Prophylactic and Therapeutic Efficacy of Prebiotic Supplementation against Intestinal Coccidiosis in Rabbits
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Rabbits
2.2. Prebiotic Product
2.3. Preparation of Eimeria Species Oocysts
2.4. Experimental Design of Prebiotic Efficacy Against Coccidiosis
2.4.1. Prophylactic Experiment
2.4.2. Therapeutic Trial
2.5. Evaluation Parameters in both Experiments
2.5.1. Clinical Signs of Eimeria Infection in Rabbits
2.5.2. Necropsy Examination
2.5.3. Oocyst Counts Per Gram Feces (OPG)
2.5.4. Growth Rate
2.5.5. Histopathological Examination
2.5.6. Biochemical Parameters
2.6. Statistical Analysis
3. Results
3.1. Prebiotic Efficacy as a Prophylaxis against Eimeria Species in Experimentally Infected Rabbits
3.1.1. Clinical Signs of Coccidiosis in Rabbits
3.1.2. Post-Mortem Lesions
3.1.3. Oocysts Per Gram of Feces
3.1.4. Body Weight of Rabbits
3.2. Treatment of Coccidiosis in Naturally Infected Rabbits with Prebiotic Supplementation
3.2.1. Oocyst Counts
3.2.2. Body Weight of Rabbits Supplemented with a Prebiotic Product
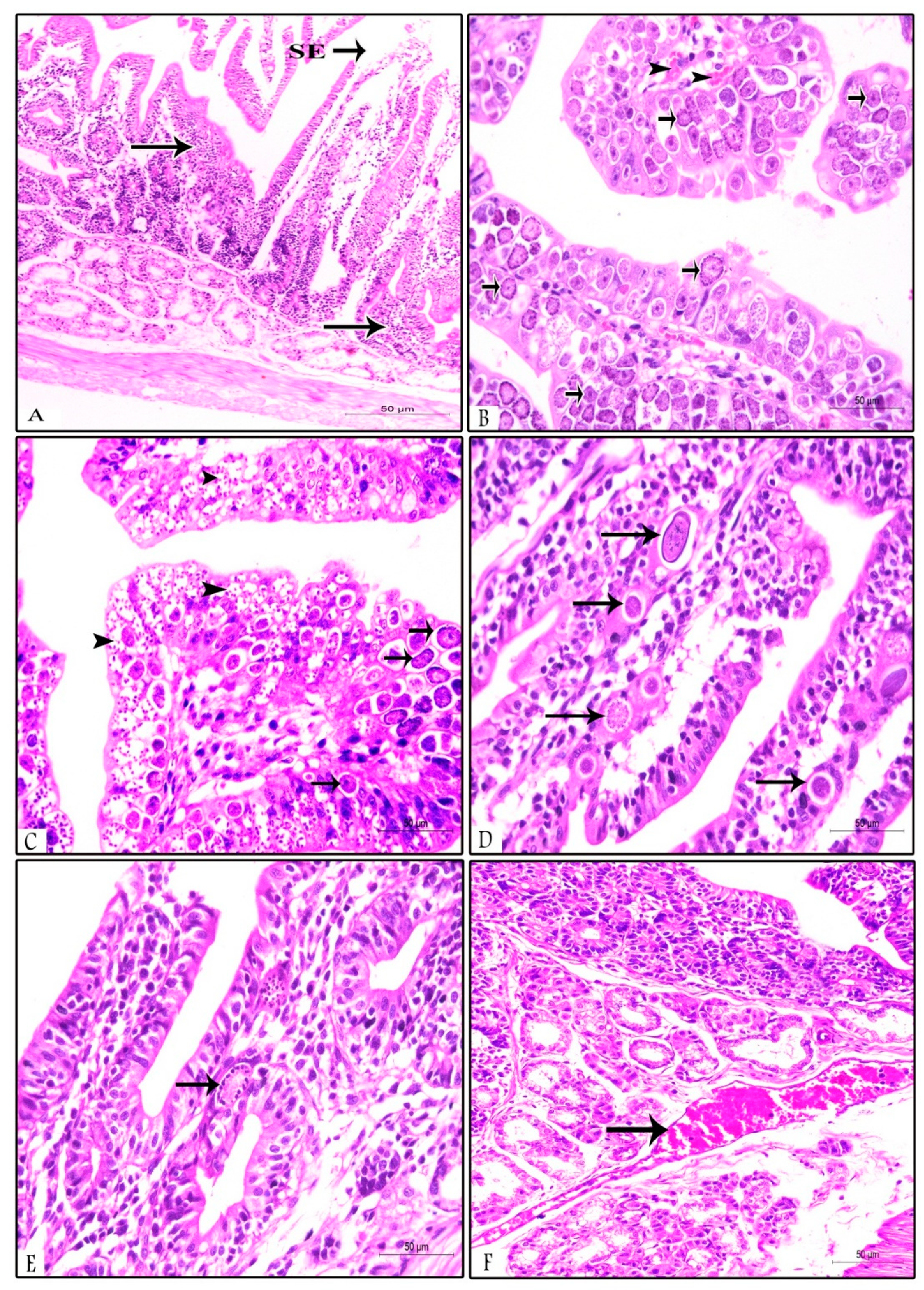
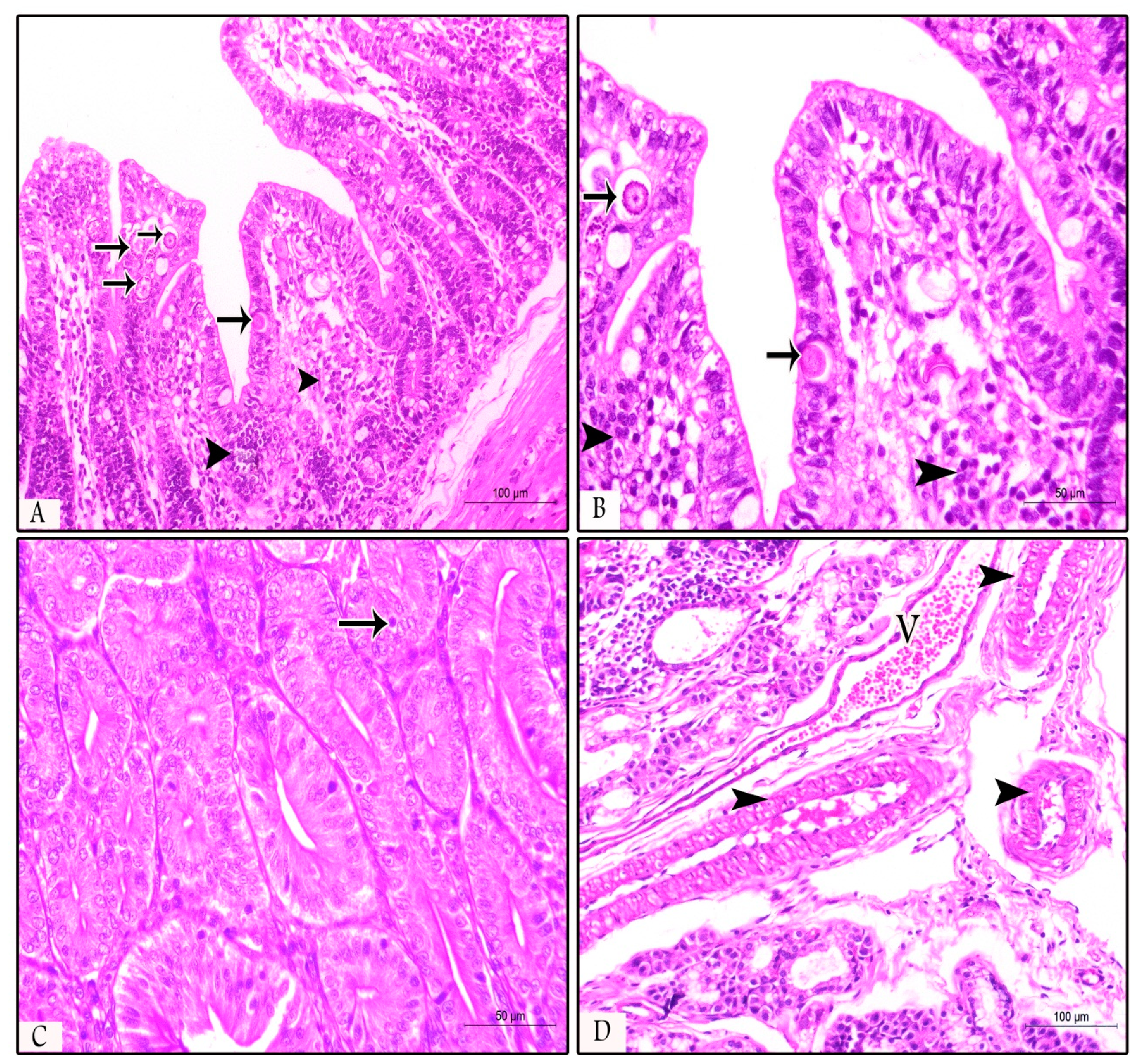
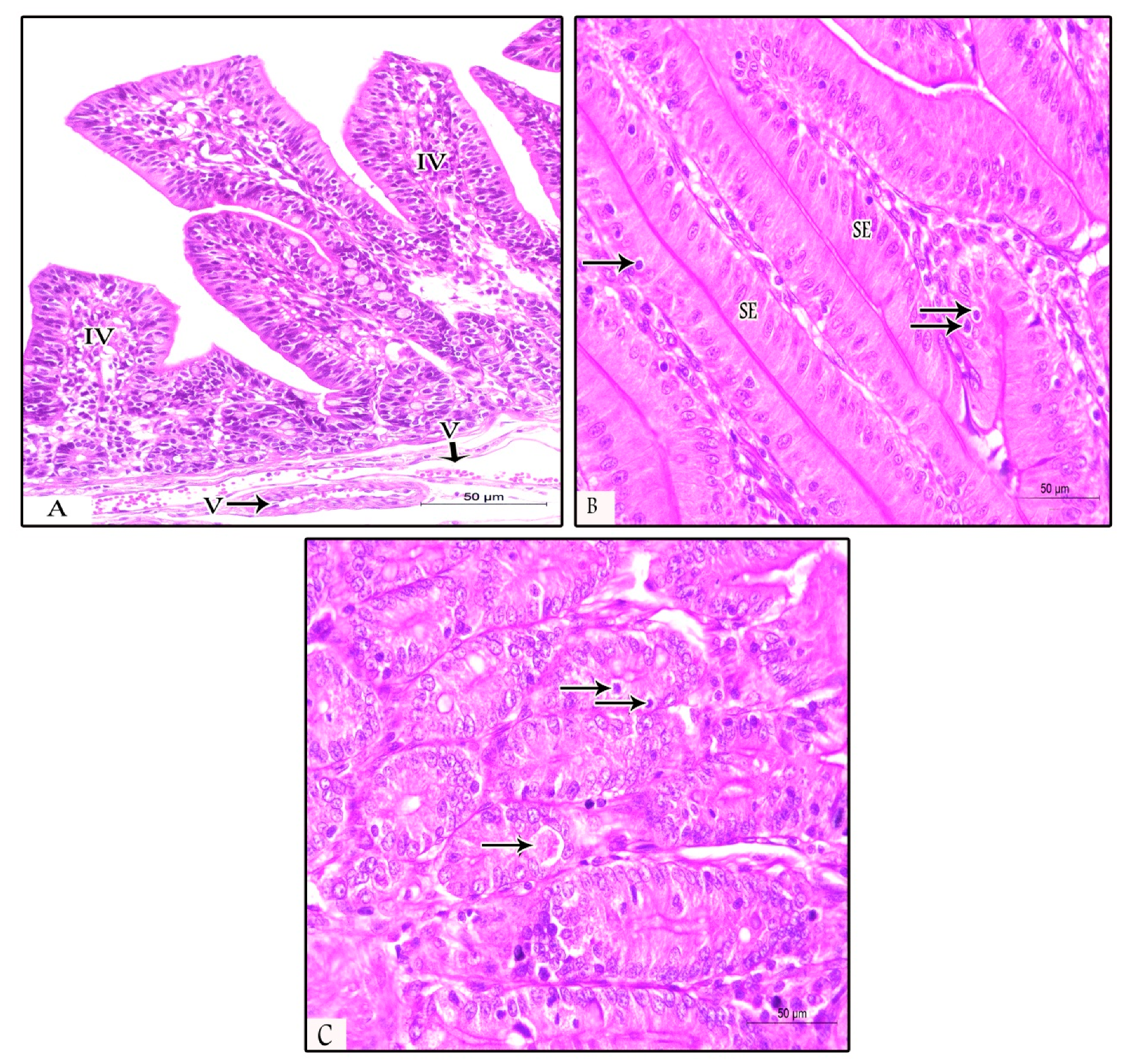
3.3. Histopathological Findings in Prophylactic and Therapeutic Experiments
3.4. Biochemical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhatt, R.S.; Agrawal, A.R.; Sahoo, A. Effect of probiotic supplementation on growth performance, nutrient utilization and carcass characteristics of growing Chinchilla rabbits. J. Appl. Anim. Res. 2017, 45, 304–309. [Google Scholar] [CrossRef]
- Aboelhadid, S.M.; El-Ashram, S.; Hassan, K.M.; Arafa, W.M.; Darwish, A.B. Hepato-protective effect of curcumin and silymarin against Eimeria stiedae in experimentally infected rabbits. Livest. Sci. 2019, 221, 33–38. [Google Scholar] [CrossRef]
- Pakandl, M. Coccidia of rabbit: A review. Folia Parasitol. 2009, 56, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Haleem, H.M.; Aboelhadid, S.M.; Sakran, T.; El-Shahawy, G.; El-Fayoumi, H.; Al-Quraishy, S.; Abdel-Baki, A.S. Gene expression, oxidative stress and apoptotic changes in rabbit ileum experimentally infected with Eimeria intestinalis. Folia Parasitol. 2017, 64, 12. [Google Scholar] [CrossRef] [PubMed]
- Lebas, F.; Coudert, P.; de Rochambeau, H.; Thebault, R.G. The Rabbit Husbandry, Health and Production; FAO Animal Production and Health Series No. 21; FAO: Rome, Italy, 1997. [Google Scholar]
- Taylor, M.A.; Coop, R.L.; Wall, R.L. Veterinary Parasitology, 3rd ed.; Blackwell Publishing Company: Hobken, NJ, USA, 2007; pp. 901–902. [Google Scholar]
- Hassan, K.M.; Arafa, W.M.; Mousa, W.M.; Shokier, K.A.; Shany, S.A.; Aboelhadid, S.M. Molecular diagnosis of Eimeria stiedae in hepatic tissue of experimentally infected rabbits. Exp. Parasitol. 2016, 169, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ogolla, K.O.; Gathumbi, P.K.; Waruiru, R.M.; Kumu, P.O.; Chebet, J.; Kitala, P.H.M. Efficacy of Sulphachloropyrazine, Amprolium Hydrochloride, Trimethoprim-Sulphamethoxazole, and Diclazuril against Experimental and Natural Rabbit Coccidiosis. J. Vet. Med. 2018, 2018, 5402469. [Google Scholar] [CrossRef] [PubMed]
- Licois, D. Domestic rabbit enteropathies. In Proceedings of the 8th World Rabbit Congress, Puebla, Mexico, 7–10 September 2004; pp. 385–403. [Google Scholar]
- Papeschi, C.; Fichi, G.; Perrucci, S. Oocysts excretion of three intestinal Eimeria species in female rabbits. World Rabbit Sci. 2013, 21, 77–83. [Google Scholar] [CrossRef]
- El-Wafa, S.A.; Sedki, A.A.; Ismail, A.M. Response of growing rabbits to diets containing black seed, garlic or onion as natural feed additives. Egypt. J. Rabbit Sci. 2002, 12, 69–83. [Google Scholar]
- El-Abasy, M.A. Studies on Sugar Cane Extract for Control of Chicken Diseases. Ph.D. Thesis, Tokyo University, Tokyo, Japan, 2002. [Google Scholar]
- Fonseca, A.; Falcao, L.; Kocher, A.; Spring, P. Effects of dietary mannon oligosaccharide in comparison to oxytetracyclin on performance of growing rabbits. In Proceedings of the Eighth World Rabbit Congress Puebla, Puebla, Mexico, 7–10 September 2004; pp. 829–833. [Google Scholar]
- Sohail, M.; Ijaz, A.; Yousaf, M.; Ashraf, K.; Zaneb, H.; Aleem, M.; Rehman, H. Alleviation of cyclic heat stress in broilers by dietary supplementation of mannan-oligosaccharide and Lactobacillus-based probiotic: Dynamics of cortisol, thyroid hormones, cholesterol, C-reactive protein, and humoral immunity. Poult. Sci. 2010, 89, 1934–1938. [Google Scholar] [CrossRef]
- Sohail, M.; Hume, M.; Byrd, J.; Nisbet, D.; Ijaz, A.; Sohail, A.; Shabbir, M.; Rehman, H. Effect of supplementation of prebiotic mannanoligosaccharides and probiotic mixture on growth performance of broilers subjected to chronic heat stress. Poult. Sci. 2012, 91, 2235–2240. [Google Scholar] [CrossRef]
- Waldroup, P.W.; Oviedo-Rondon, E.O.; Fritts, C.A. Comparison of Bio-Mos® and antibiotic feeding programs in broiler diets containing copper sulfate. Int. J. Poult. Sci. 2003, 2, 28–31. [Google Scholar]
- Kocher, A.; Garcia, P.; Tucker, L.A. Effects of Bio-Mos for laying hens 20–52 weeks under commercial conditions. In Proceedings of the Alltech’s 21st Annual Symposium on Nutritional Biotechnologies in the Feed and Food Industries, Lexington, KY, USA, 22–25 May 2005; pp. 316–317. [Google Scholar]
- Maribo, H.; Spring, P. Yeast extract as a protein source for weaning piglets. In Vitamine und Zusatzstoffe in der Ernährung von Mensch und Tier; Schubert, R., Flachowsky, G., Jahreis, G., Bitsch, R., Eds.; Friedrich-Schiller-Universität Jena: Jena Thüringen, Germany, 2003; pp. 433–437. [Google Scholar]
- Abdelhady, D.H.; El-Abasy, M.A. Effect of Prebiotic and Probiotic on Growth, Immuno-hematological responses and Biochemical Parameters of infected rabbits with Pasteurella multocida. Benha Vet. Med. J. 2015, 28, 40–51. [Google Scholar] [CrossRef]
- Soulsby, E.J.L. Helminths, Arthropods and Protozoa of Domesticated Animals, 7th ed.; Baillere Tindall: London, UK, 1986; pp. 593–614. [Google Scholar]
- El-Ashram, S.; Suo, X. Electrical cream separator coupled with vacuum filtration for the purification of eimerian oocysts and trichostrongylid eggs. Sci. Rep. 2017, 7, 43346. [Google Scholar] [CrossRef] [PubMed]
- El-Ashram, S.; Aboelhadid, S.M.; Kamel, A.A.; Mahrous, L.N.; Abdelwahab, K.H. Diversity of Parasitic Diarrhea Associated with Buxtonella Sulcata in Cattle and Buffalo Calves with Control of Buxtonellosis. Animals 2019, 9, 259. [Google Scholar] [CrossRef] [PubMed]
- Ryley, J.F.; Meade, R.; Hazelhurst, J.; Robinson, T.E. Methods in coccidiosis research: Separation of oocysts from faeces. Parasitology 1976, 73, 311–326. [Google Scholar] [CrossRef] [PubMed]
- El-Ashram, S.; Yin, Q.; Liu, H.; Al Nasr, I.; Liu, X.; Suo, X.; Barta, J. From the Macro to the Micro: Gel Mapping to Differentiate between Sporozoites of Two Immunologically Distinct Strains of Eimeria maxima (Strains M6 and Guelph). PLoS ONE. 2015, 10, e0143232. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Zhang, S.; Zhou, C.; Tang, X.; Li, C.; Wang, C.; Tang, X.; Suo, J.; Jia, Y.; El-Ashram, S.; et al. Influence of Eimeria falciformis infection on Gut Microbiota and Metabolic Pathways in Mice. Infect. Immun. 2018, 86, e00073-18. [Google Scholar] [CrossRef]
- Hanafi, M.E.; Maghraby, A.N.; Ramadan, M.M.; Mahmoud, A.M.; EL-Allawy, M.H. Aromatherapy of Cinnamomum zeylanicum Bark oil for treatment of scabies in rabbits with emphases on the productive performance. Am. Eurasian J. Agric. Environ. Sci. 2010, 7, 719–727. [Google Scholar]
- Bancroft, J.; Gamble, A. Theory and Practice of Histological Techniques, 6th ed.; Churchill-Livingstone: Edinburgh, UK; London, UK; Melbourne, Australia; New York, NY, USA, 2008. [Google Scholar]
- Tang, S.G.; Sieo, C.; Ramasamy, K.; Saad, W.Z.; Wong, H.K.; Ho, Y.W. Performance, biochemical and haematological responses, and relative organ weights of laying hens fed diets supplemented with prebiotic, probiotic and symbiotic. BMC Vet. Res. 2017, 13, 248. [Google Scholar] [CrossRef]
- Sivajothi, S.; Reddy, B.S.; Rayulu, V.C. Intestinal coccidiosis infection in domestic rabbits. Int. J. Biol. Res. 2014, 2, 48–50. [Google Scholar] [CrossRef]
- European Union Commission. Ban on Antibiotics as Growth Promoters in Animal Feed Enters into Effect; Regulation 1831/2003/ec on additives for use in animal nutrition, replacing directive 700/524//333c on additives in feedstuffs; European Union Commission: Brussels, Belgium, 2005. [Google Scholar]
- Ashayerizadeh, A.; Dabiri, N.; Ashayerizadeh, O.; Mirzadeh, K.H.; Roshanfekr, H.; Mamooee, M. Effect of dietary antibiotic, probiotic and probiotic as growth promoters on growth performance, carcass characteristics and haematological indices of broiler chickens. Pak. J. Biol. Sci. 2009, 12, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Castãneda, R.E.; Gonzźalez, E.D. Control of Avian Coccidiosis: Future and Present Natural Alternatives. BioMed Res. Int. 2015, 2015, 430610. [Google Scholar]
- Falcão-e-Cunha, L.; Castro-Solla, L.; Maertens, L. Alternatives to antibiotic growth promoters in rabbit feeding: A review. World Rabbit Sci. 2007, 15, 127–140. [Google Scholar] [CrossRef]
- Faber, T.A.; Dilger, R.N.; Hopkins, A.C.; Price, N.P.; Fahey, G.C., Jr. The effects of a galactoglucomannan oligosaccharide-arabinoxylan (GGMO-AX) complex in broiler chicks challenged with Eimeria acervulina1. Poult. Sci. 2012, 91, 1089–1096. [Google Scholar] [CrossRef]
- Servin, A.L.; Coconnier, M.H. Adhesion of probiotic strains to the intestinal mucosa and interaction with pathogens. Best Pract. Res. Clin. Gastroenterol. 2003, 17, 741–754. [Google Scholar] [CrossRef]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B.; et al. Prebiotic effects: Metabolic and health benefits. Br. J. Nutr. 2010, 104, S1–S63. [Google Scholar] [CrossRef]
- Sharma, K.G.; Vidyarthi, V.K.; Archana, K.; Zuyie, R. Probiotic Supplementation in the Diet of Rabbits—A Review. Livest. Res. Int. 2016, 4, 1–10. [Google Scholar]
- Fortun-Lamothe, L.; Drouet, V.F. Review: II Diet and immunity: Current state of knowledge and research prospect for the rabbit. World Rabbit Sci. 2002, 10, 25–39. [Google Scholar] [CrossRef]
- Teng, P.Y.; Kim, W.K. Review: Roles of Prebiotics in Intestinal Ecosystem of Broilers. Front. Vet. Sci. 2018. [Google Scholar] [CrossRef]
- Elmusharaf, M.A.; Bautista, V.; Nollet, L.; Beynen, A.C. Effect of a mannanoligosaccharide preparation on Eimeria tenella infection in broiler chickens. Int. J. Poult. Sci. 2006, 5, 583–588. [Google Scholar]
- Gomez-Verduzco, G.; Cortes-Cuevas, A.; Lopez-Coello, C.; Avila-Gonzalez, E.M.; Nava, G. Dietary supplementation of mannan-oligosaccharide enhances neonatal immune responses in chickens during natural exposure to Eimeria spp. Acta Vet. Scand. 2009, 51, 11. [Google Scholar] [CrossRef] [PubMed]
- Barberis, A.; Alloui, N.; Bennoune, O.; Ayachi, A.; Agabou, A. Effect of Using an Anticoccidial and a Prebiotic on Production Performances, Immunity Status and Coccidiosis in Broiler Chickens. Asian J. Poult. Sci. 2015, 9, 133–143. [Google Scholar] [CrossRef]
- Elmusharaf, M.A.; Peek, H.W.; Nollet, L.; Beynen, A.C. The effect of an in-feed Mannanoligosaccharide preparation (MOS) on a coccidiosis infection in broilers. Anim. Feed Sci. Technol. 2007, 134, 347–354. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, H.J.; Yu, S.H.; Wu, S.G.; Yoon, I.; Quigley, J.; Gao, Y.P.; Qi, G.H. Effects of yeast culture in broiler diets on performance and immunomodulatory functions. Poult. Sci. 2008, 87, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Al-Mansour, S.; Al-Khalf, A.; Al-Homidan, I.; Fathi, M.M. Feed efficiency and blood hematology of broiler chicks given a diet supplemented with yeast culture. Int. J. Poult. Sci. 2011, 10, 603–607. [Google Scholar]
- Yakhkeshi, S.; Rahimi, S.; Gharib Naseri, K. The effects of comparison of herbal extracts, antibiotic, probiotic and organic acid on serum lipids, immune response, GIT microbial population, intestinal morphology and performance of broilers. J. Med. Plants. 2011, 10, 80–95. [Google Scholar]
- Oso, A.O.; Idowu, O.M.O.; Haastrup, A.S.; Ajibade, A.J.; Olowonefa, K.O.; Aluko, A.O.; Ogunade, I.M.; Osho, S.O.; Bamgbose, A.M. Growth performance, apparent nutrient digestibility, caecal fermentation, ileal morphology and caecal microflora of growing rabbits fed diets containing probiotics and prebiotics. Livest. Sci. 2013, 157, 184–190. [Google Scholar] [CrossRef]
- Mourao, J.L.; Pinheiro, V.; Alves, A.; Guedes, C.M.; Pinto, L.; Saavedra, M.J.; Spring, P.; Kocher, A. Effect of mannan oligosac-Effect of mannan oligosaccharides on the performance, intestinal morphology and cecal fermentation of fattening rabbits. Anim. Feed Sci. Technol. 2006, 126, 107–120. [Google Scholar] [CrossRef]
- Piccolo, G.; Bovera, F.; Di Meo, C.; Vella, N.; Cutrignelli, M.; Nizza, A. Mannan oligosaccharides as growth promoter in finishing rabbit: Effect on in vivo performance and carcass traits. Ital. J. Anim. Sci. 2009, 8, 796–798. [Google Scholar] [CrossRef]
- Coles, B. Aids in diagnosis. In Essentials of Avian Medicine and Surgery; Coles, B., Ed.; Blackwell Publishing Ltd.: Oxford, UK, 2007; pp. 56–102. [Google Scholar]


| Group | 5 DPI | 7 DPI | 9 DPI | 10 DPI |
|---|---|---|---|---|
| NN-P * | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 0.00 ± 0.00 c |
| NI-P ** | 269 × 103 ± 50.78 a | 175.83 × 103 ± 38.68 a | 157.33 × 103 ± 43.91 a | 130.83 × 103 ± 43.38 a |
| PS-P *** | 97.33 × 103 ± 19.63 b | 83.66 × 103 ± 15.47 b | 77.83 × 103 ± 15.39 b | 57.33 × 103 ± 2.84 b |
| Group | Day 0 (Prebiotic Supplementation) | 10 DPPS | 10 DPI | WEIGHT Gain at End of Experiment |
|---|---|---|---|---|
| NN-P * | 820.00 ± 64.29 a | 1000.33 ± 3.33 a | 1080.33 ± 3.33 a | 80.00 ± 0.00 a |
| NI-P ** | 810.00 ± 37.85 a | 900.66 ± 4.66 b | 870.66 ± 6.66 c | −30.00 ± 8.81 b |
| PS-P *** | 846.66 ± 63.85 a | 980.33 ± 2.88 a | 970.33 ± 31.79 b | −10.00 ± 3.33 b |
| Group | 0 DPPT | 2 DPPT * | 3 DPPT | 4 DPPT | 5 DPPT | 7 DPPT |
|---|---|---|---|---|---|---|
| PS-T ** | 146.33 × 103 ± 29.42 | 97 × 103 ± 23.43 | 67 × 103 ± 16.37 | 30.66 × 103 ± 12.44 | 31.66 × 103 ± 21.85 | 4 × 103 ± 0.00 b |
| UI-T *** | 146 × 103 ± 30.51 | 90.66 × 103 ± 12.57 | 70 × 103 ± 18.33 | 47.33 × 103 ± 14.11 | 39.33 × 103 ± 12.34 | 32 × 103 ± 7.54 a |
| Group | 0 DPPT | 7 DPPT * | Weight Gain at End of Experiment |
|---|---|---|---|
| PS-T ** | 751.66 ± 28.91 | 756.66 ± 30.32 | 6.00 ± 2.88 |
| UI-T *** | 733.33 ± 8.81 | 706.66 ± 6.66 | −26.66 ± 8.33 |
| Group | Ileum | Duodenum | Jejunum |
|---|---|---|---|
| NN-P | 0.00 ± 0.00 c | 0.33 ± 0.33 c | 0.66 ± 0.33 d |
| NI-P * | 18.33 ± 2.02 a | 23 ± 1.15 a | 35 ± 1.15 a |
| PS-P ** | 7.00 ± 1.20 b | 5.33 ± 0.88 b | 7.66 ± 0.88 c |
| PS-T *** | 7.33 ± 1.20 b | 6.33 ± 0.88 b | 8.66 ± 1.45 c |
| UI-T **** | 16.00 ± 2.08 a | 20.33 ± 1.45 a | 30.66 ± 1.20 b |
| Group | Total Protein | ALT | AST | ALP | Total Cholesterol | Createnine |
|---|---|---|---|---|---|---|
| NN-P * | 6.42 ± 0.57 | 50.33 ± 3.17 b | 41.00 ± 4.04 b | 69.33 ± 28.58 a | 68.66 ± 14.43 a | 0.92 ± 0.18 a |
| NI-P ** | 6.39 ± 0.24 | 67.66 ± 8.56 b | 103.00 ± 6.42 a | 73.00 ± 7.00 a | 91.00 ± 11.54 a | 0.95 ± 0.25 a |
| PS-P *** | 5.78 ± 0.29 | 105.66 ± 7.53 a | 37.66 ± 7.88 b | 63.33 ± 14.52 a | 66.33 ± 12.71 a | 0.72 ± 0.14 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Ashram, S.A.; Aboelhadid, S.M.; Abdel-Kafy, E.-S.M.; Hashem, S.A.; Mahrous, L.N.; Farghly, E.M.; Moawad, U.K.; Kamel, A.A. Prophylactic and Therapeutic Efficacy of Prebiotic Supplementation against Intestinal Coccidiosis in Rabbits. Animals 2019, 9, 965. https://doi.org/10.3390/ani9110965
El-Ashram SA, Aboelhadid SM, Abdel-Kafy E-SM, Hashem SA, Mahrous LN, Farghly EM, Moawad UK, Kamel AA. Prophylactic and Therapeutic Efficacy of Prebiotic Supplementation against Intestinal Coccidiosis in Rabbits. Animals. 2019; 9(11):965. https://doi.org/10.3390/ani9110965
Chicago/Turabian StyleEl-Ashram, Saeed A., Shawky M. Aboelhadid, El-Sayed M. Abdel-Kafy, Shymaa A. Hashem, Lilian N. Mahrous, Eman M. Farghly, Usama K. Moawad, and Asmaa A. Kamel. 2019. "Prophylactic and Therapeutic Efficacy of Prebiotic Supplementation against Intestinal Coccidiosis in Rabbits" Animals 9, no. 11: 965. https://doi.org/10.3390/ani9110965
APA StyleEl-Ashram, S. A., Aboelhadid, S. M., Abdel-Kafy, E.-S. M., Hashem, S. A., Mahrous, L. N., Farghly, E. M., Moawad, U. K., & Kamel, A. A. (2019). Prophylactic and Therapeutic Efficacy of Prebiotic Supplementation against Intestinal Coccidiosis in Rabbits. Animals, 9(11), 965. https://doi.org/10.3390/ani9110965






