effect of dietary fatty acids and exercise. Am. J. Clin. Nutr. 2007, 85, 662–677. [Google Scholar]
- Kersten, S.; Seydoux, J.; Peters, J.M.; Gonzalez, F.J.; Desvergne, B.; Wahli, W. Peroxisome proliferator-activated receptor α mediatesthe adaptive response to fasting. J. Clin. Investig. 1999, 103, 1489–1498. [Google Scholar] [CrossRef]
- Djouadi, F.; Weinheimer, C.J.; Saffitz, J.E.; Pitchford, C.; Bastin, J.; Gonzalez, F.J.; Kelly, P.D. A gender-related defect in lipid metabolism and glucose homeostasis in peroxisome proliferator-activated receptor α-deficient mice. J. Clin. Investig. 1998, 102, 1083–1091. [Google Scholar] [CrossRef]
- Napal, L.; Marrero, P.F.; Haro, D. An intronic peroxisome proliferator-activated receptor-binding sequence mediates fatty acid induction of the human carnitine palmitoyl-transferase 1A. J. Mol. Biol. 2005, 354, 751–759. [Google Scholar] [CrossRef]
- Hsu, S.C.; Huang, C.J. Reduced fat mass in rats fed a high oleic acid-rich safflower oil diet is associated with changes in expression of hepatic PPARalpha and adipose SREBP-1c-regulated genes. J. Nutr. 2006, 136, 1779–1785. [Google Scholar] [CrossRef]
- Evans, R.M.; Barish, G.D.; Wang, Y.X. PPARs and the complex journey to obesity. Nat. Med. 2004, 10, 355–361. [Google Scholar] [CrossRef]
- Couvigny, B.; de Wouters, T.; Kaci, G.; Jacouton, E.; Delorme, C.; Doré, J.; Renault, P.; Blottière, H.M.; Guédon, E.; Lapaque, N. Commensal streptococcus salivarius modulates PPARγ transcriptional activity in human intestinal epithelial cells. PLoS ONE 2015, 10, e0125371. [Google Scholar] [CrossRef]
- Li, G.; Yao, W.; Jiang, H. Short-chain fatty acids enhance adipocyte differentiation in the stromal vascular fraction of porcine adipose tissue. J. Nutr. 2014, 144, 1887–1895. [Google Scholar] [CrossRef] [PubMed]
- Sale, E.M.; Atkinson, P.G.; Sale, G.J. Requirement of MAP kinase for differentiation of fibroblasts to adipocytes, for insulin activation of p90 S6 kinase and for insulin or serum stimulation of DNA synthesis. EMBO J. 1995, 14, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Menendez, J.A.; Mehmi, I.; Atlas, E.; Colomer, R.; Lupu, R. Novel signaling molecules implicated in tumor-associated fatty acid synthase-dependent breast cancer cell proliferation and survival: Role of exogenous dietary fatty acids, p53-p21WAF1/CIP1, ERK1/2 MAPK, p27KIP1, BRCA1, and NF-kappaB. Int. J. Oncol. 2004, 24, 591–608. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, A.S.; Shen, W.J.; Muliro, K.; Patel, S.; Souza, S.C.; Roth, R.A.; Kraeme, B.F. Stimulation of lipolysis and hormone-sensitive lipase via the extracellular signal-regulated kinase pathway. J. Biol. Chem. 2001, 276, 45456–45461. [Google Scholar] [CrossRef] [PubMed]
- Turcotte, L.P.; Raney, M.A.; Todd, M.K. ERK1/2 inhibition prevents contraction-induced increase in plasma membrane FAT/CD36 content and FA uptake in rodent muscle. Acta Physiol. Scand. 2005, 184, 131–139. [Google Scholar] [CrossRef]
- Aouadi, M.; Laurent, K.; Prot, M.; Marchand-Brustel, Y.L.; Binetruy, B.; Bost, F. Inhibition of p38MAPK increases adipogenesis from embryonic to adult stages. Diabetes 2006, 55, 281–289. [Google Scholar] [CrossRef]
- Hata, K.; Nishimura, R.; Ikeda, F.; Yamashita, K.; Matsubara, T.; Nokubi, T.; Yoneda, T. Differential roles of Smad1 and p38 kinase in regulation of peroxisome proliferator-activating receptor gamma during bone morphogenetic protein 2-induced adipogenesis. Mol. Boil. Cell 2003, 14, 545–555. [Google Scholar] [CrossRef]
- Vijayvargia, R.; Mann, K.; Weiss, H.R.; Pownall, H.J.; Ruan, H. JNK deficiency enhances fatty acid utilization and diverts glucose from oxidation to glycogen storage in cultured myotubes. Obesity 2010, 8, 1701–1709. [Google Scholar] [CrossRef]
- Woo, J.H.; Lim, J.H.; Kim, Y.H.; Suh, S.I.; Min, D.S.; Chang, J.S.; Lee, Y.H.; Park, J.W.; Kwon, T.K. Resveratrol inhibits phorbol myristate acetate-induced matrix metalloproteinase-9 expression by inhibiting JNK and PKC delta signal transduction. Oncogene 2004, 23, 1845–1853. [Google Scholar] [CrossRef]
- Dewulf, E.M.; Cani, P.D.; Neyrinck, A.M.; Possemiers, S.; Van Holle, A.; Muccioli, G.G.; Deldicqued, L.; Bindelsa, L.B.; Pachikiana, B.D.; Soheta, F.M.; et al. Inulin-type fructans with prebiotic properties counteract GPR43 overexpression and PPARγ-related adipogenesis in the white adipose tissue of high-fat diet-fed mice. J. Nutr. Biochem. 2011, 22, 712–722. [Google Scholar] [CrossRef]
- Fu, C.Y.; Liu, L.; Gao, Q.; Sui, X.Y.; Li, F.C. Cloning, molecular characterization, and spatial and developmental expression analysis of GPR41 and GPR43 genes in New Zealand rabbits. Animal 2017, 11, 1798–1806. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.H.; Nishimura, Y.; Hishikawa, D.; Tsuzuki, H.; Miyahara, H.; Gotoh, C.; Feng, D.D.; Chen, C.; Lee, H.G.; Katoh, K.; et al. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology 2005, 146, 5092–5099. [Google Scholar] [CrossRef] [PubMed]
- Ruderman, N.; Prentki, M. AMP kinase and malonyl-CoA: Targets for therapy of the metabolic syndrome. Nat. Rev. Drug Discov. 2004, 3, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Loftus, T.M.; Jaworsky, D.E.; Frehywot, G.L.; Townsend, C.A.; Ronnett, G.V.; Lane, M.D.; Kuhajda, F.P. Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science 2000, 288, 2379–2381. [Google Scholar] [CrossRef]
- Andersson, U.; Filipsson, K.; Abbott, C.R.; Woods, A.; Smith, K.; Bloom, S.R.; Carling, D.; Small, C.J. AMP-activated protein kinase plays a role in the control of food intake. J. Biol. Chem. 1998, 279, 12005–12008. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef]
- Hardie, D.G. AMPK and SNF1: Snuffing out stress. Cell Metab. 2007, 6, 339–340. [Google Scholar] [CrossRef]
- Liu, D.D.; Han, C.C.; Wan, H.F.; He, F.; Xu, H.Y.; Wei, S.H.; Du, X.H.; Xu, F. Effects of inhibiting PI3K-Akt-mTOR pathway on lipid metabolism homeostasis in goose primary hepatocytes. Animal 2016, 10, 1319–1327. [Google Scholar] [CrossRef]
- Skiba-Cassy, S.; Lansard, M.; Panserat, S.; Médale, F. Rainbow trout genetically selected for greater muscle fat content display increased activation of liver TOR signaling and lipogenic gene expression. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, 1421–1429. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. An emerging role of mTOR in lipid biosynthesis. Curr. Biol. 2009, 19, 1046–1052. [Google Scholar] [CrossRef]
| Gene | Genebank Accession Number | Primers Sequences (5′–3′) | Product Size (bp) |
|---|---|---|---|
| GAPDH | NM_001082253 | F: TGCCACCCACTCCTCTACCTTCG | 163 |
| R: CCGGTGGTTTGAGGGCTCTTACT | |||
| β-actin | NM_001101683.1 | F:CGCAGAAACGAGACGAGATT | 168 |
| R:GCAGAACTTTGGGGACTTTG | |||
| GPR41 | XM_002722237.2 | F:CCATCTATCTCACCTCCCTGTTC | 130 |
| R:AACCAGCAGAGCCCACTGAC | |||
| GPR43 | XM_002722218.2 | F:CGTCCAACTTCCGCTGGTA | 146 |
| R:CTTGTACTGCACGGGGTAGG | |||
| PPARγ | NM_001082148.1 | F:GGAGCAGAGCAAAGAAGTCG | 111 |
| R:CTCACAAAGCCAGGGATGTT | |||
| FATP | XM_002722970 | F:GGCCTACCTCTCTGGTGATG | 111 |
| R:TCAGTGGTGGACACGTTCTC | |||
| FABP | XM_002716060 | F:AGCTGGTGGACAGCAAGAAT | 129 |
| R:TCAGGGTGATGATGTCTCCA | |||
| CPT1 | XM_002724092.2 | F:ATTCTCACCGCTTTGGGAGG | 196 |
| R:ACGGGGTTTTCTAGGAGCAC | |||
| FAS | KF201292.1 | F:ACCACGTCCAAGGAGAGCA | 112 |
| R:AGTTCTGCACCGAGTTGAGC | |||
| HSL | XM_008249691.2 | F: CCAGGCTAAACTCGCATCCA | 119 |
| R: ATTTGGCTCTCTGGACTGGC | |||
| LPL | NM_001177330.1 | F: TTCAACCACAGCAGCAAGAC | 141 |
| R: TAACAGCCAGTCCACCACAA | |||
| PPARα | XM_002723354 | F:AGGCCCTCTTCAGAACCTGT | 122 |
| R:GTGGCTTTCTGTTCCCAGAG |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Fu, C.; Li, F. Acetate Affects the Process of Lipid Metabolism in Rabbit Liver, Skeletal Muscle and Adipose Tissue. Animals 2019, 9, 799. https://doi.org/10.3390/ani9100799
Liu L, Fu C, Li F. Acetate Affects the Process of Lipid Metabolism in Rabbit Liver, Skeletal Muscle and Adipose Tissue. Animals. 2019; 9(10):799. https://doi.org/10.3390/ani9100799
Chicago/Turabian StyleLiu, Lei, Chunyan Fu, and Fuchang Li. 2019. "Acetate Affects the Process of Lipid Metabolism in Rabbit Liver, Skeletal Muscle and Adipose Tissue" Animals 9, no. 10: 799. https://doi.org/10.3390/ani9100799
APA StyleLiu, L., Fu, C., & Li, F. (2019). Acetate Affects the Process of Lipid Metabolism in Rabbit Liver, Skeletal Muscle and Adipose Tissue. Animals, 9(10), 799. https://doi.org/10.3390/ani9100799





