Agonistic Interactions in Pigs–Comparison of Dominance Indices with Parameters Derived from Social Network Analysis in Three Age Groups
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Recording and Analysis of Agonistic Interactions
2.3. Dominance Indices
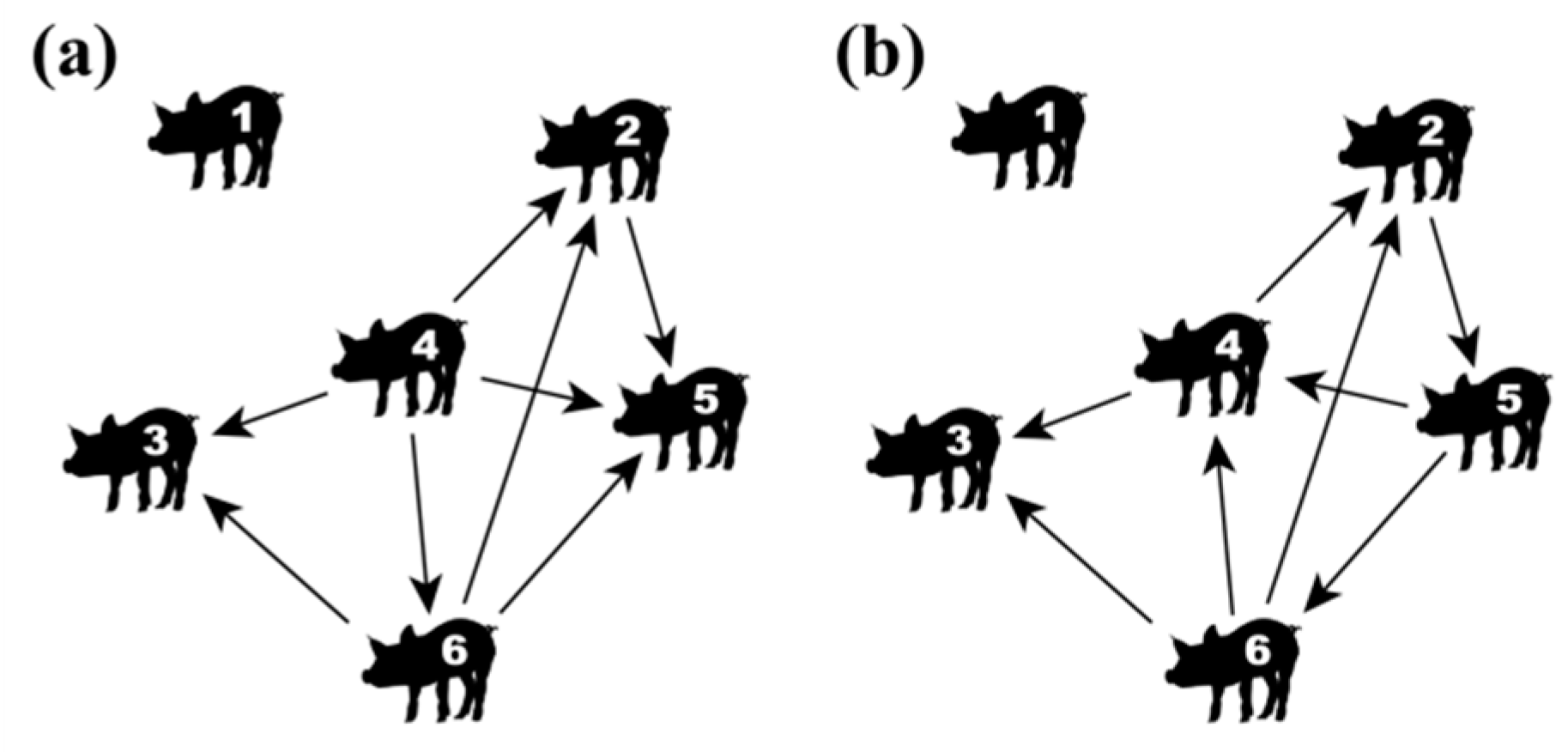
2.4. Network Construction and Centrality Parameters
2.5. Statistical Analysis
3. Results
3.1. Descriptive Statistics
3.1.1. Number of Isolated Animals
3.1.2. Relation between Initiated and Won Fights
3.1.3. Dominance Indices
3.1.4. Centrality Parameters
3.2. Spearman Rank Correlation Coefficients between the Dominance Indices and the Centrality Parameters in the Temporal Course
3.3. Rank Differences between Subsequent Rank Positions for the Winner-Loser Networks
3.4. Partial Least Squares Structural Equation Modelling (PLS-SEM)
4. Discussion
4.1. Choice of Network Type
4.2. Choice of Centrality Parameters
4.3. Advantages of Social Network Analysis
4.4. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Camerlink, I.; Turner, S.P.; Farish, M.; Arnott, G. The influence of experience on contest assessment strategies. Sci. Rep. 2017, 7, 14492. [Google Scholar] [CrossRef] [PubMed]
- Stookey, J.M.; Gonyou, H.W. The Effects of Regrouping on Behavioral and Production Parameters in Finishing Swine. J. Anim. Sci. 1994, 72, 2804–2811. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.S.L.; Shackleton, D.M.; Beames, R.M. The effect of mixing unfamiliar individuals on the growth and production of finishing pigs. Anim. Prod. 1991, 52, 201–206. [Google Scholar] [CrossRef]
- Koene, P.; Ipema, B. Social Networks and Welfare in Future Animal Management. Animals 2014, 4, 93–118. [Google Scholar] [CrossRef]
- Makagon, M.M.; McCowan, B.; Mench, J.A. How can social network analysis contribute to social behavior research in applied ethology? Appl. Anim. Behav. Sci. 2012, 138, 152–161. [Google Scholar] [CrossRef]
- Foister, S.; Doeschl-Wilson, A.; Roehe, R.; Arnott, G.; Boyle, L.; Turner, S. Social network properties predict chronic aggression in commercial pig systems. PLoS ONE 2018, 13, e0205122. [Google Scholar] [CrossRef]
- Krause, J.; Croft, D.P.; James, R. Social network theory in the behavioural sciences: Potential applications. Behav. Ecol. Sociobiol. 2007, 62, 15–27. [Google Scholar] [CrossRef]
- Newman, M.E.J. Networks. An Introduction; Oxford University Press Inc.: New York, NY, USA, 2010. [Google Scholar]
- Wasserman, S.; Faust, K. Social Network Analysis: Methods and Applications; Cambridge University Press: New York, NY, USA, 1994. [Google Scholar]
- Asher, L.; Collins, L.M.; Ortiz-Pelaez, A.; Drewe, J.A.; Nicol, C.J.; Pfeiffer, D.U. Recent advances in the analysis of behavioural organization and interpretation as indicators of animal welfare. J. Royal Soc. Interface 2009, 6, 1103–1119. [Google Scholar] [CrossRef]
- Büttner, K.; Scheffler, K.; Czycholl, I.; Krieter, J. Network characteristics and development of social structure of agonistic behaviour in pigs across three repeated rehousing and mixing events. Appl. Anim. Behav. Sci. 2015, 168, 24–30. [Google Scholar] [CrossRef]
- Büttner, K.; Scheffler, K.; Czycholl, I.; Krieter, J. Social network analysis - centrality parameters and individual network positions of agonistic behavior in pigs over three different age levels. Springerplus 2015, 4, 185. [Google Scholar] [CrossRef]
- Boyland, N.K.; Mlynski, D.T.; James, R.; Brent, L.J.; Croft, D.P. The social network structure of a dynamic group of dairy cows: From individual to group level patterns. Appl. Anim. Behav. Sci. 2016, 174, 1–10. [Google Scholar] [CrossRef]
- Foris, B.; Zebunke, M.; Langbein, J.; Melzer, N. Comprehensive analysis of affiliative and agonistic social networks in lactating dairy cattle groups. Appl. Anim. Behav. Sci. 2019, 210, 60–67. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Johnston, L.; Martin, W. Understanding Tail-Biting in Pigs through Social Network Analysis. Animals 2018, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Stukenborg, A.; Traulsen, I.; Puppe, B.; Presuhn, U.; Krieter, J. Agonistic behaviour after mixing in pigs under commercial farm conditions. Appl. Anim. Behav. Sci. 2011, 129, 28–35. [Google Scholar] [CrossRef]
- Meese, G.B.; Ewbank, R. The establishment and nature of the dominance hierarchy in the domesticated pig. Anim. Behav. 1973, 21, 326–334. [Google Scholar] [CrossRef]
- Friend, T.H.; Knabe, D.A.; Tanksley, T.D.J. Behavior and performance of pigs grouped by three different methods at weaning. J. Anim. Sci. 1983, 57, 1406–1411. [Google Scholar] [CrossRef]
- Jensen, P. An ethogram of social interaction patterns in group-housed dry sows. Appl. Anim. Ethol. 1980, 6, 341–350. [Google Scholar] [CrossRef]
- Jensen, P. An analysis of agonistic interaction patterns in group-housed dry sows—Aggression regulation through an “avoidance order”. Appl. Anim. Ethol. 1982, 9, 47–61. [Google Scholar] [CrossRef]
- Langbein, J.; Puppe, B. Analysing dominance relationships by sociometric methods—A plea for a more standardised and precise approach in farm animals. Appl. Anim. Behav. Sci. 2004, 87, 293–315. [Google Scholar] [CrossRef]
- McGlone, J.J. Agonstic behavior in food animals: Review of research and techniques. J. Anim. Sci. 1986, 62, 1130–1139. [Google Scholar] [CrossRef]
- Samarakone, T.S.; Gonyou, H.W. Domestic pigs alter their social strategy in response to social group size. Appl. Anim. Behav. Sci. 2009, 121, 8–15. [Google Scholar]
- Bowen, D.W.; Brooks, R.J. Social Organization of confined male collared lemmings (Dicrostonyx groenlandicus). Anim. Behav. 1978, 26, 1126–1135. [Google Scholar] [CrossRef]
- Borberg, C.; Hoy, S. Mixing of sows with or without the presence of a boar. Livest. Sci. 2009, 125, 314–317. [Google Scholar] [CrossRef]
- Fels, M.; Hoy, S.; Hartung, J. Influence of origin litter on social rank, agonistic behaviour and growth performance of piglets after weaning. Appl. Anim. Behav. Sci. 2012, 139, 225–232. [Google Scholar] [CrossRef]
- Hagberg, A.; Schult, D.; Swart, P.J. Exploring network structure, dynamics and function using NetworkX. In Proceedings of the 7th Python in Science Conference (SciPy2008), Pasadena, CA, USA, 19–24 August 2008; pp. 11–16. [Google Scholar]
- Freeman, L.C. A Set of Measures of Centrality Based on Betweenness. Sociometry 1977, 40, 35–41. [Google Scholar] [CrossRef]
- Bavelas, A. Communication patterns in task-oriented groups. J. Acoust. Soc. Am. 1950, 22, 725–730. [Google Scholar] [CrossRef]
- Sabidussi, G. The centrality index of a graph. Psychometrika 1966, 31, 581–603. [Google Scholar] [CrossRef]
- SAS® Institute Inc. Base SAS® 9.4 Procedures Guide: Statistical Procedures, 2nd ed.; Statistical Analysis System Institute Inc.: Cary, NC, USA, 2013. [Google Scholar]
- Martin, P.; Bateson, P. Measuring Behaviour. An Introductory Guide, 3rd ed.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Holm, S. A Simple Sequentially Rejective Multiple Test Procedure. Scand. J. Statist. 1979, 6, 65–70. [Google Scholar]
- Ringle, C.M.; Wende, S.; Becker, J.-M. SmartPLS; SmartPLS GmbH: Boenningstedt, Germany, 2015. [Google Scholar]
- Hair, J.F.; Ringle, C.M.; Sarstedt, M. PLS-SEM: Indeed a Silver Bullet. J. Market. Theor. Pract. 2011, 19, 139–152. [Google Scholar]
- Henseler, J.; Ringle, C.M.; Sinkovics, R.R. The Use of Partial Least Squares Path Modeling in International Marketing. Adv. Int. Mark. 2009, 20, 277–319. [Google Scholar]
- Hessing, M.J.C.; Hagelsø, A.M.; Schouten, W.G.P.; Wiepkema, P.R.; van Beek, J.A.M. Individual Behavioral and Physiological Strategies in Pigs. Physiol. Behav. 1994, 55, 39–46. [Google Scholar] [CrossRef]
- Hessing, M.J.C.; Hagelsø, A.M.; van Beek, J.A.M.; Wiepkema, P.R.; Schouten, W.G.P.; Krukow, R. Individual behavioural characteristics in pigs. Appl. Anim. Behav. Sci. 1993, 37, 285–295. [Google Scholar] [CrossRef]
- Flack, J.C.; Girvan, M.; de Waal, F.B.; Krakauer, D.C. Policing stabilizes construction of social niches in primates. Nature 2006, 439, 426–429. [Google Scholar] [CrossRef] [PubMed]
- Brent, L.J.N. Friends of friends: Are indirect connections in social networks important to animal behaviour? Anim. Behav. 2015, 103, 211–222. [Google Scholar] [CrossRef]
- Klass, K.; Cords, M. Effect of unknown relationships on linearity, steepness and rank ordering of dominance hierarchies: Simulation studies based on data from wild monkeys. Behav. Process. 2011, 88, 168–176. [Google Scholar] [CrossRef]
- Douglas, P.H.; Ngonga Ngomo, A.-C.; Hohmann, G. A novel approach for dominance assessment in gregarious species: ADAGIO. Anim. Behav. 2017, 123, 21–32. [Google Scholar] [CrossRef]
- Funkhouser, J.A.; Mayhew, J.A.; Mulcahy, J.B. Social network and dominance hierarchy analyses at Chimpanzee Sanctuary Northwest. PLoS ONE 2018, 13, e0191898. [Google Scholar] [CrossRef]
- Sih, A.; Hanser, S.F.; McHugh, K.A. Social network theory: New insights and issues for behavioral ecologists. Behav. Ecol. Sociobiol. 2009, 63, 975–988. [Google Scholar] [CrossRef]
- Puppe, B.; Langbein, J.; Bauer, J.; Hoy, S. A comparative view on social hierarchy formation at different stages of pig production using sociometric measures. Livest. Sci. 2008, 113, 155–162. [Google Scholar] [CrossRef]
- Puppe, B. Effects of familiarity and relatedness on agonistic pair relationships in newly mixed domestic pigs. Appl. Anim. Behav. Sci. 1998, 58, 233–239. [Google Scholar] [CrossRef]
- Arey, D.S.; Franklin, M.F. Effects of straw and unfamiliarity on fighting between newly mixed growing pigs. Appl. Anim. Behav. Sci. 1995, 45, 23–30. [Google Scholar] [CrossRef]
- Nielsen, B.L.; Lawrence, A.B.; Whittemore, C.T. Effect of group size on feeding behaviour, social behaviour, and performance of growing pigs using single-space feeders. Livest. Prod. Sci. 1995, 44, 73–85. [Google Scholar] [CrossRef]
- Turner, S.P.; Horgan, G.W.; Edwards, S.A. Effect of social group size on aggressive behaviour between unacquainted domestic pigs. Appl. Anim. Behav. Sci. 2001, 74, 203–215. [Google Scholar] [CrossRef]
- Andersen, I.L.; Nævdal, E.; Bakken, M.; Bøe, K.E. Aggression and group size in domesticated pigs, Sus scrofa: ‘When the winner takes it all and the loser is standing small’. Anim. Behav. 2004, 68, 965–975. [Google Scholar] [CrossRef]
- Marchant-Forde, J.N.; Marchant-Forde, R.M. Minimizing inter-pig aggression during mixing. Pig News Inf. 2005, 26, 63N–71N. [Google Scholar]
- Schmolke, S.A.; Li, Y.Z.; Gonyou, H.W. Effects of group size on social behavior following regrouping of growing–finishing pigs. Appl. Anim. Behav. Sci. 2004, 88, 27–38. [Google Scholar] [CrossRef]
- Lindberg, A.C. Group life. In Social Behaviour in Farm Animals; Keeling, L.J., Gonyou, H.W., Eds.; CAB International: Oxon, UK, 2001; pp. 37–76. [Google Scholar]
- Hunter, E.J.; Broom, D.M.; Edwards, S.A.; Sibly, R.M. Social hierarchy and feeder access in a group of 20 sows using a computer-controlled feeder. Anim. Prod. 1988, 47, 139–148. [Google Scholar] [CrossRef]
- Meese, G.B.; Ewbank, R. A note on instability of the dominance hierarchy and variations in level of aggression within groups of fattening pigs. Anim. Sci. 1972, 14, 359–362. [Google Scholar] [CrossRef]





| Parameter | Weaned Piglets | Fattening Pigs | Gilts |
|---|---|---|---|
| Age at time of mixing | 26 days | 68 days | 154 days |
| Level of familiarity 1 | 0 animals | 2 animals | 5 animals |
| Compartment | Flatdeck | Fattening unit | Breeding unit |
| Dimensions | 2.05 m × 1.36 m | 3.25 m × 2.40 m | 7.20 m × 5.40 m |
| Floor | Concrete & metal | Half-slatted & -solid | Half-slatted & -solid |
| Feed | Solid pelleted feed | Mash feeding machine | Mash feeding machine |
| Water | Nipple drinkers | Nipple drinkers | Nipple drinkers |
| Number of animals | 829 | 543 | 249 |
| Number of pens | 93 | 26 | 12 |
| Number of animals/pen | 8.9 ± 0.6 (6–11) | 20.9 ± 1.7 (17–25) | 20.8 ± 3.4 (16–27) |
| Average male:female ratio | 1:1.14 | 1:1 | - |
| Video observation | 28 h | 17 h | 17 h |
| Observation periods | Day 1: 12:00–18:00 | Day 1: 12:00–18:00 | Day 1: 12:00–18:00 |
| Day 2: 07:00–18:00 | Day 2: 07:00–18:00 | Day 2: 07:00–18:00 | |
| Day 3: 07:00–18:00 | - | - |
| Parameter | Description |
|---|---|
| In- & Out-degree | Number of ingoing (in-degree) or outgoing (out-degree) interactions. In the initiator-receiver networks, in-degree describes the number of received fights and out-degree describes the number of initiated fights. In the winner-loser networks, in-degree describes the number of fights lost and out-degree describes the number of fights won [8]. |
| Betweenness | Measures the extent to which an animal lies on shortest paths to other animals [9,28]. |
| Ingoing & Outgoing closeness | Mean distance from all reachable animals to any other specific animal (ingoing closeness) or mean distance from one specific animal to all other reachable animals (outgoing closeness) [9,29,30]. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Büttner, K.; Czycholl, I.; Mees, K.; Krieter, J. Agonistic Interactions in Pigs–Comparison of Dominance Indices with Parameters Derived from Social Network Analysis in Three Age Groups. Animals 2019, 9, 929. https://doi.org/10.3390/ani9110929
Büttner K, Czycholl I, Mees K, Krieter J. Agonistic Interactions in Pigs–Comparison of Dominance Indices with Parameters Derived from Social Network Analysis in Three Age Groups. Animals. 2019; 9(11):929. https://doi.org/10.3390/ani9110929
Chicago/Turabian StyleBüttner, Kathrin, Irena Czycholl, Katharina Mees, and Joachim Krieter. 2019. "Agonistic Interactions in Pigs–Comparison of Dominance Indices with Parameters Derived from Social Network Analysis in Three Age Groups" Animals 9, no. 11: 929. https://doi.org/10.3390/ani9110929
APA StyleBüttner, K., Czycholl, I., Mees, K., & Krieter, J. (2019). Agonistic Interactions in Pigs–Comparison of Dominance Indices with Parameters Derived from Social Network Analysis in Three Age Groups. Animals, 9(11), 929. https://doi.org/10.3390/ani9110929




