Potential of Molecular Weight and Structure of Tannins to Reduce Methane Emissions from Ruminants: A Review
Simple Summary
Abstract
1. Introduction
2. Production and Mitigation of Enteric Methane
3. Sources of Tannin and Global Perspectives
3.1. Sources and Chemical Diversity of Tannins
3.2. Global Perspectives for Tannin-Containing Feeds
4. Effects of Tannins in Ruminant Nutrition
4.1. Binding Effects of Tannins
4.1.1. Negative Effects of Tannins
4.1.2. Beneficial Effects of Tannins
4.2. Rumen Fermentation and Enteric Methane Production (in Vitro and in Vivo)
4.2.1. Mode of Action
4.2.2. The Mechanistic Effect of MW, Source and Subunit Interactions on in Vitro CH4 Reduction
4.2.3. Effects of MW, Source and Subunit Interactions on in Vivo CH4 Reduction
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Intergovernmental Panel on Climate Change (IPCC). Guidelines for National Greenhouse Gas Inventories. In Prepared by the National Greenhouse Gas Inventories Programme; Eggleston, H.S., Buendia, L., Miwa, K., Ngara, T., Tanabe, K., Eds.; IGES: Hayama, Kanagawa, Japan, 2006. [Google Scholar]
- Gerber, P.J.; Steinfeld, H.; Henderson, B.; Mottet, A.; Opio, C.; Dijkman, J.; Falcucci, A.; Tempio, G. Tackling Climate Change through Livestock: A Global Assessment of Emissions and Mitigation Opportunities; FAO: Rome, Italy, 2013. [Google Scholar]
- Johnson, K.A.; Johnson, D.E. Methane emissions from cattle. J. Anim. Sci. 1995, 73, 2483–2492. [Google Scholar] [CrossRef]
- Calsamiglia, S.; Ferret, A.; Reynolds, C.K.; Kristensen, N.B.; Van Vuuren, A.M. Strategies for optimizing nitrogen use by ruminants. Animal 2010, 4, 1184–1196. [Google Scholar] [CrossRef]
- Dijkstra, J.; Oenema, O.; van Groenigen, J.W.; Spek, J.W.; van Vuuren, A.M.; Bannink, A. Diet effects on urine composition of cattle and N2O emissions. Animal 2013, 7, 292–302. [Google Scholar] [CrossRef]
- Grainger, C.; Clarke, T.; Auldist, M.J.; Beauchemin, K.A.; McGinn, S.M.; Waghorn, G.C.; Eckard, R.J. Potential use of Acacia mearnsii condensed tannins to reduce methane emissions and nitrogen excretion from grazing dairy cows. Can. J. Anim. Sci. 2009, 89, 241–251. [Google Scholar] [CrossRef]
- Aboagye, I.A.; Oba, M.; Castillo, A.R.; Koenig, K.M.; Iwaasa, A.D.; Beauchemin, K.A. Effects of hydrolysable tannin with or without condensed tannin on methane emissions, nitrogen use, and performance of beef cattle fed a high forage diet. J. Anim. Sci. 2018, 96, 5276–5286. [Google Scholar] [CrossRef] [PubMed]
- Aboagye, I.A.; Oba, M.; Koenig, K.M.; Zhao, G.Y.; Beauchemin, K.A. Use of gallic acid and hydrolysable tannins to reduce methane emission and nitrogen excretion in beef cattle fed a diet containing alfalfa silage. J. Anim. Sci. 2019, 97, 2230–2244. [Google Scholar] [CrossRef] [PubMed]
- Bhatta, R.; Uyeno, Y.; Tajima, K.; Takenaka, A.; Yabumoto, Y.; Nonaka, I.; Enishi, O.; Kurihara, M. Difference in the nature of tannins on in vitro ruminal methane and volatile fatty acid production and on methanogenic archaea and protozoal populations. J. Dairy Sci. 2009, 92, 5512–5522. [Google Scholar] [CrossRef] [PubMed]
- Jayanegara, A.; Goel, G.; Makkar, H.P.S.; Becker, K. Divergence between purified hydrolysable and condensed tannin effects on methane emission, rumen fermentation and microbial population in vitro. Anim. Feed Sci. Technol. 2015, 209, 60–68. [Google Scholar] [CrossRef]
- Jayanegara, A.; Leiber, F.; Kreuzer, M. Meta-analysis of the relationship between dietary tannin level and methane formation in ruminants from in vivo and in vitro experiments. J. Anim. Physiol. Anim. Nutr. 2012, 96, 365–375. [Google Scholar] [CrossRef]
- Sliwinski, B.J.; Kreuzer, M.; Wettstein, H.R.; Machmuller, A. Rumen fermentation and nitrogen balance of lambs fed diets containing plant extracts rich in tannins and saponins, and associated emissions of nitrogen and methane. Arch. Anim. Nutr. 2002, 56, 379–392. [Google Scholar]
- Zeller, W.E. Activity, purification, and analysis of condensed tannins: Current state of affairs and future endeavors. Crop Sci. 2019, 59, 886–904. [Google Scholar] [CrossRef]
- Naumann, H.D.; Tedeschi, L.O.; Huntley, N.F. The role of condensed tannins in ruminant animal production: Advances, limitations and future directions. Rev. Bras. Zootec. 2017, 46, 929–949. [Google Scholar] [CrossRef]
- Naumann, H.; Sepela, R.; Rezaire, A.; Masih, S.E.; Zeller, W.E.; Reinhardt, L.A.; Robe, J.T.; Sullivan, M.L.; Hagerman, A.E. Relationships between structures of condensed tannins from Texas legumes and methane production during in vitro rumen digestion. Molecules 2018, 23, 2123. [Google Scholar] [CrossRef] [PubMed]
- Thauer, R. Biochemistry of methanogenesis: A tribute to Marjory Stephenson. Microbiology 1998, 144, 2377–2406. [Google Scholar] [CrossRef]
- Paul, K.; Nonoh, J.O.; Mikulski, L.; Brune, A. Methanoplasmatales, Thermoplasmatales-related archaea in termite guts and other environments: Are the seventh order of methanogens. Appl. Environ. Microbiol. 2012, 78, 8245–8253. [Google Scholar] [CrossRef]
- Poulsen, M.; Schwab, C.; Jensen, B.B.; Engberg, R.M.; Spang, A.; Canibe, N.; Hojberg, O.; Milinovich, G.; Fragner, L.; Schleper, C.; et al. Methylotrophic methanogenic Thermoplasmata implicated in reduced methane emissions from bovine rumen. Nat. Commun. 2013, 4, 1428. [Google Scholar] [CrossRef]
- Morgavi, D.P.; Forano, E.; Martin, C.; Newbold, C.J. Microbial ecosystem and methanogenesis in ruminants. Animal 2010, 4, 1024–1036. [Google Scholar] [CrossRef]
- Hegarty, R.S.; Gerdes, R. Hydrogen production and transfer in the rumen. Rec. Adv. Anim. Nutr. Austr. 1999, 12, 37–44. [Google Scholar]
- Hristov, A.N.; Oh, J.; Firkins, J.L.; Dijkstra, J.; Kebreab, E.; Waghorn, G.; Makkar, H.P.S.; Adesogan, A.T.; Yang, W.; Lee, C.; et al. Special topics-Mitigation of methane and nitrous oxide emissions from animal operations: I. A review of enteric methane mitigation options. J. Anim. Sci. 2013, 91, 5045–5069. [Google Scholar] [CrossRef]
- Eckard, R.J.; Grainger, C.; de Klein, C.A.M. Options for the abatement of methane and nitrous oxide from ruminant production: A review. Livest. Sci. 2010, 130, 47–56. [Google Scholar] [CrossRef]
- Patra, A.K.; Saxena, J. Exploitation of dietary tannins to improve rumen metabolism and ruminant nutrition. J. Sci. Food Agric. 2011, 91, 24–37. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, X.; Zhao, G.; Hu, T.; Wang, Y. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim. Nutr. 2018, 4, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Mueller-Harvey, I.; Bee, G.; Dohme-Meier, F.; Hoste, H.; Karonen, M.; Kölliker, R.; Lüscher, A.; Niderkorn, V.; Pellikaan, W.F.; Salminen, J.-P.; et al. Benefits of condensed tannins in forage legumes fed to ruminants: Importance of structure, concentration and diet composition. Crop Sci. 2019, 59, 861–885. [Google Scholar] [CrossRef]
- Hagerman, A.E. Tannin Handbook; Department of Chemistry and Biochemistry, Miami University: Oxford, OH, USA, 2011; Available online: http://www.users.muohio.edu/hagermae (accessed on 8 August 2019).
- Herbert, R.B. The biosynthesis of secondary metabolites; Chapman and Hall: London, UK, 1989. [Google Scholar]
- Waghorn, G. Beneficial and detrimental effects of dietary condensed tannins for sustainable sheep and goat production: Progress and challenges. Anim. Feed Sci. Technol. 2008, 147, 116–139. [Google Scholar] [CrossRef]
- Top, S.; Preston, C.; Dukes, J.S.; Tharayil, N. Climate influences the content and chemical composition of foliar tannins in green and senesced tissues of Quercus rubra. Front. Plant Sci. 2017, 8, 423. [Google Scholar] [CrossRef]
- Iwaasa, A.D.; Sottie, E.; Svendsen, E.; Coulman, B.; Biligetu, B.; Acharya, S.; Dyck, D.; Jefferson, P. Sainfoin for Western Canada; Agriculture and Agri-Food Canada: Swift Current, SK, Canada, 2018. [Google Scholar]
- Kardel, M.; Taube, F.; Schulz, H.; Schütze, W.; Gierus, M. Different approaches to evaluate tannin content and structure of selected plant extracts–review and new aspects. J. Appl. Bot. Food Qual. 2013, 86, 154–166. [Google Scholar]
- Terrill, T.H.; Rowan, A.M.; Douglas, G.B.; Barry, T.N. Determination of extractable and bound condensed tannin concentrations in forage plants, protein concentrate meals and cereal grains. J. Sci. Food Agric. 1992, 58, 321–329. [Google Scholar] [CrossRef]
- Jackson, F.S.; McNabb, W.C.; Barry, T.N.; Foo, L.Y.; Peters, J.S. The condensed tannin content of a range of subtropical and temperate forages and the reactivity of condensed tannin with ribulose 1,5-bisphosphate carboxylase (rubisco) protein. J. Sci. Food Agric. 1996, 72, 483–492. [Google Scholar] [CrossRef]
- Schreurs, N.M.; Tavendale, M.H.; Lane, G.A.; Barry, T.N.; Lopez-Villalobos, N.; McNabb, W.C. Effect of different condensed tannin-containing forages, forage maturity and nitrogen fertilizer application on the formation of indole and skatole in in vitro rumen fermentation. J. Sci. Food Agric. 2007, 87, 1076–1087. [Google Scholar] [CrossRef]
- Berard, N.C.; Wang, Y.; Wittenberg, K.M.; Krause, D.O.; Coulman, B.E.; McAllister, T.A.; Ominski, K.H. Condensed tannin concentrations found in vegetative and mature forage legumes grown in western Canada. Can. J. Plant Sci. 2011, 91, 669–675. [Google Scholar] [CrossRef]
- McMahon, L.R.; Majak, W.; McAllister, T.A.; Hall, J.W.; Jones, G.; Popp, J.D.; Cheng, K.-J. Effect of sainfoin on in vitro digestion of fresh alfalfa and bloat in steers. Can. J. Anim. Sci. 1999, 79, 203–212. [Google Scholar] [CrossRef]
- Waghorn, G.C.; Tavendale, M.H.; Woodfield, D.R. Methanogenesis from forages fed to New Zealand ruminants. Proc. N. Z. Grassl. Assoc. 2002, 64, 167–171. [Google Scholar]
- Hove, L.; Topps, J.H.; Sibanda, S.; Ndlovu, L.R. Nutrient intake and utilization by goats fed dried leaves of the shrub legumes Acacia angustissima, Calliandra calothyrsus and Leucaena leucocephala as supplements to native pasture hay. Anim. Feed Sci. Technol. 2001, 91, 95–106. [Google Scholar] [CrossRef]
- Priolo, A.; Waghorn, G.C.; Lanza, M.; Biondi, L.; Pennisi, P. Polyethylene glycol as a means to reducing the impact of condenced tannins in carob pulp: Effect on lamb growth and meat quality. J. Anim. Sci. 2000, 78, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Silanikove, N.; Landau, S.; Or, D.; Kababya, D.; Bruckental, I.; Nitsan, Z. Analytical approach and effects of condensed tannins in carob pod (Ceratonia siliqua) on feed intake, digestive and metabolic responses of kids. Livest. Sci. 2006, 99, 29–38. [Google Scholar] [CrossRef]
- Barahona, R.; Lascano, C.E.; Cochran, R.; Morrill, J.; Titgemeyer, E.C. Intake, digestion, and nitrogen utilization by sheep fed tropical legumes with contrasting tannin concentration and astringency. J. Anim. Sci. 1997, 75, 1633–1640. [Google Scholar] [CrossRef]
- Smith, A.H.; Odenyo, A.A.; Osuji, P.O.; Wallig, M.A.; Kandil, F.E.; Seigler, D.S.; Mackie, R.I. Evaluation of toxicity of Acacia angustissima in a rat bioassay. Anim. Feed. Sci. Technol. 2001, 91, 41–57. [Google Scholar] [CrossRef]
- Norton, B.W. Nutritive value of tree legumes. In Forage Tree Legumes in Tropical Agriculture; Gutteridge, R.C., Shelton, H.M., Eds.; CAB International: Wallingford, UK, 1994; pp. 177–191. [Google Scholar]
- Gemeda, B.S.; Hassen, A. Effect of tannin and species variation on in vitro digestibility, gas and methane production of tropical browse plants. Asian Australas. J. Anim. Sci. 2015, 28, 188–199. [Google Scholar] [CrossRef]
- Tahrouch, S.; Andary, C.; Rapior, S.; Mondolot, L.; Gargadennec, A.; Fruchier, A. Polyphenol investigation of Argania spinosa (Sapotaceae) endemic tree from Morocco. Acta Bot. Gall. 2000, 147, 225–232. [Google Scholar] [CrossRef]
- Makkar, H.P.S. Effects and fate of tannins in ruminant animals, adaptation to tannins, and strategies to overcome detrimental effects of feeding tannin-rich feeds. Small Rumin. Res. 2003, 49, 241–256. [Google Scholar] [CrossRef]
- Jerónimo, E.; Pinheiro, C.; Lamy, E.; Dentinho, M.T.; Sales-Baptista, E.; Lopes, O.; Silva, F.C. Tannins in ruminant nutrition: Impact on animal performance and quality of edible products. In Tannins: Biochemistry, Food Sources and Nutritional Properties; Combs, C.A., Ed.; Nova Science Publisher Inc.: Hauppauge, NY, USA, 2016; pp. 121–168. [Google Scholar]
- Min, B.R.; Pinchak, W.E.; Hernandez, K.; Hernandez, C.; Hume, M.E.; Valencia, E.; Fulford, J.D. The effect of plant tannins supplementation on animal responses and in vivo ruminal bacterial populations associated with bloat in heifers grazing wheat forage. Prof. Anim. Sci. 2012, 28, 464–472. [Google Scholar] [CrossRef]
- Martínez-Ortíz-de-Montellano, C.; Vargas-Maga˜na, J.J.; Canul-Ku, H.L.; Miranda-Soberanis, R.; Capetillo-Leal, C.; Sandoval-Castro, C.A.; Hoste, H.; Torres-Acosta, J.F.J. Effect of a tropical tannin-rich plant Lysiloma latisiliquum on adult populations of Haemonchus contortus in sheep. Vet. Parasitol. 2010, 172, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Woodward, S.L.; Waghorn, G.C.; Laboyrie, P.G. Condensed tannins in birdsfoot trefoil (Lotus corniculatus) reduce methane emissions from dairy cows. Proc. N. Z. Soc. Anim. Prod. 2004, 64, 160–164. [Google Scholar]
- Dschaak, C.M.; Williams, C.M.; Holt, M.S.; Eun, J.S.; Young, A.J.; Min, B.R. Effects of supplementing condensed tannin extract on intake, digestion, ruminal fermentation, and milk production of lactating dairy cows. J. Dairy Sci. 2011, 94, 2508–2519. [Google Scholar] [CrossRef] [PubMed]
- Henke, A.; Dickhoefer, U.; Westreicher-Kristen, E.; Knappstein, K.; Molkentin, J.; Hasler, M.; Susenbeth, A. Effect of dietary Quebracho tannin extract on feed intake, digestibility, excretion of urinary purine derivatives and milk production in dairy cows. Arch. Anim. Nutr. 2017, 71, 37–53. [Google Scholar] [CrossRef]
- Robins, C.; Brooker, J.D. The effects of Acacia aneura feeding on abomasal and intestinal structure and function in sheep. Anim. Feed Sci. Technol. 2005, 121, 205–215. [Google Scholar] [CrossRef]
- Garg, S.K.; Makkar, H.P.S.; Nagal, K.B.; Sharma, S.K.; Wadhwa, D.R.; Singh, B. Toxicological investigations into oak (Quercus incana) leaf poisoning in cattle. Vet. Hum. Toxicol. 1992, 34, 161–164. [Google Scholar]
- Kumar, R.; Singh, M. Tannins: Their adverse role in ruminant nutrition. J. Agric. Food Chem. 1984, 32, 447–453. [Google Scholar] [CrossRef]
- Lesschaeve, I.; Noble, A.C. Polyphenols: Factors influencing their sensory properties and their effects on food and beverage preferences. Am. J. Clin. Nutr. 2005, 81, 330S–335S. [Google Scholar] [CrossRef]
- Reed, J.D. Nutritional toxicology of tannins and related polyphenols in forage legumes. J. Anim. Sci. 1995, 73, 1516–1528. [Google Scholar] [CrossRef]
- Murdiati, T.B.; McSweeney, C.S.; Lowry, J.B. Metabolism in sheep of gallic acid, tannic acid, and hydrolysable tannin from Terminalia oblongata. Aust. J. Agric. Res. 1992, 43, 1307–1319. [Google Scholar] [CrossRef]
- Min, B.R.; Barry, T.N.; Attwood, G.T.; McNabb, W.C. The effect of condensed tannins on the nutrition and health of ruminants fed fresh temperate forages. A review. Anim. Feed Sci. Technol. 2003, 106, 3–19. [Google Scholar] [CrossRef]
- Gladine, C.; Rock, E.; Morand, C.; Bauchart, D.; Durand, D. Bioavailability and antioxidant capacity of plant extracts rich in polyphenols, given as a single acute dose, in sheep made highly susceptible to lipoperoxidation. Br. J. Nutr. 2007, 98, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Díaz, M.A.; Torres-Acosta, J.F.J.; Sandoval-Castro, C.A.; Hoste, H. Tannins in tropical tree fodders fed to small ruminants: A friendly foe? Small. Rumin. Res. 2010, 89, 164–173. [Google Scholar] [CrossRef]
- Lamy, E.; da Costa, G.; Santos, R.; Capela Silva, F.; Potes, J.; Pereira, A.; Coelho, A.V.; Sales- Baptista, E. Effect of condensed tannin ingestion in sheep and goat parotid saliva proteome. J. Anim. Physiol. Anim. Nutr. 2011, 95, 304–312. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; McGinn, S.M.; Martinez, T.F.; McAllister, T.A. Use of condensed tannin extract from quebracho trees to reduce methane emissions from cattle. J. Anim. Sci. 2007, 85, 1990–1996. [Google Scholar] [CrossRef]
- Deschamps, A.M.; Mohudeau, G.; Conti, M.; Lebeault, L.M. Bacteria degrading tannic acid and related compounds. J. Ferment Technol. 1980, 58, 93–97. [Google Scholar]
- Krumholz, L.R.; Bryant, M.P. Eubacterium oxidoreducens sp. nov. requiring H2 or formate to degrade gallate, pyrogallol, phloroglucinol and quercetin. Arch. Microbiol. 1986, 144, 8–14. [Google Scholar]
- Bhat, T.K.; Singh, B.; Sharma, O.P. Microbial degradation of tannins–a current perspective. Biodegradation 1998, 9, 343–357. [Google Scholar] [CrossRef]
- Field, J.A.; Kortekaas, S.; Lettinga, G. The tannin theory of methanogenic toxicity. Biol. Wastes 1989, 29, 241–262. [Google Scholar] [CrossRef]
- Carrasco, J.M.D.; Cabral, C.; Redondo, L.M.; Viso, N.D.P.; Colombatto, D.; Farber, M.D.; Miyakawa, M.E.F. Impact of chestnut and quebracho tannins on rumen microbiota of bovines. BioMed. Res. Int. 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Carulla, J.E.; Kreuzer, M.; Machmüller, A.; Hess, H.D. Supplementation of Acacia mearnsii tannins decreases methanogenesis and urinary nitrogen in forage-fed sheep. Austr. J. Agric. Res. 2005, 56, 961–970. [Google Scholar] [CrossRef]
- Becker, P.M.; Wikselaar, P.G.; Franssen, M.C.R.; Vos, R.C.H.; Hall, R.D.; Beekwilder, J. Evidence for a hydrogen-sink mechanism of (+) catechin-mediated emission reduction of the ruminant greenhouse gas methane. Metabolomics 2014, 10, 179–189. [Google Scholar] [CrossRef]
- Naumann, H.D.; Tedeschi, L.O.; Muir, J.P.; Lambert, B.D.; Kothmann, M.M. Effect of molecular weight of condensed tannins from warm-season perennial legumes on ruminal methane production in vitro. Biochem. Syst. Ecol. 2013, 50, 154–162. [Google Scholar] [CrossRef]
- Hassanat, F.; Benchaar, C. Assessment of the effect of condensed (acacia and quebracho) and hydrolysable (chestnut and valonea) tannins on rumen fermentation and methane production in vitro. J. Sci. Food Agric. 2013, 3, 332–339. [Google Scholar] [CrossRef]
- Mengistu, G.; Karonen, M.; Salminen, J.-P.; Hendriks, W.H.; Pellikaan, W.F. In vitro fermentation of browse species using goat rumen fluid in relation to browse polyphenol content and composition. Anim. Feed Sci. Technol. 2017, 231, 1–11. [Google Scholar] [CrossRef]
- Pellikaan, W.F.; Stringano, E.; Leenaars, J.; Bongers, D.J.G.M.; Van Laar-van Schuppen, S.; Plant, J.; Mueller-Harvey, I. Evaluating effects of tannins on extent and rate of in vitro gas and CH4 production using an automated pressure evaluation system (APES). Anim. Feed Sci. Technol. 2011, 166, 377–390. [Google Scholar] [CrossRef]
- Rira, M.; Morgavi, D.P.; Genestoux, L.; Djibiri, S.; Sekhri, I.; Doreau, M. Methanogenic potential of tropical feeds rich in hydrolysable tannins. J. Anim. Sci. 2019, 97, 2700–2710. [Google Scholar] [CrossRef]
- Saminathan, M.; Sieo, C.C.; Gan, H.M.; Abdullah, N.; Wong, C.M.V.L.; Ho, Y.W. Effects of condensed tannin fractions of different molecular weights on population and diversity of bovine rumen methanogenic archaea in vitro, as determined by high-throughput sequencing. Anim. Feed Sci. Technol. 2016, 216, 146–160. [Google Scholar] [CrossRef]
- Saminathan, M.; Sieo, C.C.; Abdullah, N.; Wong, C.; Ho, Y.W. Effects of condensed tannin fractions of different molecular weights from a Leucaena leucocephala hybrid on in vitro methane production an rumen fermentation. J. Sci. Food Agric. 2015, 95, 2742–2749. [Google Scholar] [CrossRef]
- Soltan, Y.A.; Morsy, A.S.; Sallam, S.M.A.; Louvandini, H.; Abdalla, A.L. Comparative in vitro evaluation of forage legumes (prosopis, acacia, atriplex, and leucaena) on ruminal fermentation and methanogenesis. J. Anim. Feed Sci. 2012, 21, 759–772. [Google Scholar] [CrossRef]
- Tan, H.Y.; Sieo, C.C.; Abdullah, N.; Liang, J.B.; Huang, X.D.; Ho, Y.W. Effects of condensed tannins from Leucaena on methane production, rumen fermentation and populations of methanogens and protozoa in vitro. Anim. Feed Sci. Technol. 2011, 169, 185–193. [Google Scholar] [CrossRef]
- Tavendale, M.H.; Meagher, L.P.; Pacheco, D.; Walker, N.; Attwood, G.T.; Sivakumaran, S. Methane production from in vitro rumen incubations with Lotus pedunculatus and Medicago sativa, and effects of extractable condensed tannin fractions on methanogenesis. Anim. Feed Sci. Technol. 2005, 123, 403–419. [Google Scholar] [CrossRef]
- Ebert, P.J.; Bailey, E.A.; Shreck, A.L.; Jennings, J.S.; Cole, N.A. Effect of condensed tannin extract supplementation on growth performance, nitrogen balance, gas emissions, and energetic losses of beef steers. J. Anim. Sci. 2017, 95, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Lima, P.R.; Apdini, T.; Freire, A.S.; Santana, A.S.; Moura, L.M.L.; Nascimento, J.C.S.; Rodrigues, R.T.S.; Dijkstra, J.; Garcez Neto, A.F.; Queiroz, M.A.A.; et al. Dietary supplementation with tannin and soybean oil on intake, digestibility, feeding behavior, ruminal protozoa and methane emission in sheep. Anim. Feed Sci. Technol. 2019, 249, 10–17. [Google Scholar] [CrossRef]
- Liu, H.Y.; Puchala, R.; LeShure, S.; Gipson, T.A.; Goetsch, A.L. Effects of lespedeza condensed tannins alone or with monensin, soybean oil, and coconut oil on feed intake, growth, digestion, ruminal methane mission, and heat energy by yearling Alpine doelings. J Anim Sci. 2019, 97, 885–889. [Google Scholar] [CrossRef]
- Malik, P.K.; Kolte, A.P.; Baruah, L.; Saravanan, M.; Bakshi, B.; Bhatta, R. Enteric methane mitigation in sheep through leaves of selected tanniniferous tropical tree species. Livest. Sci. 2017, 200, 29–34. [Google Scholar] [CrossRef]
- Stewart, E.K.; Beauchemin, K.A.; Dai, X.; MacAdam, J.W.; Christensen, R.G.; Villalba, J.J. Effect of tannin-containing hays on enteric methane emissions and nitrogen partitioning in beef cattle. J. Anim. Sci. 2019, 97, 3286–3299. [Google Scholar] [CrossRef]
- Supapong, C.; Cherdthonga, A.; Seankamsorna, A.; Khonkhaenga, B.; Wanapata, M.; Gununb, N.; Gununc, P.; Chanjulad, P.; Polyorach, S. Effect of Delonix regia seed meal supplementation in Thai native beef cattle on feed intake, rumen fermentation characteristics and methane production. Anim. Feed Sci. Technol. 2017, 232, 40–48. [Google Scholar] [CrossRef]
- Yang, K.; Wei, C.; Zhao, G.Y.; Xu, Z.W.; Lin, S.X. Effects of dietary supplementing tannic acid in the ration of beef cattle on rumen fermentation, methane emission, microbial flora and nutrient digestibility. J. Anim. Physiol. Anim. Nutr. 2016, 101, 302–310. [Google Scholar] [CrossRef]
- Silanikove, N. The physiological basis of adaptation in goats to harsh environments. Small Rumin. Res. 2000, 35, 181–193. [Google Scholar] [CrossRef]
- Vyas, D.; Alemu, A.W.; McGinn, S.M.; Duval, S.M.; Kindermann, M.; Beauchemin, K.A. The combined effects of supplementing monensin and 3-nitrooxypropanol on methane emissions, growth rate, and feed conversion efficiency in beef cattle fed high forage and high grain diets. J. Anim. Sci. 2018, 96, 2923–2938. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T. Salivary proteins as a defense against dietary tannins. J. Chem. Ecol. 2006, 32, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
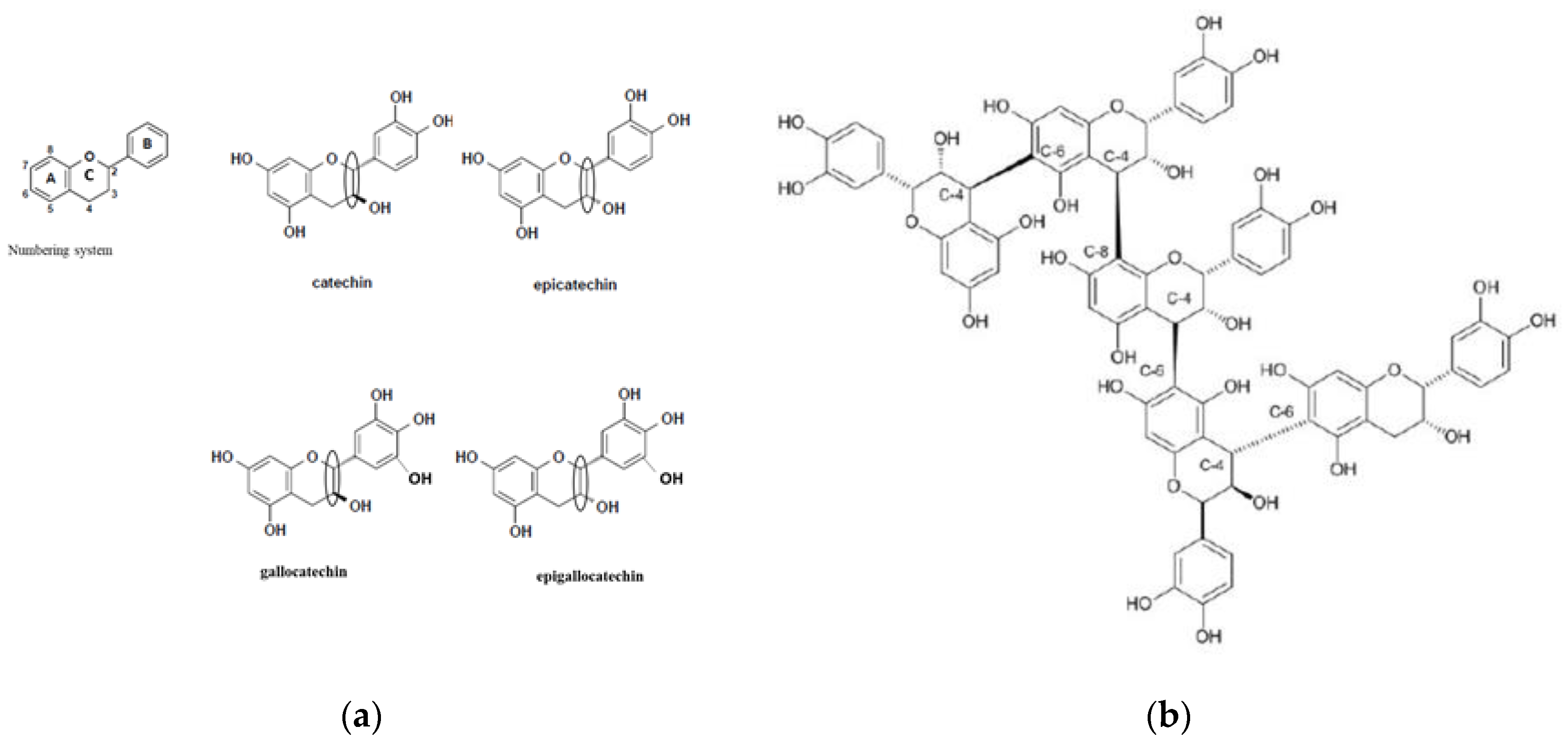
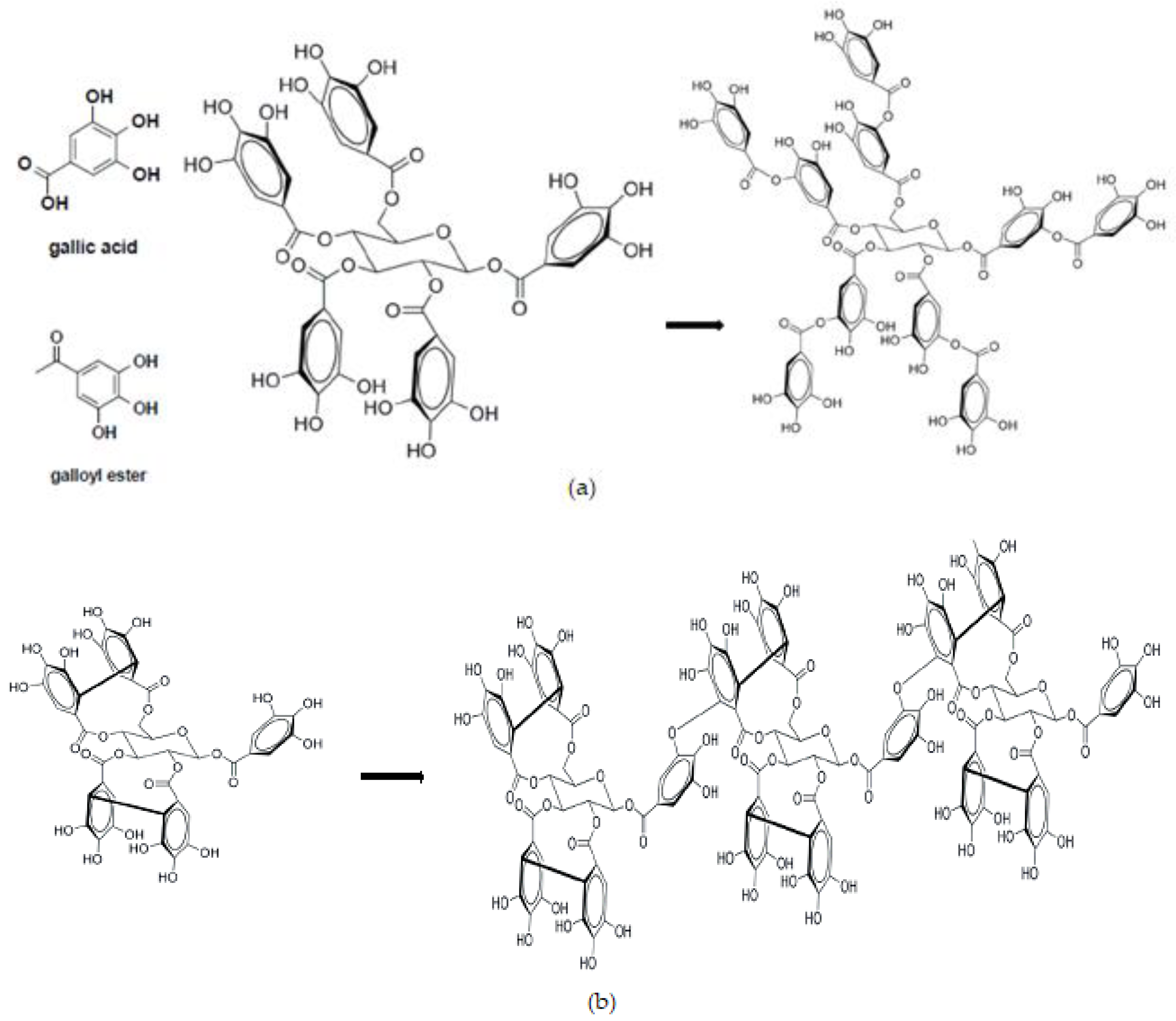

| Source | Forages | Tannin 1 | |
|---|---|---|---|
| (g/100 g DM) | Type | ||
| Legumes (temperate) | |||
| Terrill et al. [32], Jackson et al. [33] | Birdsfoot trefoil (Lotus corniculatus) | 0.7 to 4.0 | CT |
| Terrill et al. [32] | Crownvetch (Coronilla varia) | 1.6 | CT |
| Terrill et al. [32], Schreurs et al. [34] | Greater birdsfoot trefoil (Lotus pedunculatus) | 6.1 to 9.9 | CT |
| Berard et al. [35] | Purple prairie clover (Dalea purpurea) | 3.8 to 9.3 | CT |
| Jackson et al. [33], Berard et al. [35] | Red clover (Trifolium pratense) | 0.04 to 1.53 | CT |
| Berard et al. [35], McMahon et al. [36] | Sainfoin (Onobrychis viciifolia) | 1.6 to 9.4 | CT |
| Terrill et al. [32] | Serradella (Ornithopus sativus) | 0.4 | CT |
| Terrill et al. [32], Jackson et al. [33], Waghorn et al. [37] | Sulla (Hedysarum coronarium) | 3.3 to 6.8 | CT |
| Schreurs et al. [34], Berard et al. [35] | White clover (Trifolium repens) | 0.1 to 1.2 | CT |
| Legumes (tropical) | |||
| Jackson et al. [33], Hove et al. [38] | Calliandra (Calliandra calothyrsus) | 11.6 to 19.6 | CT |
| Priolo et al. [39], Silanikove et al. [40] | Carob tree (Ceratonia silique) | 3.0 to 17.0 | CT |
| Jackson et al. [33], Barahona et al. [41] | Desmodium (Desmodium ovalifolium) | 9.4 to 23.8 | CT |
| Jackson et al. [33], Hove et al. [38] | Leucaena (Leucaena leucocephala) | 5.4 to 13.4 | CT |
| Smith et al. [42], Norton [43] | White ball acacia (Acacia angustissima) | 0.7 to 17.4 | CT |
| Trees (Tropical) | |||
| Gemeda and Hassen [44] | African milkbush (Euphorbia tirucalli) | 7.6 | HT |
| Gemeda and Hassen [44] | African sumac (Rhus lancea) | 13.9 | HT |
| Tahrouch et al. [45] | Argan tree (Argania spinose) | 14.0 | CT |
| Gemeda and Hassen [44] | Northern red oak (Quercus rubica | 8.8 | HT |
| Gemeda and Hassen [44] | Sacred fig (Ficus religiosa) | 9.3 | HT |
| Source | Tannin | Effect | ||
|---|---|---|---|---|
| Plant/Extract | Type 1 | g/100 g DM 2 | ||
| Beneficial | ||||
| Min et al. [48] | Chestnut and mimosa extracts | HT and CT, respectively | 0.0 to 1.5 | Decreased the number of days that heifers experienced bloat by 81% and 77%, respectively, compared with control (no tannin diet). Increased average daily gain of heifers by 20% and 6%, respectively, compared with the control animals. |
| Martínez-Ortíz-de-Montellano et al. [49] | Tzalam (Lysiloma latisiliquum) | CT | 5.5 | Worm fecal egg count decreased by 33% for lambs fed L. latisiliquum compared with those fed control diet (no tannin) after day 36 of dosing lambs with Haemonchus contortus. |
| Aboagye et al. [7] | Chestnut and quebracho extracts | HT and CT, respectively | 0.0 to 1.5 | Rumen ammonia N decreased by 44% for beef cattle fed tannin supplements compared with the control (no tannin diet) |
| Aboagye et al. [8] | Tannic acid, chestnut and gallic acid | HT sources and HT subunit, respectively | 0.0 to 2.0 | Tannic acid and chestnut increased the proportion of N excreted in feces and decreased the proportion in urine in growing beef cattle compared with control animals (43.9% vs. 37.8% and 56.1% vs. 62.2%; respectively). |
| Woodward et al. [50] | Birdsfoot trefoil (Lotus corniculatus) | CT | 2.6 | Lotus corniculatus increased milk production by 33% in dairy cattle compared with those fed ryegrass (no tannin). Methane production per unit of DMI also decreased by 17% for dairy cattle fed L. corniculatus relative those fed ryegrass. |
| Negative | ||||
| Dschaak et al. [51] | Quebracho extracts | CT | 0.0 or 3.0 | Supplementing tannin decreased DMI by 6% in dairy cows fed either a high forage or low forage diet. |
| Henke et al. [52] | Quebracho extracts | CT | 0.0, 1.5, or 3.0 | There was no negative effect with tannin added at 1.5 g/100 g DM, but at 3.0 g/100 g DM, tannin decreased nutrient digestibilities with greater effect on crude protein digestibility; and so, milk yield, milk fat and protein contents decreased for dairy cows. |
| Garg et al. [53] | Oak (Quercus incana) | HT and CT mixture | 9.8 and 0.6, respectively | Cattle fed Q. incana had anorexia, severe constipation and brisket edema with 70% mortality. |
| Robins and Broker [54] | Mulga (Acacia aneura) or oaten hay chaff | CT | 7.5 and 0.03 respectively | In sheep fed a A. aneura diet, DMI and body weight were reduced with tissue fragility at discrete areas of the abomasum compared with sheep fed the oaten hay chaff. |
| Source | Animal (Rumen Fluid) | Forage Substrate and Level 1 | Tannin | Effects 5 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Plant/Extract | Type 2 | g/100 g 3 | Molecular weight 4 | VFA | NH3 | CH4 Yield 6 | Degradability | Microbes | |||
| Jayanegara et al. [10] | Cattle | Hay:concentrate (0.38 g; 70:30). | Extracts of chestnut, sumach, mimosa, quebracho | HT, HT, CT, CT, respectively | 0.0 to 1.0 mg/mL | NR but HT < CT | ↓ except sumach | NR | ↓ (4.3 % for HT and 2.5% for CT). | ↓ OM | ↓ methanogens (only for the 1 mg/mL). |
| Nauman et al. [15] Nauman et al. [71] | Cattle | Same plants (0.2 g; 100%) | Leucaena retusa, Desmanthus illinoensis, Neptunia lutea, Acacia angustissima, Lespedeza stuevei, and Desmodium paniculatum | CT | 3.3, 8.2, 8.3, 8.7, 11.7 and 12.5, respectively | 1745 Da, 1369 Da, 3025 Da, 1241 Da, 1473 Da and 2065 Da, respectively | ↓ except for L. retusa | NR | ↓ (70% relative to Arachis glabrata, 0.6% CT) except for L. retusa | NR | NR |
| Gemeda and Hassen [44] | Sheep | Same plants (0.4 g ± PEG; 100%). | Melia azedarach, Peltrophorum africanum, Rhus lancea | Mixture of HT and CT | HT = 0.68 to 13.9; CT = 0.65 to 6.0 | NR | ↓ | ↓ | ↓ (59%) | ↓ OM | NR |
| Hassanatand Benchaar [72] | Cattle | Forage:concentrate (65:35; 0.2 g) | Acacia, quebracho; and chestnut, valonea extracts | CT; and HT, respectively | 0.0 to 20.0 | NR | ↓ except for valonea at 50 g/kg DM | ↓ | ↓ (40 relative to control | NR | NR |
| Mengistu et al. [73] | Goat | Same plants (0.5 g ± PEG; 100%). | Euclea racemose, Rhus natalensis, Maytenus senegalensis | CT | > 20.0 | NR | ↓ | ↓ | ↓ (42%) | ↑ OM | NR |
| Pellikaan et al. [74] | Cattle | Alfalfa (0.4 g ± PEG; 100%) | Green tea, quebracho, grape seed; and chestnut, valonea myrabolan, tara extracts | CT; and HT, respectively | CT = 6.6 to 17.0; HT = 2.9 to 19.0 | CT = 481.8 to 2237.4 Da; HT = 655.5 to 2191.0 Da | ↓ | ↓ | ↓ (21%) | NR | NR |
| Rira et al. [75] | Sheep | Dichanthium spp (0.4 g; 100%) | Acacia nilotica (leaves or pods) | HT | 17.8 to 35.0 | NR | ↓ | NR | ↓ (55 to 64%) | ↑ DM for leaves and pods | NR |
| Saminathanet al. [76], Saminathanet al. [77] | Cattle | Guinea grass (0.5 g; 100%) | Leucaena leucocephala extract | Unfraction-ated CT (F0) Fractionat-ed CT (F1 to F5) | 0.0 and 3.0 | F0 = 1293.0 Da; F1 = 1265.8 Da; F2 = 1028.6 Da; F3 = 652.2 Da; F4 = 562.2 Da; F5 = 469.6 Da. | ↓ For F0 and F1 | NR | ↓ for all MW of CT (i.e., F0 to F5; average = 28%). | − DM; ↓N for only F1. | ↓ total methanogens with increasing MW but in relative abundance, the rumen cluster C was the most abundant archaeal community and it ↑ with increasing MW of CT. |
| Soltan et al. [78] | Sheep | Same plants (0.5 g; 100%). | Acacia saligna, and Leucaena leucocephala | CT | 6.3 and 4.6, respectively | NR | − | − | ↓ (37% relative to Tifton hay, 0% tannin) | ↑ undigested ruminal protein compared with Tifton hay | ↓ protozoa relative to Tifton hay |
| Tan et al. [79] | Cattle | Guinea grass (0.5 g; 100%) | Leucaena leucocephala extract | CT | 0.0 to 6.0 | NR | ↓ with increasing CT dosage | NR | ↓ with increasing CT dosage (average = 52%). | ↓DM and N with increasing CT dosage. | ↓methanogens and protozoa with increasing dose of CT. |
| Tavendale et al. [80] | Sheep | Same plant (0.5 g ± PEG; 100%). | Lotus pedunculatus | CT | 10.0 | NR | − | ↓ | ↓ (20%) | NR | Oligomeric fractions were inactive against Methanobrevibacter ruminantium relative to polymeric fraction in broth culture. |
| Source | Animal (rumen fluid) | Forage substrate and level 1 | Tannin | Effects 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Plant/Extract | Type 2 | g/100 g DM 3 | VFA | NH3 | CH4 yield 5 | Digestibility | Microbes | |||
| Aboagye al. [7] | Cattle | Alfalfa silage:barley silage (50:50; 95%) | Chestnut and Quebracho extracts | HT and CT, respectively | 0.0 to 1.5 | − | ↓ | ↓ (6% for 1.5% HT and CT combination). | NR | − protozoa |
| Aboagye al. [8] | Cattle | Alfalfa silage:barley silage (79:21; 95%) | Tannic acid chestnut and gallic acid | HT sources and HT subunit, respectively | 0.0 to 2.0 | ↑for only gallic acid | ↓ for only tannic acid | ↓ (9 % for gallic acid). | − nutrients, except ↓crude protein for HT sources | − protozoa |
| Ebert et al. [81] | Cattle | Sorghum stalk: concentrate (8.5:91.5) | Quebracho extract | CT | 0.0, 0.5, or 1.0 | NR | NR | − | − DM and OM | NR |
| Lima et al. [82] | Sheep | Elephant grass: concentrate (60:40). | Mimosa tenuiflora extract | CT | 0.0 and 3.0 | NR | NR | − | − nutrients | ↓protozoa |
| Liu et al. [83] | Goat | Forage: concentrate (75:25). | Lespedeza cuneate with quebracho extract | CT | 7.5 to 9.0 | ↓ | − | ↓ (54 to 58% relative to a control, i.e., an alfalfa based diet). | ↓ for all nutrients | − bacteria but ↓protozoa |
| Malik et al. [84] | Sheep | Forage: concentrate (60:40). | Artocarpus heterophyllus, Azadirachta indica and Ficus benghalensis | CT | 7.2 to 10.9 | ↓ | − | ↓ (24% relative to wheat bran control diet). | ↓ DM for Azadirachta indica relative to the other tannin-containing and control (no tannin) diets. | − bacteria but ↓protozoa |
| Stewart et al. [85] | Cattle | Same plants (100%) | Birdsfoot trefoil, sainfoin and small burnet | CT, CT and HT respectively | 0.6, 2.5 and 4.5, respectively | NR | NR | ↓ (39% for HT relative to CT). | Sainfoin and small burnet ↓nutrients and crude protein relative to birdsfoot trefoil. | NR |
| Supapong et al. [86] | Cattle | Rice straw: concentrate (80:20) | Delonix regia seed meal | CT | 0.0, 9.0, 18.0 or 27.0 | − | ↑ with increased CT | ↓ (16% relative to no tannin). | ↓DM and OM with increasing CT concentration. | ↓protozoa |
| Yang et al. [87] | Cattle | Corn silage: concentrate (50:50) | Tannic acid | HT | 0, 0.65, 1.3 or 2.6 | ↓ | ↓ | ↓ (11, 15, and 34%, respectively relative to no tannin) | ↓DM, OM and protein. | ↓protozoa and methanogens (only for the 2.6% DM). |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aboagye, I.A.; Beauchemin, K.A. Potential of Molecular Weight and Structure of Tannins to Reduce Methane Emissions from Ruminants: A Review. Animals 2019, 9, 856. https://doi.org/10.3390/ani9110856
Aboagye IA, Beauchemin KA. Potential of Molecular Weight and Structure of Tannins to Reduce Methane Emissions from Ruminants: A Review. Animals. 2019; 9(11):856. https://doi.org/10.3390/ani9110856
Chicago/Turabian StyleAboagye, Isaac A., and Karen A. Beauchemin. 2019. "Potential of Molecular Weight and Structure of Tannins to Reduce Methane Emissions from Ruminants: A Review" Animals 9, no. 11: 856. https://doi.org/10.3390/ani9110856
APA StyleAboagye, I. A., & Beauchemin, K. A. (2019). Potential of Molecular Weight and Structure of Tannins to Reduce Methane Emissions from Ruminants: A Review. Animals, 9(11), 856. https://doi.org/10.3390/ani9110856





